Abstract
Aims and Objectives:
Platelet rich fibrin is widely used in stimulation and acceleration of soft tissue and bone healing because of local and continuous delivery of growth factors and proteins, mimicking the needs of the physiological wound healing and reparative tissue processes. This article will serve to introduce a second generation platelet concentrate, platelet-rich fibrin (PRF).
Materials and Methods:
Fifteen cases are presented in which conventional endodontic therapy failed to resolve the problem and periapical root-end surgery was required.
Results:
At the end of six months, all patients showed complete bone regeneration.
Conclusion:
Production of a dense, cross-linked, physically robust PRF made of intact platelets and fibrin by high-speed centrifugation in the absence of exogenous thrombin, yields an ideal scaffold for use in tissue repair.
Keywords: Periapical surgery, platelet growth factor, platelet rich fibrin, wound healing
INTRODUCTION
The success of endodontic therapy depends on complete periapical repair and regeneration. Most of the time teeth with periapical lesions heal satisfactorily after non-surgical endodontic intervention. Abramovitz et al. discussed the guidelines of case selection for apical surgery and non-surgical retreatment. They reported that treatment of 24.5% of the cases was impossible without surgical therapy.[1]
The healing of hard and soft tissue is mediated by wide range of intra and extracellular events that are regulated by signalling proteins. Platelets play a crucial role not only in hemostasis, but also in wound healing process.
Platelets are formed in bone marrow from megakaryocytes, and discoidal and anuclear structures. Their lifespan is 8 to 10 days, and the cytoplasm contains many granules whose contents are secreted at the time of activation.
α-Granules contain many proteins, platelet specific (such as β-thromboglobulin) or nonplatelet specific (fibronectin, thrombospondin, fibrinogen, and other factors of coagulation, growth promoters, fibrinolysis inhibitors, immunoglobulins, etc.). The dense granules contain calcium, serotonin etc.
Activation is fundamental to initiate and support hemostasis because of aggregation on the injured site and interactions with coagulation mechanisms. However, degranulation also implies the release of cytokines able to stimulate cell migration and proliferation within the fibrin matrix, launching the first stage of healing.[2]
MATERIALS AND METHODS
Fifteen patients, aged between 20 to 50 years, were selected for the study. The patients had been diagnosed with periradicular lesions of endodontic origin based on clinical signs and symptoms, intraoral and echographic findings. The patients were scheduled to be treated in the Department of Conservative Dentistry and Endodontics, Darshan dental college and hospital, Udaipur, Rajasthan.
The selected cases included those in which conventional endodontic therapy failed to resolve the problem and periapical root-end surgery was required. The bony defect had to be confined to the apical area with the bone covering the entire root surface coronally with an intact lingual cortical plate. Patients who agreed to take part in the study were asked to sign an informed consent form before undergoing the treatment. The first step of the treatment plan was to complete the root canal therapy in cases where dry canals existed and in others obturation was done on the day of surgery. The surgical protocol included a routine medical history followed by blood investigations. The surgical procedure included reflection of a full thickness mucoperiosteal flap by sulcular incision and two relieving vertical incisions. Debridement of tissues at the defect site was followed by irrigation with sterile saline solution.
Armamentarium and technique
PRF preparation requires an adequate table centrifuge and collection kit including: A 18 gauge needle and 10 ml blood collection tubes.
Protocol for PRF preparation
10 ml of venous blood was drawn from the patient. Whole blood was drawn into the tubes without anticoagulant and immediately centrifuged at 3,000 rpm for 10 minutes.
Within a few minutes, the absence of anticoagulant allows activation of the majority of platelets contained in the sample to trigger a coagulation cascade. The result is a fibrin clot containing the platelets located in the middle of the tube, just between the red blood cell layer at the bottom and acellular plasma at the top.
This clot was removed from the tube and the attached red blood cells scraped off and discarded [Figure 1].
Figure 1.
