Abstract
Background:
Conventional non-surgical periodontal therapy has been proven to be an effective treatment for patients with chronic periodontitis. Tea tree oil (TTO) can be used as adjunct to conventional periodontal therapy in patient with chronic periodontitis. The aim of this study was to evaluate the effectiveness of adjunctive treatment of TTO on the clinical parameters and the level of pentraxin-3 (PTX3) in chronic periodontitis.
Materials and Methods:
A total of 40 patients with moderate to severe chronic periodontitis were divided into two groups, Group I received scaling and root planing (SRP) only, Group II received SRP and TTO gel. Clinical parameters were recorded and gingival crevicular fluid (GCF) samples were collected from each subject for measuring PTX3 levels at baseline, 1, 3 and 6 months after treatment.
Results:
In all evaluation periods, there was statistically significant reduction in each of the studied clinical parameters and PTX3 level in Group II as compared with Group I.
Conclusions:
The local delivery of TTO gel in case of chronic periodontitis may have some beneficial effects to augment the results of the conventional periodontal therapy. Moreover, it places a focus on the value of monitoring GCF levels of PTX3 as a marker of periodontal tissue healing.
Keywords: Chronic periodontitis, pentraxin-3, tea tree oil
INTRODUCTION
Periodontitis, a local chronic inflammation in the supporting tissues of the teeth leading to progressive loss of the periodontal ligament and bone, is believed to result from disruption of the homeostatic balance between periodontopathic bacteria and the host response to these microorganisms.[1]
The critical objectives of periodontal therapy include stopping the disease progression and resolving the inflammation.[2] Mechanical debridement has been the most widely used approach, but many studies have shown that mechanical debridement alone cannot effectively eliminate most of the causative bacteria and it is usually associated with high recurrence rate.[3,4]
Several systemic antibiotics have been used for periodontal treatment to suppress or eliminate residual periodontal pathogens and thus serve as an adjunct to the conventional mechanical therapy.[5] Unwanted side effects such as resistance, toxicity, sensitivity, growth of opportunistic infection and interaction with other medications are often encountered.[3] Therefore, the local administration of antimicrobials can provide a useful solution to those complications.[6]
Local drug delivery results in a high concentration of the administrated drug in the pocket, which can penetrate into the periodontal tissues as well. This dual effect on pocket microflora as well as on pathogens invading the tissue can result in enhanced clinical results without any systemic side effects and bacterial resistance. In addition, it provides 100-fold higher therapeutic doses of the agent in subgingival areas than systemic therapy.[7,8]
Natural products have served as a major source of drugs for centuries and about half of pharmaceuticals in use today are derived from natural products.[9] Oriental medicines have been evaluated for their effects on periodontal disease, regarding the antibacterial and anti-inflammatory effects as well as the periodontal tissue regeneration.[10] Among these materials, tea tree oil (TTO) is derived from the paper bark tea tree.[11] TTO has a broad-spectrum antimicrobial, antifungal, antiviral, antioxidant and anti-inflammatory effect.[12,13,14,15,16,17] A few studies have shown the useful effect of the local application of TTO on periodontal diseases.[11]
Pentraxin-3 (PXT3) is an inflammatory molecule that belongs to the same family of C-reactive protein (CRP), which is indeed a short PTX.[18,19] While CRP is produced in the liver mainly in response to interleukin-6 (IL-6), PTX3 is produced by the widely distributed innate immune cells including neutrophils, fibroblasts, dendritic cells, epithelial cells, macrophages and vascular endothelial cells in response to inflammatory mediators, such as IL-1, tumors necrosis factor-α (TNF-α) and bacterial products.[20] Human PTX3 plasma levels increase in several pathological conditions including sepsis, acute myocardial infarction and small-vessel vasculitis.[19,21]
Gingival crevicular fluid (GCF) contains a variety of microbial and host products, including neutrophils, antibodies, complement proteins and cytokines.[22] Recent studies by Pradeep et al.,[23] and Fujita et al.,[24] found increased concentration of PTX3 in GCF and they used it as a diagnostic marker for periodontal disease in patients with chronic periodontitis.
