Abstract
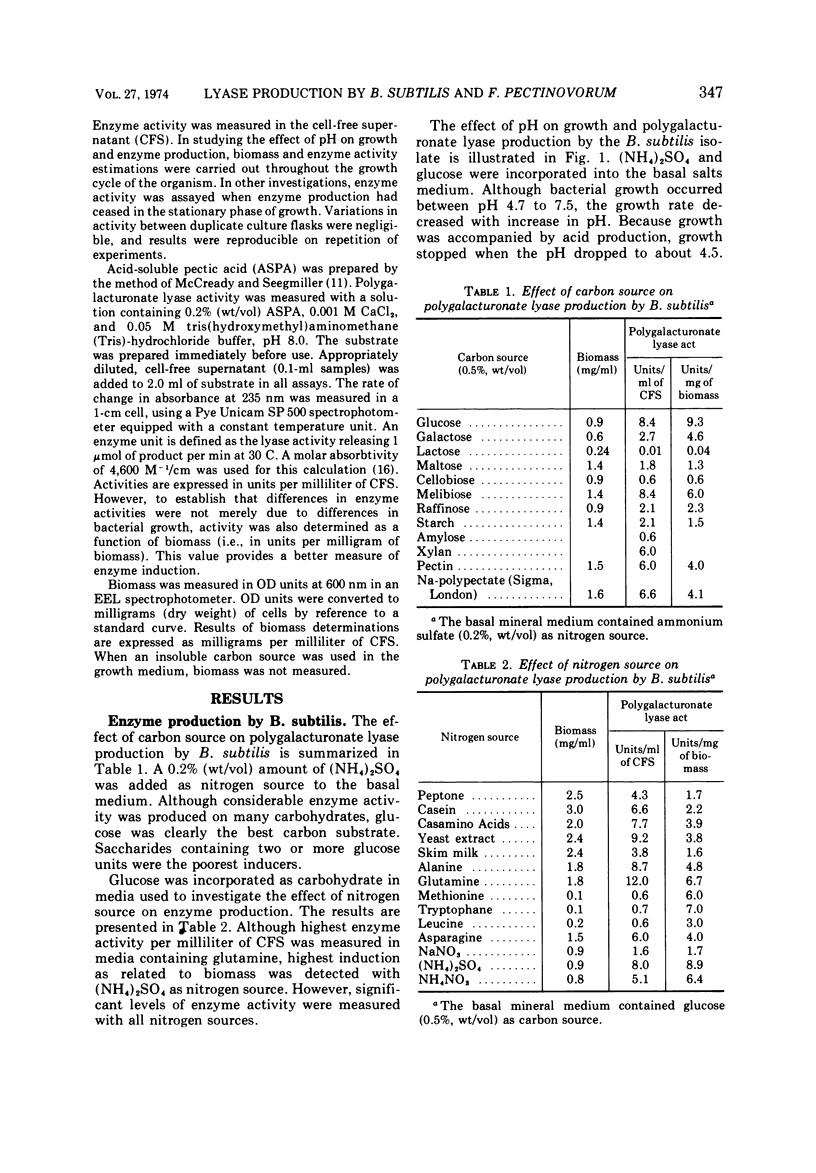
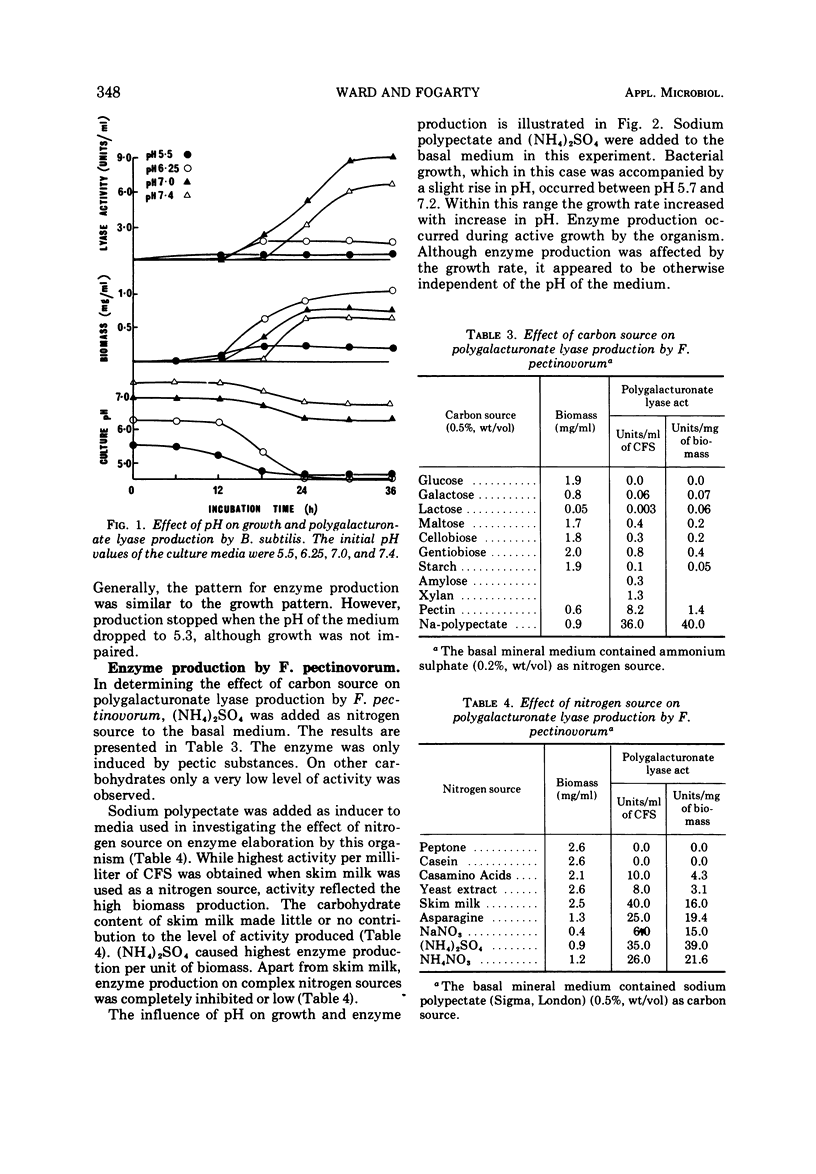
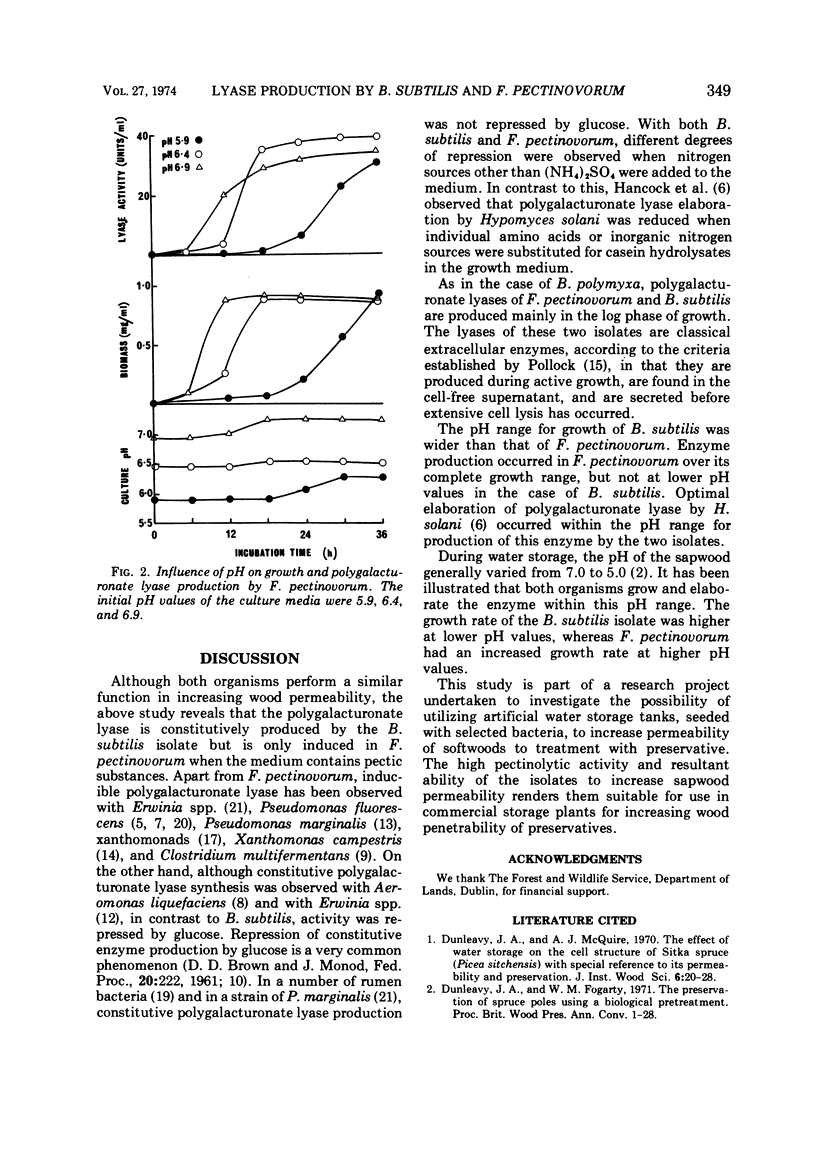
Nutritional factors relating to the production of polygalacturonate lyases by strains of Bacillus subtilis and Flavobacterium pectinovorum were examined. Studies were carried out in shake flask cultures. In the case of B. subtilis the enzyme was produced constitutively, whereas in the case of F. pectinovorum it was only produced in quantity in the presence of pectic substances. Glucose was the most suitable carbon source for production of the polygalacturonate lyase of B. subtilis; of the nitrogen sources examined, the highest activities per milliliter of supernatant and per milligram of cells were obtained with glutamine and ammonium sulfate, respectively. The pattern of enzyme production and growth was similar although enzyme production ceased at pH 5.3. Sodium polypectate was the best inducer of polygalacturonate lyase with F. pectinovorum. Highest activity per milliliter of cell-free supernatant was obtained with skin milk powder as nitrogen source, although ammonium sulfate gave highest enzyme production