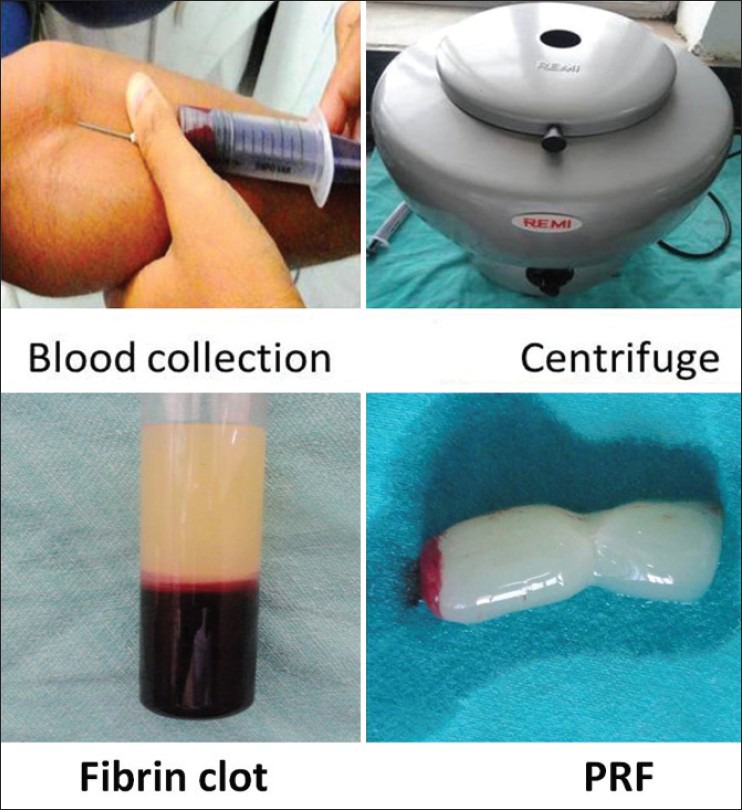
PRF preparation
PRF gel was carefully placed into the cavity till the entire cavity was filled. Wound closure was performed with a 3-0 black silk suture. Analgesics (Paracetamol + Nimusulidebd-3 days), Antibiotics (Amoxicillin + Clavulanic acid [Co-amoxyclav-625 mg] tid-5 days) were prescribed post-operatively.
The sutures were removed after seven days. The patients were reviewed after one week and one, two and six months. Standard IOPA radiography was done using the paralleling cone technique.
Case 1
24 year old male patient came to the Department of Conservative Dentistry and Endodontics Darshan dental college and hospital, Udaipur, Rajasthan, with the complaint of fractured tooth of upper front region of jaw since five year.
On investigation, periapical radiolucency involving 21, 22 was seen on IOPA and Color Doppler Echographic imaging revealed a granuloma of diameter 13 × 5 mm in size [Figure 2].
Figure 2.
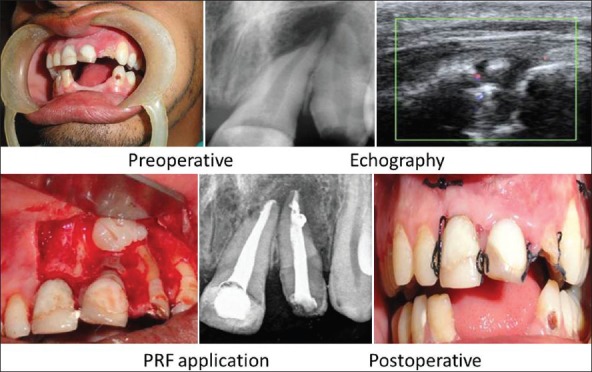
Case 1
Case 2
32 year old male patient came to the Department of Conservative Dentistry and Endodontics with the complaint of draining sinus from upper front region of jaw. Patient had undergone Root canal treatment elsewhere two years ago in the same tooth.
On investigation, periapical radiolucency involving 12 was seen on IOPA and Color Doppler Echographic imaging showed granuloma of diameter 10 × 8 mm in size [Figure 3].
Figure 3.