The current study was conducted to evaluate the effect of local application of TTO gel as an adjunctive treatment of moderate to severe chronic periodontitis regarding the clinical parameters and the level of long PXT3 in GCF.
MATERIALS AND METHODS
After approval of the Research Ethical Committee of Tanta University Egypt, we recruited 40 adult patients with chronic periodontitis (21 males and 19 females). The cases were selected from the outpatient Department of Oral Medicine, Faculty of Dentistry, 6th October University and Tanta University from February 2011 to January 2012.
Inclusion criteria
Male and female subjects between the ages of 30 and 60 years were included in this study if they had:
Untreated moderate to severe chronic or recurrent periodontitis without periodontal surgery at least for the last 24 months
Single rooted teeth with probing pocket depth (PPD) of 5-8 mm, without any recession, that bled on probing at the initial visit.
Exclusion criteria
Subjects were excluded if they:
Had any systemic disease that affects the periodontium
Were pregnant or postmenopausal women
Had received any anti-inflammatory drugs, antibiotics or vitamins within the previous 3 months
Were heavy smokers (>10 cigarettes/day)
History of alcohol abuse
Had previous participation in other clinical trials
Had received scaling and root planing (SRP) subgingival instrumentation less than 2 months prior to the baseline examination
Had any teeth with a periodontal pocket extending to the apex because of possible endodontic/periodontal complications.
Materials
Gel formulation was prepared from TTO (Melaleuca alternifolia) to be used as a locally delivered sustained release subgingival gel was prepared as follows:
To make the methyl cellulose gel, 7 g of methyl cellulose powder was dissolved in 100 ml boiled water. To make the TTO gel 5%, 5 ml of TTO (Sigma® Steinheim, Germany) was then mixed into the methyl cellulose gel. The gel was sterilized in the autoclave at 110°C for 20 min.[25]
Patient groups
Patients were divided randomly by random number table drawn up by a professor of statistics into two equal groups.
Group I (control group)
Twenty patients were received oral hygiene instructions and full mouth SRP with hand instruments.
Group II (test group)
Twenty patients were received oral hygiene instructions and full mouth SRP with hand instruments. After drying the area, the deepest selected periodontal pocket was filled with 5% TTO gel (immediately after completion of SRP) until the gel could be observed at the gingival margin. The gel applied sites were covered with periodontal pack (Coe-Pack). The patients were asked to return for replacement of the periodontal dressings if the dressing were dislodged before 7 days. No antibiotics, antiplaque or anti-inflammatory agents were prescribed after treatment. The patients were instructed to refrain from chewing hard or sticky foods, brushing near the TTO or using inter-dental aids and eating crusty foods for 1 week.
The dressing was removed after 7 days and the operation site was washed with sterile saline. Adverse effects were noted at recall visits (7 days, 1, 3, 6 months) and any supragingival deposits were removed.
Collection of GCF
GCF samples were collected using paper strips from the deepest diseased site (pocket ≥5 mm). After isolation with cotton rolls to prevent contamination with saliva, supragingival plaque was removed with cotton pellets and the tooth was air-dried. The paper strips were carefully inserted into the crevice until mild resistance was felt and were left in place for 30 s. Samples visibly contaminated with blood were discarded. Three GCF samples from the same site were pooled. Paper strips with absorbed GCF were placed in microcentrifuge tubes containing 500 μl of phosphate-buffered saline. Tubes were vortexed for 45 min and centrifuged at 8000 rpm for 5 min. The supernatants were transferred into new microcentrifuge tubes and stored at −80°C until analysis.