per unit of biomass. Growth of F. pectinovorum occurred between pH 5.7 and 7.2. Enzyme production occurred during active growth and was independent of the pH of the medium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fuchs A. The trans-eliminative breakdown of Na-polygalacturonate by Pseudomonas fluorescens. Antonie Van Leeuwenhoek. 1965;31(3):323–340. doi: 10.1007/BF02045912. [DOI] [PubMed] [Google Scholar]
- Hancock J. G., Eldridge C., Alexander M. Characteristics of pectate lyase formation by Hypomyces solani f. sp. cucurbitae. Can J Microbiol. 1970 Feb;16(2):69–74. doi: 10.1139/m70-013. [DOI] [PubMed] [Google Scholar]
- Hsu E. J., Vaughn R. H. Production and catabolite repression of the constitutive polygalacturonic acid trans-eliminase of Aeromonas liquefaciens. J Bacteriol. 1969 Apr;98(1):172–181. doi: 10.1128/jb.98.1.172-181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACMILLAN J. D., VAUGHN R. H. PURIFICATION AND PROPERTIES OF A POLYGALACTURONIC ACID-TRANS-ELIMINASE PRODUCED BY CLOSTRIDIUM MULTIFERMENTANS. Biochemistry. 1964 Apr;3:564–572. doi: 10.1021/bi00892a016. [DOI] [PubMed] [Google Scholar]