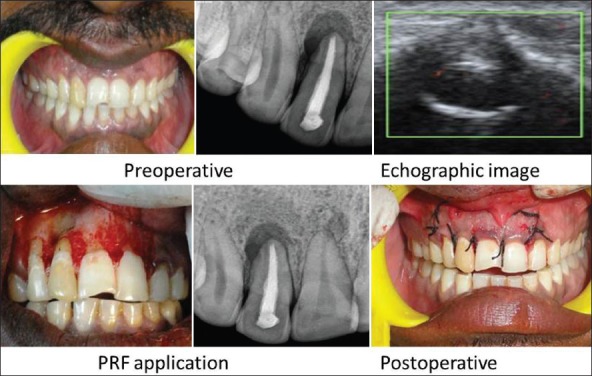
Case 2
Case 3
35 year old male patient came, with a complaint of discoloured tooth of upper front region of jaw since 8 years. Patient had history of trauma 17 years ago.
On investigation, periapical radiolucency involving 21, 22 was seen on IOPA and Color Doppler Echographic imaging showing periapical cyst of diameter 17 × 15 mm in size [Figure 4].
Figure 4.
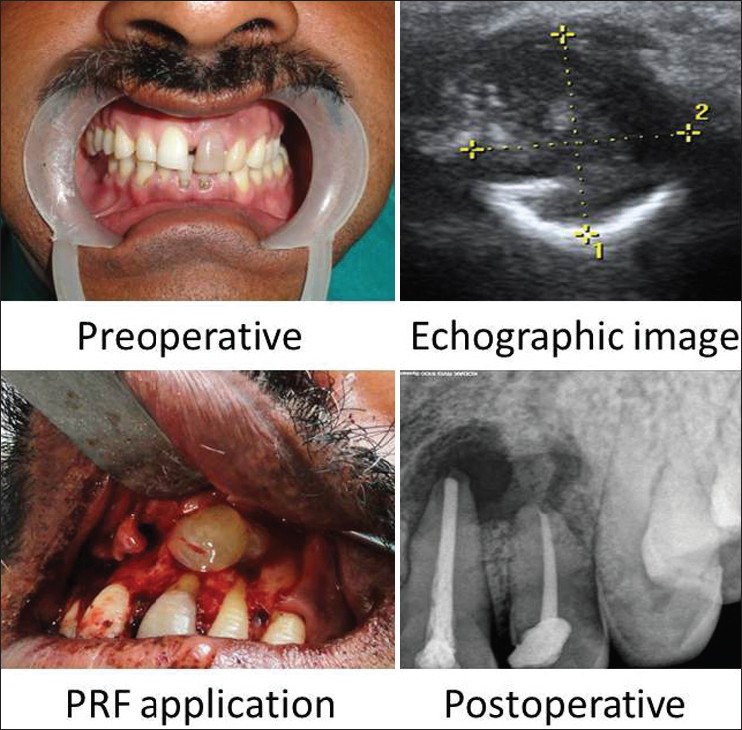
Case 3
RESULTS
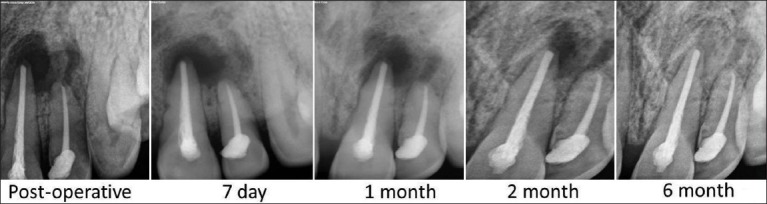
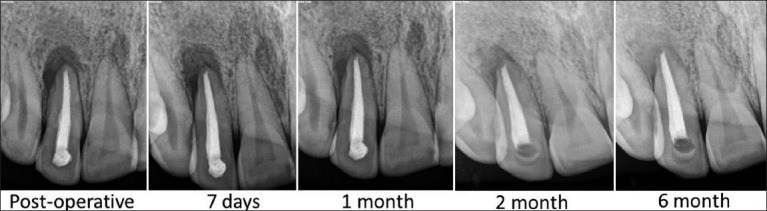
The patients did not complain of any unusual or severe pain. There were no signs of infection, untoward reaction, wound dehiscence or extrusion of material in any of the patients. Radiographically all patients showed complete bone regeneration at the end of six months [Figures 5-7].