PTX3 immunoassay
The PTX3 level in 20 μl of the GCF sample was determined using a commercial solid-phase sandwich enzyme-linked immunosorbent assay (Quantikine® DPT × 30; R and D Systems Inc., Minneapolis, MN, USA) according to the manufacturer's protocol. It detects up to 0.65 ng/ml PTX3; with intra-assay and inter-assay CVs range from 3.8% to 4.4% and 4.1% to 6.1%, respectively. GCF was obtained from each site at baseline, 1, 3 and 6 months after treatment.
Clinical measurement
The measurements described below were obtained only from the diseased site, at baseline, 1, 3 and 6 months after treatment:
Statistical analysis
All the results were tabulated and statistically analyzed using Statistical Package for Social Science (SPSS version 12). The difference between groups was statistically analyzed using independent sample t-test and intra-group analysis using paired t-test.
RESULTS
All the patients completed the study without any complications. The patient in Group II complained of intensive and unpleasant taste of the TTO gel. At baseline, there was no significant difference in clinical parameters between the groups (P > 0.01).
PI and GI
The mean changes overtime in PI and GI are given in Tables 1 and 2 respectively.
Table 1.
Mean values of PI, GI and PPD among the study groups at base line, 1, 3 and 6 months after treatment
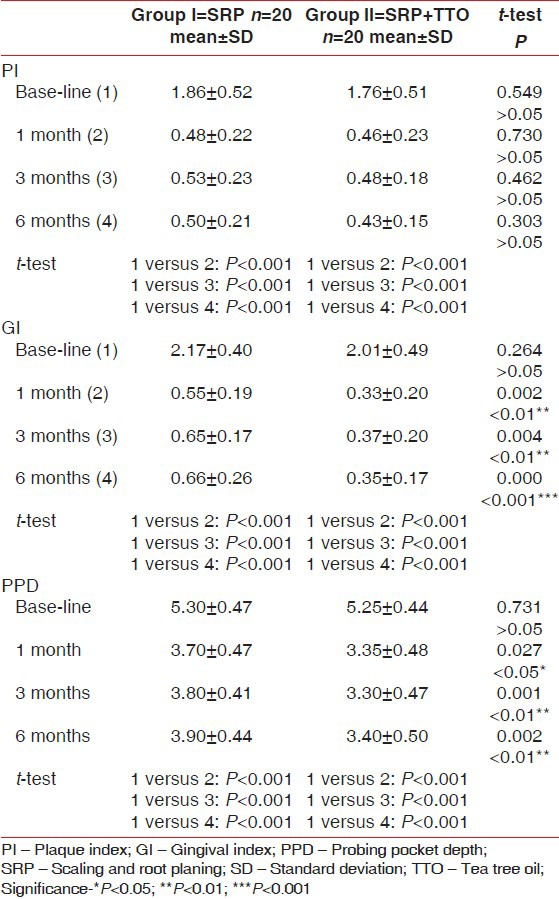
Table 2.
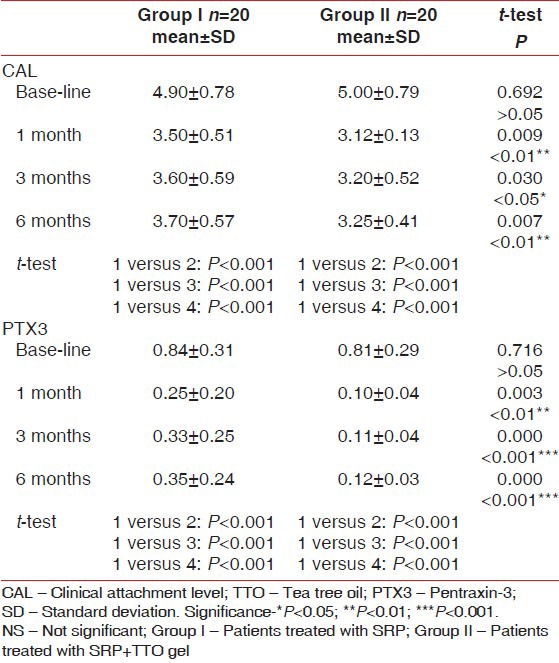
Mean values of CAL and PTX3 level among the study groups at base line, 1, 3 and 6 months after treatment
All subjects showed a statistically very highly clinical significant improvement in both gingival and plaque indices at follow-up visits when compared with the baseline levels. The mean reduction in PI score from baseline to 1, 3 and 6 months was (1.86 ± 0.52, 0.48 ± 0.22, 0.53 ± 0.23 and 0.50 ± 0.21 respectively) in Group I while in Group II was (1.67 ± 0.51, 0.46 ± 0.23, 0.48 ± 0.18 and 0.43 ± 0.15 respectively) (P < 0.001).
