Abstract
Purpose:
Fracture healing involves complex processes of cell and tissue proliferation and differentiation. Many factors are involved, including growth factors, inflammatory cytokines, antioxidants, bone breakdown (osteoclast) and bone building (osteoblast) cells, hormones, amino acids, and uncounted nutrients. We studied the osteogenic potential of Cissus quadrangularis (CQ), a plant that has been customarily used in the Indian subcontinent to hasten the process of healing in bone fractures.
Materials and Methods:
Total of 60 patients (age, 20-35 years) of mandible fracture was divided in two groups. Patients of group 1 were given capsules of CQ and fracture healing was assessed with osteopontin expression during treatment. Group 2 was control group.
Results:
Clinical and radiological analysis in our study was suggestive of better healing of fractures in group 1. All the samples of group 1 examined for osteopontin expression using western blot analysis and flow cytometry showed significant levels of expression of osteopontin protein and CD4+ T cells expressing osteopontin, respectively.
Conclusion:
We conclude that CQ accelerates fracture healing and also causes early remodeling of fracture callus.
Keywords: Cissus quadrangularis, flow cytometry, osteopontin, western blot
INTRODUCTION
Cissus quadrangularis (CQ) has been used by common man in India for fastening of fracture healing. and well-known as “Hadjod.” It is also known as Vitis quadrangularis wall which belongs to family Vitaceae. It has been prescribed in ayurveda as an alternative, anthelmintic, dyspeptic, digestive, tonic, analgesic in eye and ear diseases, and in the treatment of irregular menstruation and asthma. In some parts of world, the whole plant is used in oral rehydration, while the leaf, stem, and root extracts of this plant are important in the management of various ailments. Some other reports on CQ justifies its effectiveness in management of obesity and complications associated with metabolic disorders,[1] as well as its antioxidant and free radical scavenging activity in vitro.[2,3]
The plant and its medicinal properties have been described in ancient books such as Bhawa Prakash.[4] The fresh stem and leaves of CQ are used for the treatment of hemorrhoids, menstrual disorders, scurvy, and flatulence. Extracts of this plant are reported to contain phytoestrogenic steroids, ascorbic acid, carotene, calcium and anabolic steroids.[4,5,6] Murthy et al.,[7] reported the antibacterial and antioxidant activities of the CQ extract. CQ was also recently shown to have significant effects in periodontal regenerative therapy.[8]
CQ, a rambling shrub, characterized by a thick quadrangular fleshy stout stem, commonly known as the “Bone Setter,” the plant is referred to as “Asthisamdhani” in Sanskrit and “Hadjod” in Hindi because of its ability to join bones. A phytogenic isolated steroid is believed to be the main constituent in CQ. The plant extract has been shown to have bone fracture healing properties in several in vivo studies.[9,10,11] Studies on fracture healing suggest that this unidentified anabolic steroid may act on estrogenic receptors of the bone. Efficacy of CQ on early ossification and remodeling of bones have been reported and it has been observed that CQ acts by stimulation of metabolism and increased uptake of the minerals calcium, sulfur, and strontium by the osteoblasts in fracture healing.[12,13] Using an ovariectomized rat model for osteoporosis, Shirwaiker et al.,[11] found that the ethanolic extract of CQ has bone-healing properties. Recently, it has been shown that the petroleum ether extract of CQ enhances fetal bone growth and ossification.[14]
Phytochemical analyses of CQ have revealed high contents of ascorbic acid, carotene, anabolic steroidal substances, and calcium.[5] The stem contains two asymmetric tetracyclic triterpenoids and two steroidal principles. The presence of β-sitosterol, δ-amyrin, δ-amyrone, and flavanoids (quercetin) has also been reported.[3,15] All of these components have potentially different metabolic and physiologic effects.
Osteopontin is an extracellular matrix protein with diverse localization and functionality that has multiple, stage-dependent roles during fracture healing. Study by Duvall et al.,[16] specifically indicates the role of osteopontin in early callus formation, neovascularization, biomechanical strength and ductility, and in remodeling of fracture callus. So, we used osteopontin as biochemical marker in our study to assess the fracture healing in patients treated with CQ.
MATERIALS AND METHODS
A total of 60 patients of simple mandible fracture were included in this study and patients were randomly divided into two groups (30 patients in each group).
The grouping was made on the basis of coded drugs these patients received.
Group 1 capsules of CQ-two capsules B.D
Group 2 capsules of placebo-two capsules B.D.
