Abstract
Background and Objectives:
This study was designed to compare inorganic salivary calcium, phosphate, flow rate and pH of un-stimulated saliva and oral hygiene of healthy subjects, patients with periodontitis and dental caries and to correlate salivary calcium level with the number of intact teeth.
Materials and Methods:
The present study consisted of 90 patients aged between 18 and 55 years and were divided into three groups, periodontitis, dental caries and controls. Oral hygiene index-simplified, probing pocket depth, clinical attachment level and number of teeth present, teeth with active carious lesions were recorded. Salivary flow rate and pH was recorded and subjected to biochemical investigation. Estimation of inorganic calcium and phosphate was performed by colorimetric method.
Results:
Results showed statistically significant increase in salivary inorganic calcium and phosphate levels, poor oral hygiene status, pH and salivary flow rate in patients with periodontitis when compared with dental caries group and controls.
Interpretation and Conclusion:
Individuals who have increased salivary inorganic calcium, phosphate, pH, flow rate and maintain poor oral hygiene could be at a higher risk for developing periodontitis and may have less dental caries and more number of intact teeth.
Keywords: Flow rate, pH, phosphate, salivary calcium
INTRODUCTION
Saliva is not one of the popular bodily fluids. It lacks the drama of blood, sincerity of sweat and the emotional appeal of tears.[1] Saliva is the glandular secretion, which constantly bathes the teeth and the oral mucosa. The presence of saliva is vital for the maintenance of healthy oral tissues. Severe reduction of salivary output not only results in a rapid deterioration in oral health, but also has a detrimental impact on quality-of-life. Patients suffering from dry mouth experience difficulty in maintaining oral hygiene and rapidly progressive dental caries.[2]
Mineralization favoring factors are known to maintain the integrity of enamel surfaces and intraoral mineralization capacity has been a matter of scientific interest for decades. In the presence of low plaque pH, demineralization of enamel and dental decay may occur. Far less attention has been paid to the opposite pattern of mineral exchange in the oral cavity, i.e., calculus formation. Earlier, calculus was regarded as one of the main etiologic factors of periodontitis, but the interest later faded. The reason for this is probably due to the fact that in experimental conditions, microbial plaque was shown to induce gingivitis.[3] Periodontal research began to focus on microbiology and periodontitis was thought to be induced by specific microbes.
Positive correlations have been shown between high salivary calcium content and periodontitis,[4] and between high salivary calcium content and number of intact teeth.[5] It was also found that subjects with periodontitis have more intact teeth and more intact molars than subjects who are free of the disease.[6] Therefore, the present concept is that periodontitis affected subjects have higher intraoral mineralization potential. Moreover, it is demonstrated that several mineralization favoring factors are prominent in periodontitis-affected subjects when compared with those who are periodontitis-free.[4,7] An inverse relationship between caries and mineral content of plaque has been shown earlier.[8] Furthermore, the hypothesis of indirect correlation between dental decay and periodontal disease has been presented earlier.[9]
This study was designed to estimate and compare inorganic salivary calcium, phosphate, salivary flow rate and salivary pH of un-stimulated saliva and oral hygiene status of periodontally healthy subjects, patients with periodontitis and dental caries; to compare inorganic salivary calcium and phosphate levels, salivary pH, salivary flow rate levels and oral hygiene status in patients having periodontitis, dental caries and controls; and to correlate salivary calcium level and number of intact teeth in patients having periodontitis, dental caries and controls.
Armamentarium
The armamentarium included mouth mask, disposable latex gloves, mouth mirror, tweezers, William's graduated periodontal probe, kidney tray, graduated jar for collection of saliva and estimating flow rate, 5 ml glass bottle for transfer of specimen to the laboratory [Figure 1], salivary calcium (Agappe diagnostics, USA) and phosphorus estimation kit (Teco diagnostics, USA) [Figure 2], pH meter [Figure 3] and colorimeter [Figure 4].
Figure 1.
Armamentarium for clinical examination and collection of salivary sample
Figure 2.