Along with the evaluation periods, t-test showed a statistically very highly significant reduction of GI scores of Group I at 1, 3 and 6 months (0.55 ± 0.19, 0.65 ± 0.17 and 0.66 ± 0.26 respectively) and as compared with baseline values (2.17 ± 0.40). In addition, results of Group II revealed a statistically very highly significant reduction of GI scores, which reduced from (2.02 ± 0.40) at baseline to (0.33 ± 0.20, 0.37 ± 0.20 and 0.35 ± 0.17) at 1, 3 and 6 months respectively (P < 0.001).
Intergroup comparison showed a statistically insignificant difference of the mean PI between the two treated groups at any evaluation periods when compared with each other (P > 0.05). Comparing Group I and Group II at 1 and 3 months evaluation periods, results showed that the mean GI reduction was statistically highly significant (P < 0.01) while at 6 months, the mean GI reduction was statistically very highly significant comparing the two tested groups (P < 0.001) [Table 1].
PPD and CAL
Tables 1 and 2 show changes in PPD and CAL for each treatment group. At baseline, there was no statistically significant difference in PDD or CAL of the treated sites.
All sites showed very highly significant changes in PDD and CAL from baseline to 1, 3 and 6 months. Mean reduction of PDD from baseline to 1, 3 and 6 months was (5.30 ± 0.47, 3.70 ± 0.47, 3.80 ± 0.41, 3.90 ± 0.44 respectively) in Group I and (5.25 ± 0.44, 3.35 ± 0.48, 3.30 ± 0.47 and 3.40 ± 0.50 respectively) in Group II. Also, the improvement in CAL in Group I was highly significant changed from (4.90 ± 0.78) at baseline to (3.50 ± 0.51, 3.60 ± 0.59, 3.70 ± 0.57) at 1, 3 and 6 months respectively while in Group II changed from (5.00 ± 0.79) at baseline to (3.12 ± 0.13, 3.20 ± 0.52, 3.25 ± 0.41) at 1, 3 and 6 months respectively (P < 0.001).
Comparing Group I and Group II at 1, 3 and 6 months evaluation periods, t-test showed that the mean PPD reduction was statistically significant (P < 0.05, P < 0.01). Regarding CAL, when comparing Group I and Group II results showed a statistically significant improvement at 1, 3 and 6 months evaluation periods (P < 0.001, P < 0.05).
PTX3 level
Results of Group I revealed that there was a very highly statistically significant reduction of PTX3 level at 1, 3 and 6 months post treatment. Results of Group II, showed a remarkable statistically significant reduction in the PTX3 level, which continued up to 6 months as compared with the mean baseline value (0.81 ± 0.29) while the mean value at 1, 3 and 6 months were 0.10 ± 0.04, 0.11 ± 0.04 and 0.12 ± 0.03. Intergroup comparison showed that the mean of PTX3 level at baseline was statistically insignificant between all treated groups. t-test showed a statistically highly significant improvement at 1 month evaluation period (P < 0.01) while the mean PTX3 level showed a very remarkable reduction at 3 and 6 months when comparing the two tested groups (P < 0.001).