Figure 5.
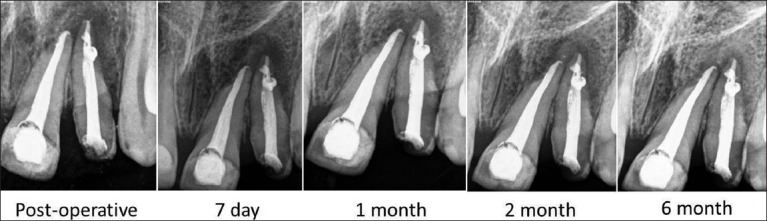
Case 1-Follow up
Figure 7.
Case 3-Follow up
Figure 6.
Case 2-Follow up
DISCUSSION
Recent bone healing studies are aimed to accelerate bone regeneration to maximize predictability as well as the volume of regenerated bone. It is difficult to state whether Bone morphogenetic protein-2 (BMP-2), recombinant platelet derived growth factor-BB (rhPDGF-BB), platelet rich plasma (PRP), plasma rich in growth factors (PRGF), or a combination of all four are responsible for bone healing. This article will serve to introduce a second generation platelet concentrate i.e., a platelet-rich fibrin (PRF). PRF is easy to obtain, inexpensive, and possibly a very beneficial ingredient to add to the “regenerative mix.”
Tissue damage and vascular exposure result in platelet activation which secretes platelet-α-granule Fuse with Platelet cell membrane Transforms secretory protein into Bioactive state proteins (PDGF, TGF-β, VEGF, EGF and IGF-1) Secreted and bind to transmembrane receptor of target cellActivates Intracellular signaling proteins results in expression of gene sequence directs cellular proliferation, collagen synthesis, and osteoid production The first surgical additives to be used were fibrin sealants which were commercially available in Europe since late 1970s. It was also known as fibrin glue or fibrin tissue adhesive.[3]
Fibrin glue is composed of two separate solutions of fibrinogen and thrombin. When mixed together, these agents mimic the last stages of the clotting cascade to form a fibrin clot. Fibrinogen can be obtained from pooled, single-donor, and autologous blood donors and is usually isolated by the process of cryoprecipitation. The thrombin component is generally derived from commercial bovine sources. Some investigators have added calcium chloride and/or antifibrinolytics (i.e., aminocaproic acid, aprotinin) to their preparations.
The major drawback to its use is the risk of transmitted serological disease from pooled and single-donor blood donors. The safest preparations use the patient's own blood to prepare fibrin glue.[3]
Platelet-rich plasma (PRP)- first generation platelet concentrate was proposed as a method of introducing concentrated growth factors PDGF, TGF-ß, and IGF-1 to the surgical site, enriching the natural blood clot in order to expedite wound healing and stimulate bone regeneration.[4] PRP was developed by Whitmen et al., in 1997. A natural human blood clot contains 95% red blood cells (RBCs), 5% platelets, less than 1% white blood cells (WBCs), and numerous amounts of fibrin strands. A PRP blood clot, on the other hand, contains 4% RBCs, 95% platelets, and 1% WBCs. The classic PRP production protocol requires blood collection with anticoagulant, 2 steps of centrifugation, and artificial polymerization of the platelet concentrate using calcium chloride and bovine thrombin.[5] Since its introduction, PRP has been used in conjunction with different grafting materials in bone augmentation procedures.[3,4,5,6,7]
PRF represents a new step in the platelet gel therapeutic concept with simplified processing minus artificial biochemical modification. Unlike other platelet concentrates, this technique requires neither anticoagulants nor bovine thrombin (nor any other gelifying agent), making it no more than centrifuged natural blood without additives. PRF consists of a fibrin matrix polymerized in a tetra molecular structure, with incorporation of Cytokines, platelet, leucocytes and circulating stem cells.[6]
Developed in France by Choukroun et al. in 2001, the PRF production protocol attempts to accumulate platelets and released cytokines in a fibrin clot. Though platelets and leukocyte cytokines play an important part in the biology of this biomaterial, the fibrin matrix supporting them certainly constitutes the determining element responsible for the real therapeutic potential of PRF.[8] Cytokines are quickly used and destroyed in a healing wound. The synergy between cytokines and their supporting fibrin matrix has much more importance than any other parameter. A physiologic fibrin matrix (such as PRF) will have very different effects than a fibrin glue enriched with cytokines (such as PRP), which will have a massively uncontrollable and short-term effect.