One capsule of CQ = 300 mg powder of dried stem of CQ, capsules of placebo were made of starch powder. The drugs were provided in capsules form by International Institute of Herbal Medicine and they are certified organic, they are free from any pesticides and chemicals and heavy metals. The drugs were given in a double-blind fashion and the code was decoded at the end of study only or at the time of some serious side effects, noted, if any.
Patients were diagnosed on the basis of clinical examination and radiographic interpretation. The cause of trauma, interval from injury to trial of drug, average age, gender, and site distribution were all reviewed and recorded on a set proforma. A thorough clinical, radiological, biochemical, and hematological profile was carried out. Subsequent investigations were carried out during the period of healing till the mobility at the fracture site became zero. Follow-up was done at 1, 3, 6, and 8 weeks. Results were assessed on the basis of clinical, biochemical, and radiological findings at 1st week, 3rd week, 6th week, and 3 months based on the following parameters pain, swelling, mobility, bite force recording, OPG, serum calcium, serum phosphates, serum alkaline phosphatase, and osteopontin.
For osteopontin expression, blood samples of patients were collected and send for the analysis to the Indian Institute of Toxicology Research, Lucknow, India.
Flow cytometric analysis for the expression of osteopontin: Blood sample (1.0 mL) containing ethylenediaminetetraacetic acid was immediately mixed with equal amount of nutrient mixture F-12 (Ham) medium supplemented with 10% fetal bovine serum, 0.2% sodium bicarbonate and antibiotic and antimycotic solution (10×, 1 mL/100 mL of medium, Invitrogen, Life Technologies, USA) and 40 μg/mL brefeldin (Sigma, St Louis, MO, USA). The cells were then incubated for 24 h in 5% CO2 -95% atmosphere in high humidity at 37°C. Following the incubation, cells were washed with ice-cold PBS and cells were recovered by centrifugation and adjusted to 5 × 105 white blood cells per test. The fixation and permiabilization of cells were performed using Intra-Prep™ reagent. The cells were then stained with antihuman osteopontin monoclonal antibody (Immuno-Biological Laboratories, Fujioka, Japan), PE-labeled antimouse immunoglobulin G (IgG) antibody (BD Pharmingen, San Diego, CA, USA), and FITC-labeled anti-human CD4 antibody (BD Pharmingen) and processed for flowcytometric analysis. The flow cytometric analysis was performed to evaluate the expression of osteopontin using a FACScan™ instrument (Becton Dickinson, San Jose, CA, USA). Nonspecific staining with the isotype-matched control with monoclonal antibody was ascertained prior to perform the experimental analysis.
Western blot analysis for the expression of osteopontin: The recovery process for white blood cells from the blood samples was similar as described earlier in case of flow cytometric analysis. Western blot analysis was carried out to study the expression levels of osteopontin protein in CD4+ T cells of peripheral blood of volunteers enrolled in the study at three time points following the first treatment. Cells were pelleted and lysed using CelLytic M Cell Lysis Reagent (Catalog no. C2978, Sigma, USA) in the presence of 1 × protein inhibitor cocktail (Catalog no. P8340, Sigma, USA). Protein estimation was done by BCA Protein Assay Kit (catalog no. G1002, Lamda Biotech, Inc., St. Louis, MO, USA). Equal amounts (50 μg/well) of proteins were loaded in 10% tricine-SDS gel and blotted on polyvinylidene fluoride membranes using wet transfer system. After blocking for 2 h at 37°C, the membranes were incubated overnight at 4°C with antihuman osteopontin monoclonal antibody (1:1000, Immuno-Biological Laboratories, Fujioka, Japan) in blocking buffer (pH 7.5). The membrane was then incubated for 2 h at room temperature with secondary antiprimary IgG-conjugated with horseradish peroxidase (Calbiochem, USA). The blots were developed using either luminol (catalog no. 34080, Thermo Scientific, USA) or by TMB-H2O2 (Sigma, USA), and densitometry for protein specific bands was done in Gel Documentation System (Alpha Innotech, USA) with the help of AlphaEase FC Stand Alone V. 4.0.0 software. Actin-β was used as an internal control to normalize the data. Data of second and third sampling were compared with data of first sampling and expressed in relative quantities (fold change).
RESULTS
There was significant reduction in pain, swelling, and mobility at fracture site at different follow-ups in CQ group when compared to control. Incisor and molar biting force increase was more in patients of Cissus group when compared to controls at different follow-up. In biochemical analysis, serum calcium level and alkaline phosphatase level were significantly increased in Cissus group.