- McCREADY R. M., SEEGMILLER C. G. Action of pectic enzymes on oligogalacturonic acids and some of their derivatives. Arch Biochem Biophys. 1954 Jun;50(2):440–450. doi: 10.1016/0003-9861(54)90060-0. [DOI] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Pectic enzymes of pseudomonas marginalis. Phytopathology. 1966 Dec;56(12):1414–1415. [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonic acid trans-eliminase of Xanthomonas campestris. Biochem J. 1967 Jul;104(1):178–185. doi: 10.1042/bj1040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchinger H., Wieland T. Suche nach einem Metaboliten bei Vergiftung mit Desmethylphalloin (DMP) Eur J Biochem. 1969 Nov;11(1):1–6. doi: 10.1111/j.1432-1033.1969.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Wojciechowicz M. Partial characterization of pectinolytic enzymes of Bacteroides ruminicola isolated from the rumen of a sheep. Acta Microbiol Pol A. 1971;3(1):45–56. [PubMed] [Google Scholar]
- Zucker M., Hankin L. Inducible pectate lyase synthesis and phytopathogenicity of Pseudomonas fluorescens. Can J Microbiol. 1971 Oct;17(10):1313–1318. doi: 10.1139/m71-210. [DOI] [PubMed] [Google Scholar]