PRF results from a natural and progressive polymerization occurring during centrifugation. The fibrin network thus formed presents a particularly homogeneous 3-dimensional organization, even more highly coherent than natural fibrin clots.
Progressive polymerization mode signifies increased incorporation of the circulating cytokines in the fibrin meshes (intrinsic cytokines). The intrinsic incorporation of cytokines within the fibrin mesh allows for their progressive release over time (7-11 days), as the network of fibrin disintegrates.[9] Such a configuration implies an increased lifespan for these cytokines, because they will be released and used only at the time of initial cicatricial matrix remodelling (long term effect). The cytokines are thus maintained available in situ for a convenient period, when the cells start cicatricial matrix remodelling, i.e., when they have to be stimulated to launch injured site reconstruction.
Slow polymerization with physiologic thrombin concentrations implies very elastic matricial architecture (equilateral junctions between fibrin fibrillae particularly favourable to cell migration and soluble molecule retention). The easily applied PRF membrane acts much like a fibrin bandage, serving as a matrix to accelerate the healing of wound edges.
CONCLUSIONS
Early publications and clinical experience seem to indicate that PRF improves early wound closure, maturation of bone, and the final aesthetic result of the periodontal soft tissues.
Production of a dense, cross-linked, physically robust PRF made of intact platelets and fibrin by high-speed centrifugation in the absence of exogenous thrombin, yields an ideal scaffold for use in tissue repair.
It requires around 1 year for complete healing to occur after the periapical surgery while with the use of PRF, healing is fastened and requires approx 6 months for complete regeneration of bone.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Abramovitz I, Better H, Shacham A, Shalomi B, Metzger Z. Case Selection for apical surgery: A retrospective evaluation of associated factors and rational. J Endod. 2002;28:527–30. doi: 10.1097/00004770-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:45–50. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Thomson DF, Letassy NA, Thomson GD. Fibrin glue: A review of its preparation, efficacy, and adverse effects as a topical haemostat. Drug Intell Clin Pharm. 1988;22:946–52. doi: 10.1177/106002808802201203. [DOI] [PubMed] [Google Scholar]
- 4.Soffer E, Ouhayoun JP, Anagnostou F. Fibrin sealants and platelet preparations in bone and periodontal healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:521–8. doi: 10.1067/moe.2003.152. [DOI] [PubMed] [Google Scholar]
- 5.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 6.Toffler M, Toscano N, Holtzclaw D, Corso MD, Ehrenfest DD. Introducing Choukroun's Platelet Rich Fibrin (PRF) to the Reconstructive Surgery Milieu. Implant Dent. 2009;1:21–30. [Google Scholar]
- 7.Sanchez AR, Sheridan PJ, Kupp LI. Is platelet rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18:93–103. [PubMed] [Google Scholar]
- 8.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299–303. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Simonpieri A, Del Corso M, Sammartino G, Dohan Ehrenfest DM. The Relevance of Choukroun's Platelet-Rich Fibrin and Metronidazole during Complex Maxillary Rehabilitations Using Bone Allograft. Part I: A New Grafting Protocol. Implant Dent. 2009;18:102–11. doi: 10.1097/ID.0b013e318198cf00. [DOI] [PubMed] [Google Scholar]