We examined the frequency of osteopontin-expressing CD4+ T cells among the T cells by flow cytometry. All the samples of group 1 were having significant percent of CD4+ T cells expressing osteopontin. The percent numbers of cells expressing the osteopontin were more or less similar in all the three points of sample collection, that is, 0, 4, and 6 weeks after therapeutic interventions. The interindividual variation between different volunteers for osteopontin expressing CD4+ T cells was also nonsignificant and shown linearity response.
Osteopontin expression using western blot analysis showed significant levels of expression of osteopontin protein. However, therapeutic interventions could not induce the alterations in the magnitude of expression of osteopontin protein at any point of sample collection, that is, 0, 4, and 6 weeks after the first intervention. [Figures 1 and 2] The data gathered from flow cytometric analysis and western blot analyses were having good correlation and linearity.
Figure 1.
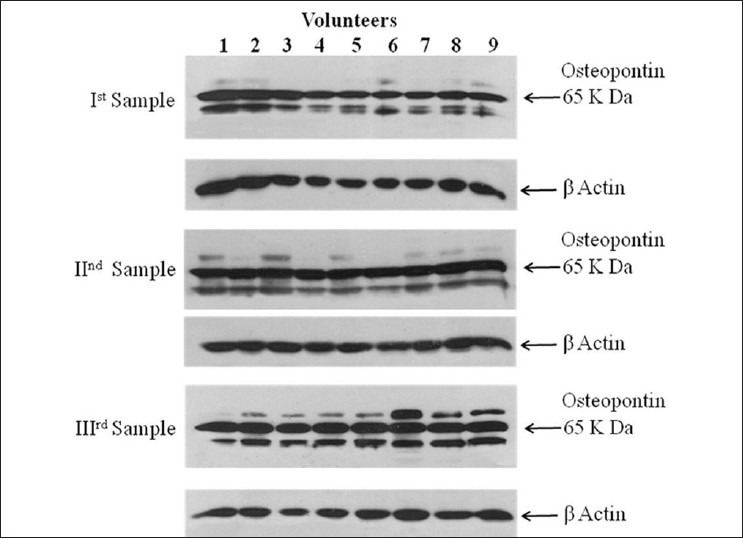
Western blot analysis of osteopontin expression in the CD4+ T cells of peripheral blood of human volunteers at different time intervals following the therapeutic interventions
Figure 2.
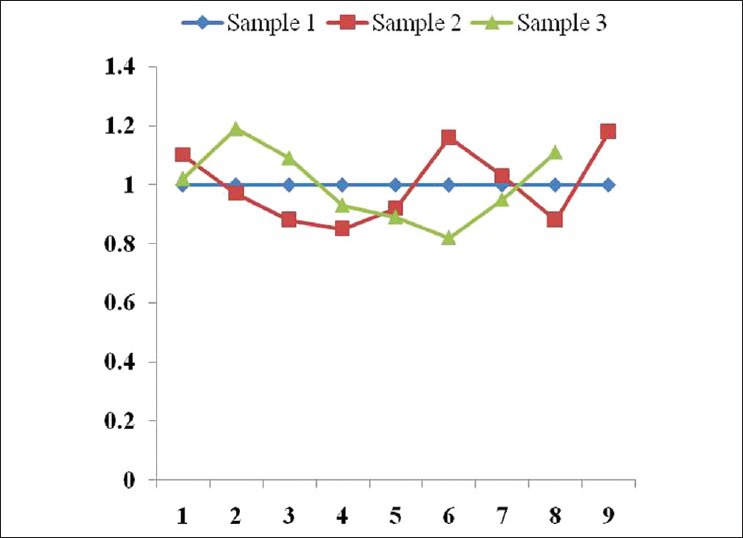
Fold change in the expression (western blot analysis) of osteopontin protein considering protein expression in sample collected at first time as basal level
DISCUSSION
Plants have long been the principal tools of traditional medical systems. Although ancient in origins, many traditional medical paradigms and their pharmacopoeias have evolved into quite sophisticated healing systems, using thousands of plants and other natural materials such as minerals and animal products. In many parts of the world, traditional medicine is still used to provide the major part of primary health care. The World Health Organization has estimated that the majority of the world's population depends on botanical medicines for basic health care needs. Reasons for this include the fact that these local systems are culturally acceptable, cheaper for many conditions as compared to biomedicine, and efficacious in many of the circumstances in which they are used.