DISCUSSION
In the present study, the local TTO gel was investigated as a delivery system for an active agent to treat cases of chronic periodontitis. A part from the bad taste of the TTO, the patients didn’t show any complications throughout the study periods. The studied groups consisted of untreated moderate chronic periodontitis patients; therefore, all of received the standard Phase I periodontal therapy. The treated teeth were single rooted to provide the standardization of mechanical instrumentation. It has reported that the surface irregularities, developmental difference in root anatomy and difficulties in getting better clinical outcomes, especially for the closed surgical interventions.
In this study, we examined the effect of TTO gel, on the clinical parameters and PTX3 level in patients with moderate to severe chronic periodontitis.
However, our results showed that there was a statistically significant reduction in the mean PI scores during the study periods in both groups as compared with baseline values and there was no significant difference between the two groups during the study periods. The reduction in plaque scores could be due to the proper oral hygiene maintenance after SRP. This was in agreement with the clinical results reported by Arweiler et al.,[29] who described the efficacy of a TTO used as a mouthwash on plaque formation. They found that the PI in the group treated by TTO mouth wash did not differ from placebo mouthwash on any day from the study periods. Also, our results were in agreement with the other clinical results reported by Soukoulis and Hirsch,[11] who performed a double-blind, longitudinal study to evaluate the effect of TTO gel (2.5%) applied to toothbrush twice daily. They found that TTO did not reduce plaque scores.
It was apparent in the present study that Group II (SRP plus TTO gel) showed a statistically significant reduction in gingival inflammation, which was maintained until the end of the study period as compared with Group I (SRP only). This could be attributed to the anti-inflammatory and antibacterial effect of the TTO. The antimicrobial action of TTO results from its ability to disrupt the permeability barrier of microbial membrane structures.[30] These results are consistent with the study by Soukoulis and Hirsch,[11] that revealed when the TTO gel was used twice daily with a toothbrush as a dentifrice, a significant reduction of gingival inflammation and bleeding was reported with severe gingivitis. In addition, improvement in gingival inflammation due to the anti-inflammatory activity of TTO has been also reported in other studies.[16,31,32] Furthermore TTO may mediate its anti-inflammatory functions through the production of superoxide by human monocytes.[16] Clinical improvements, reduction in PPD and gain in CAL seen following extensive SRP (alone) in Group I, are apparently due to reduction of inflammation secondary to alteration in the subgingival bacteria.[33] In addition to the elimination of the local etiologic factors, it has been proposed that a scaling procedure may also elicit a local and systemic host response that would aid in eliminating local infection and promote healing.[34]
In the current project, there was a statistically significant reduction in PPD and CAL in Group II compared to Group I during all evaluation periods. Arweiler et al.,[29] and Saxer et al.,[35] found that TTO was effective in reducing the oral bacteria. Furthermore, the component of TTO is known to have lipophilic properties, which facilitate its diffusion through epithelium.[36] If TTO was readily absorbed after topical application into the gingival tissues and succeeded to enter the connective tissues, it would be a unique, non-toxic agent that could be a useful addition to the current range of chemotherapeutic periodontal treatment option.[11]
The results of PX3 revealed that there was a statistically significant reduction in PX3 level in Group II when compared with Group I at all post treatment evaluation periods. Since, PTX3 is produced in response to inflammatory mediators, such as IL-1, TNF and bacterial products,[20] our results could be in agreement with that of Juergens et al., who showed a significant decrease in the production of different cytokines (TNF-α and IL-1 β) from lipopolysaccharides (LPS) stimulated monocytes after administration of Soledum® (containing 1, 8-cineole).[31] Similar effects in vitro were reported on monocytes by other component of TTO like terpinen-4-ol.[16,32] Also, the reduction of PXT3 level in TTO group may be attributed to the antimicrobial effect of TTO, that decrease production of bacterial products like LPS which are responsible for stimulation of PTX3 production by immune cells.[35]
CONCLUSIONS
The findings of the current study suggest that, local application of TTO gel may provide some beneficial therapeutic effects to augment the results of SRP. Also, it emphasizes the potential therapeutic value of TTO as an anti-inflammatory agent, which is able to reduce inflammation and promote healing of periodontal tissues. Moreover, it places a focus on the value of monitoring GCF levels of PTX3 as a marker of periodontal tissue healing following various periodontal therapeutic approaches.