Systemic use of CQ in rats caused complete restoration of normal composition of bone, after fracture in 4 weeks, while the controls required 6 weeks. There was a shortening of about 2 weeks in the bone-healing duration. The total weight of the fractured bone also came down toward normal much earlier than the controls indicating quickest bone remodeling. All the events namely fibroblastic phase (1st week), collagen phase (2nd week) and osteochondroital phase (3rd and 4th weeks) were hastened by about 10-14 days in the treated group. This hastening in the fracture healing was attributed to the stimulation of all the cells of mesenchyma origin, namely the fibroblasts, the chondroblasts and osteoblasts by CQ. It has greater impact on osteoblastic proliferation than other cellular responses. In both the models, the mucopolysaccharide and collagen levels of the bones in the treated group came down to normal at the end of only 4 weeks, while the control required 6 weeks as confirmed with histological and histochemical observations.[17,18,19]
Paste of alcoholic extract of CQ was locally as well as intramuscularly facilitates rapid healing of fracture in albino rats.[20] Ethanol extract (95%) of CQ enhances the development of cortical bone and trabeculae in fetal fumor, which may be related to rich content of calcium, phosphorous and phytoestrogenic steroids, and shown to influence early regeneration and quick mineralization of bone fracture healing process. Ethanol extract (95%) of whole plant possess antiosteoporotic activity in ovariectomized rat model of osteoporosis at two different dose levels of 500 and 750 mg/kg/weight.[21]
Bone marrow mesenchymal stem cells are the source of bone-forming osteoblast.[22] The petroleum ether extract of CQ can stimulate the differentiation of mesenchymal stem cells into osteoblasts in a dose-dependent manner even in the absence of osteogenic conditioning media. This activity of the plant extract is increased further in the presence of osteogenic media. The plant extract also facilitated extracellular matrix mineralization, which was more pronounced in the presence of osteogenic media.
Clinical and radiological analysis in our study was suggestive of better healing of fractures in group 1 treated with indegineous herb when compared to control group. To get more conclusive and definitive results, we did the analysis of biochemical marker (osteopontin).
In our study, all the blood samples of patients of group 1 analyzed for osteopontin expression by flow cytometric analysis and western blot analysis were having significant percent of CD4+ T cells expressing osteopontin. However, there was no significant variation in percent change in the osteopontin expressing CD4+ T cells in all the samples analyzed at different time of collection. This may be justified as OPN is multifunctional during fracture healing and has role in various phases of fracture healing. Increased OPN level in early samples collected at 1st and 4th weeks is suggestive of early neovascularization and early callus formation.[16]
Duvall et al.,[16] showed multiple, stage-dependent roles of OPN during fracture healing. They conclude that OPN deficiency alters the functionality of multiple cell types, resulting in delayed early vascularization, altered matrix organization and late remodeling, and reduced biomechanical properties. Their study indicates that OPN plays multiple roles during early callus formation, neovascularization, and late stage remodeling. The increased expression of osteopontin level in our study indicates early recovery from injury and bone remodeling.[16] Study by Terai et al.,[23] strongly suggests that OPN is an important factor triggering bone remodeling. Our findings also show increased OPN level in sample collected at 6th week, which may be due to callus remodeling.
However, therapeutic interventions could not induce the significant alterations in the magnitude of expression of osteopontin protein at any point of sample collection, that is, 0, 4, and 6 weeks after the first intervention. Interestingly, the trend for the osteopontin expression was almost similar in the samples collected from all the volunteers. This may be because of the low sample size which was analyzed for osteopontin as this was not available in our university and the cost of the investigation was high in other central laboratories. A much larger sample size could be more informative and conclusive in this regard.
CONCLUSION
In recent years, ethnobotanical and traditional uses of natural compounds, especially of plant origin received much attention as they are well-tested for their efficacy and generally believed to be safe for human use. Needless to mention that the root and stem extracts of the plant CQ have therapeutic efficacy and are known to possess antioxidant, antimicrobial activity, and are routinely used to accelerate the process of bone fracture healing. Our study proved that CQ accelerates fracture healing and also causes early remodeling of fracture callus; thus, it can be utilized for faster and enhanced fracture healing along with advanced surgical techniques of fracture management.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Oben J, Kuate D, Agbor G, Momo C, Talla X. The use of a cissus quadrangularis formulation in the management of weight loss and metabolic syndrome. Lipids Health Dis. 2006;5:24. doi: 10.1186/1476-511X-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallika J, Shyamala CS. In vitro and In vivo evaluation of free radical scavenging potential of Cissus quadrangularis. Afr J Biomed Res. 2005;8:95–9. [Google Scholar]
- 3.Mehta M, Kaur N, Bhutani KK. Determination of marker constituents from Cissus quadrangularis Linn and their quantitation by HPTLC and HPLC. Phytochem Anal. 2001;12:91–5. doi: 10.1002/pca.569. [DOI] [PubMed] [Google Scholar]
- 4.Udupa KN, Prasad G. Biomechanical and Calcium-45 studies on the effect of Cissus quadrangularis in fracture repair. Indian J Med Res. 1964;52:480–7. [PubMed] [Google Scholar]
- 5.Sen SP. Preliminary clinical studies of Cissus quadrangularis. J Med Res. 1963;4:26. [Google Scholar]
- 6.Singh LM, Udupa KN. Studies on “Cissus quadrangularis” in fracture by using phosphorus 32. III. Ind J Med Sci. 1962;16:926–31. [PubMed] [Google Scholar]
- 7.Chidambara Murthy KN, Vanitha A, Mahadeva Swamy M, Ravishankar GA. Antioxidant and antimicrobial activity of Cissus quadrangularis. J Med Food. 2003;6:99–105. doi: 10.1089/109662003322233495. [DOI] [PubMed] [Google Scholar]
- 8.Jain A, Dixit J, Prakash D. Modulatory effects of Cissus quadrangularis on periodontal regeneration by bovine-derived hydroxyapatite in intrabony defects: Exploratory clinical trial. J Int Acad Periodontol. 2008;10:59–65. [PubMed] [Google Scholar]
- 9.Chopra SS, Patel MR, Gupta LP, Datta IC. Studies on Cissus quadrangularis in experimental fracture repair: Effect of chemical parameters in blood. Indian J Med Res. 1975;63:824–8. [PubMed] [Google Scholar]
- 10.Sanyal A, Ahmad A, Sastry M. Calcite growth in Cissus quadrangularis plant extract, a traditional Indian bone healing aid. Curr Sci. 2005;89:1742–5. [Google Scholar]
- 11.Shirwaiker A, Khan S, Malini S. Antiosteoporotic activity of Ethanol extract of Cissus quadrangularis Linn on ovariectomized rat. J Ethnopharmocol. 2003;89:245–50. doi: 10.1016/j.jep.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Prasad GC, Udupa KN. Pathways and site of action of a phytogenic steroid from Cissus quadrangularis. J Res Indian Med. 1972;4:132. [Google Scholar]
- 13.Udupa KN, Prasad G, Sen SP. The effect of phytogenic steroid in the accleration of fracture repair. Life Sci. 1965;4:317–27. doi: 10.1016/0024-3205(65)90148-7. [DOI] [PubMed] [Google Scholar]
- 14.Potu BK, Rao MS, Kutty GN, Bhat MR, Chamallamudi MR, Nayak SR. Petroleum ether extract of Cissus quadrangularis (LINN) stimulates the growth of fetal bone during intrauterine developmental period: A morphometric analysis. Clinics (Sao Paulo) 2008;63:815–20. doi: 10.1590/S1807-59322008000600018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakikasem S, Limsiriwong P, Kajsongkarm T, Sontorntanasart T. Phytochemical study of Cissus quadrangularis. Thai J Pharm Sci. 2000;24:5. [Google Scholar]
- 16.Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res. 2007;22:286–97. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- 17.Singh LM, Udupa KN. Studies of Cissus quadrangularis in fracture by using phosphorus 32 III. Indian J Med Sci. 1962;76:926–31. [PubMed] [Google Scholar]
- 18.Sen SP, Udupa KN, Prasad G. Studies on the active constituents. 10 Further studies on the effect of Cissus quadrangularis in accelerating fracture healing. Indian J Med Res. 1966;52:26–35. [PubMed] [Google Scholar]
- 19.Kausch AP, Horner HT. A comparison of calcium oxalate crystals isolated from callus cultures and their explant sources. Scan Electron Microsc. 1982;1:199–211. [Google Scholar]
- 20.Prasad GC, Udupa KN. Effect of Cissus quadrangularis on the healing of cortisone treated fracture. Indian J Med Res. 1963;51:667–76. [PubMed] [Google Scholar]
- 21.Rao MS, Bhagath KP, Narayana Swamy VB, Gopalan KN. Cissus quadrangularis plant extract enhances the development of cortical bone and trabeculae in the fetal femur. Pharmacol Online. 2007;3:190–202. [Google Scholar]
- 22.Manolagas SC. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–37. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 23.Terai K, Takano-Yamamoto T, Ohba Y, Hiura K, Sugimoto M, Sato M, et al. Role of osteopontin in bone remodeling caused by mechanical stress. J Bone Miner Res. 1999;14:839–49. doi: 10.1359/jbmr.1999.14.6.839. [DOI] [PubMed] [Google Scholar]