RECOMMENDATIONS
Further human research is warranted to investigate the value of local application of TTO to augment different treatment modalities for periodontitis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Haffajee AD, Socransky SS, Smith C, Dibart S. Microbial risk indicators for periodontal attachment loss. J Periodontal Res. 1991;26:293–6. doi: 10.1111/j.1600-0765.1991.tb01662.x. [DOI] [PubMed] [Google Scholar]
- 2.Greenstein G, Caton J. Periodontal disease activity: A critical assessment. J Periodontol. 1990;61:543–52. doi: 10.1902/jop.1990.61.9.543. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg D, Friedman M, Soskolne A, Sela MN. A new degradable controlled release device for treatment of periodontal disease: In vitro release study. J Periodontol. 1990;61:393–8. doi: 10.1902/jop.1990.61.7.393. [DOI] [PubMed] [Google Scholar]
- 4.Feng Z, Weinberg A. Role of bacteria in health and disease of periodontal tissues. Periodontol 2000. 2006;40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 5.Greenstein G. Clinical significance of bacterial resistance to tetracyclines in the treatment of periodontal diseases. J Periodontol. 1995;66:925–32. doi: 10.1902/jop.1995.66.11.925. [DOI] [PubMed] [Google Scholar]
- 6.Hanes PJ, Purvis JP. Local anti-infective therapy: Pharmacological agents. A systematic review. Ann Periodontol. 2003;8:79–98. doi: 10.1902/annals.2003.8.1.79. [DOI] [PubMed] [Google Scholar]
- 7.Greenstein G, Polson A. The role of local drug delivery in the management of periodontal diseases: A comprehensive review. J Periodontol. 1998;69:507–20. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen MG, Slots J. Responsible use of antimicrobials in periodontics. J Calif Dent Assoc. 2000;28:185–93. [PubMed] [Google Scholar]
- 9.Clark AM. Natural products as a resource for new drugs. Pharm Res. 1996;13:1133–44. doi: 10.1023/a:1016091631721. [DOI] [PubMed] [Google Scholar]
- 10.Cao CF, Sun XP. Herbal medicine for periodontal diseases. Int Dent J. 1998;48:316–22. doi: 10.1111/j.1875-595x.1998.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 11.Soukoulis S, Hirsch R. The effects of a tea tree oil-containing gel on plaque and chronic gingivitis. Aust Dent J. 2004;49:78–83. doi: 10.1111/j.1834-7819.2004.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 12.Carson CF, Riley TV. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J Appl Bacteriol. 1995;78:264–9. doi: 10.1111/j.1365-2672.1995.tb05025.x. [DOI] [PubMed] [Google Scholar]
- 13.Jandourek A, Vaishampayan JK, Vazquez JA. Efficacy of melaleuca oral solution for the treatment of fluconazole refractory oral candidiasis in AIDS patients. AIDS. 1998;12:1033–7. [PubMed] [Google Scholar]
- 14.Carson CF, Ashton L, Dry L, Smith DW, Riley TV. Melaleuca alternifolia (tea tree) oil gel (6%) for the treatment of recurrent herpes labialis. J Antimicrob Chemother. 2001;48:450–1. doi: 10.1093/jac/48.3.450. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Chen F, Wu C, Wang X, Chung HY, Jin Z. Evaluation of antioxidant activity of Australian tea tree (Melaleuca alternifolia) oil and its components. J Agric Food Chem. 2004;52:2849–54. doi: 10.1021/jf035377d. [DOI] [PubMed] [Google Scholar]
- 16.Brand C, Ferrante A, Prager RH, Riley TV, Carson CF, Finlay-Jones JJ, et al. The water-soluble components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm Res. 2001;50:213–9. doi: 10.1007/s000110050746. [DOI] [PubMed] [Google Scholar]
- 17.Koh KJ, Pearce AL, Marshman G, Finlay-Jones JJ, Hart PH. Tea tree oil reduces histamine-induced skin inflammation. Br J Dermatol. 2002;147:1212–7. doi: 10.1046/j.1365-2133.2002.05034.x. [DOI] [PubMed] [Google Scholar]
- 18.Breviario F, d’Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–7. [PubMed] [Google Scholar]
- 19.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–66. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 20.Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–93. [PubMed] [Google Scholar]
- 21.Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, et al. PTX3, a prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–41. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 22.Lamster IB, Grbic JT. Diagnosis of periodontal disease based on analysis of the host response. Periodontol 2000. 1995;7:83–99. doi: 10.1111/j.1600-0757.1995.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 23.Pradeep AR, Kathariya R, Raghavendra NM, Sharma A. Levels of pentraxin-3 in gingival crevicular fluid and plasma in periodontal health and disease. J Periodontol. 2011;82:734–41. doi: 10.1902/jop.2010.100526. [DOI] [PubMed] [Google Scholar]
- 24.Fujita Y, Ito H, Sekino S, Numabe Y. Correlations between pentraxin 3 or cytokine levels in gingival crevicular fluid and clinical parameters of chronic periodontitis. Odontology. 2012;100:215–21. doi: 10.1007/s10266-011-0042-1. [DOI] [PubMed] [Google Scholar]
- 25.Enshaieh S, Jooya A, Siadat AH, Iraji F. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: A randomized, double-blind placebo-controlled study. Indian J Dermatol Venereol Leprol. 2007;73:22–5. doi: 10.4103/0378-6323.30646. [DOI] [PubMed] [Google Scholar]
- 26.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 27.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 28.Ramfjord SP. The Periodontal disease index (PDI) J Periodontol. 1967;38:602–10. doi: 10.1902/jop.1967.38.6.602. [DOI] [PubMed] [Google Scholar]
- 29.Arweiler NB, Donos N, Netuschil L, Reich E, Sculean A. Clinical and antibacterial effect of tea tree oil – A pilot study. Clin Oral Investig. 2000;4:70–3. doi: 10.1007/s007840050118. [DOI] [PubMed] [Google Scholar]
- 30.Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil) J Appl Microbiol. 2000;88:170–5. doi: 10.1046/j.1365-2672.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 31.Juergens UR, Stöber M, Vetter H. Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8-cineole) in human blood monocytes in vitro. Eur J Med Res. 1998;3:508–10. [PubMed] [Google Scholar]
- 32.Hart PH, Brand C, Carson CF, Riley TV, Prager RH, Finlay-Jones JJ. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm Res. 2000;49:619–26. doi: 10.1007/s000110050639. [DOI] [PubMed] [Google Scholar]
- 33.Llambés F, Silvestre FJ, Hernández-Mijares A, Guiha R, Caffesse R. Effect of non-surgical periodontal treatment with or without doxycycline on the periodontium of type 1 diabetic patients. J Clin Periodontol. 2005;32:915–20. doi: 10.1111/j.1600-051X.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 34.Offenbacher S. Periodontal diseases: Pathogenesis. Ann Periodontol. 1996;1:821–78. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 35.Saxer UP, Stäuble A, Szabo SH, Menghini G. Effect of mouthwashing with tea tree oil on plaque and inflammation. Schweiz Monatsschr Zahnmed. 2003;113:985–96. [PubMed] [Google Scholar]
- 36.Williams AC, Barry BW. Terpenes and the lipid-protein-partitioning theory of skin penetration enhancement. Pharm Res. 1991;8:17–24. doi: 10.1023/a:1015813803205. [DOI] [PubMed] [Google Scholar]