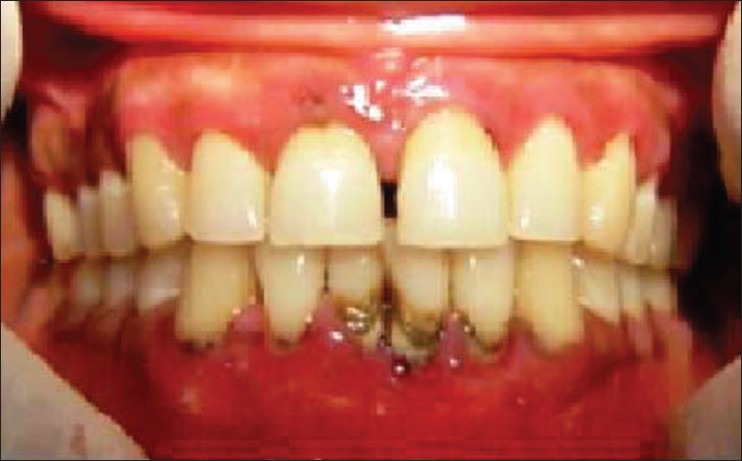
Clinical picture showing periodontitis
Figure 3.
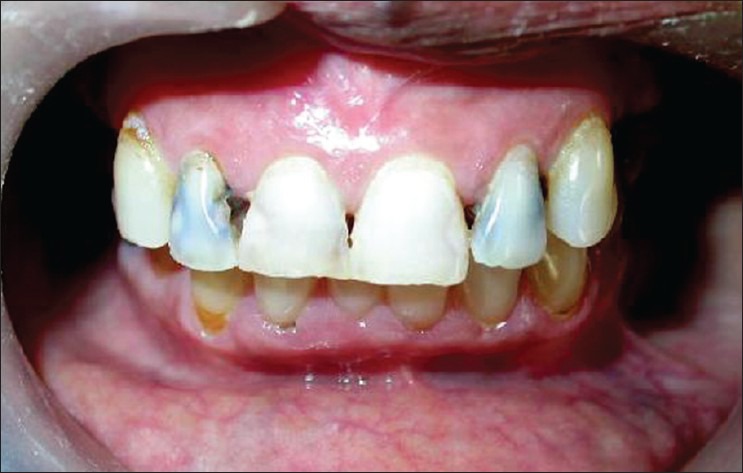
Clinical picture showing dental caries
Figure 4.
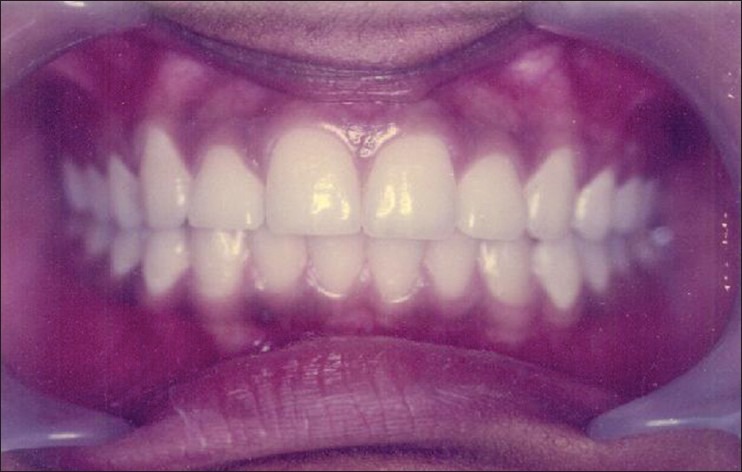
Clinical picture showing the control
MATERIALS AND METHODS
Ninety subjects in the age group of 18-55 years attending the Department of Periodontics, A.B. Shetty Memorial Institute of Dental Sciences, Mangalore, India comprising 30 patients affected with periodontitis, [Figure 5] 30 patients affected by dental caries and [Figure 6] 30 subjects as a control group were included in this study [Figure 7].
Figure 5.
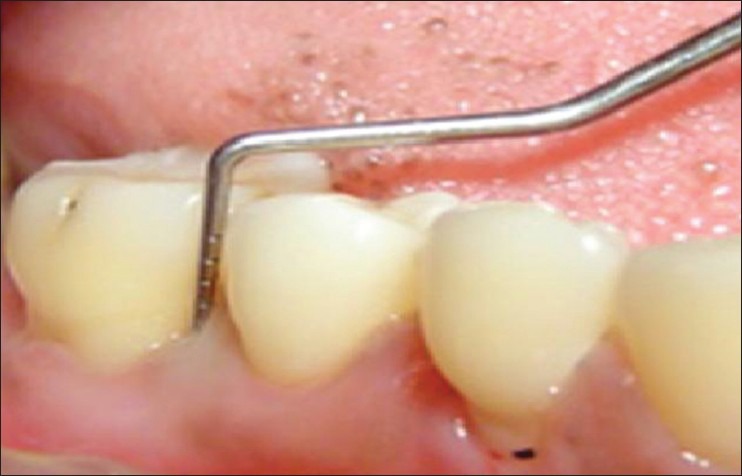
Method of measuring probing pocket depth
Figure 6.
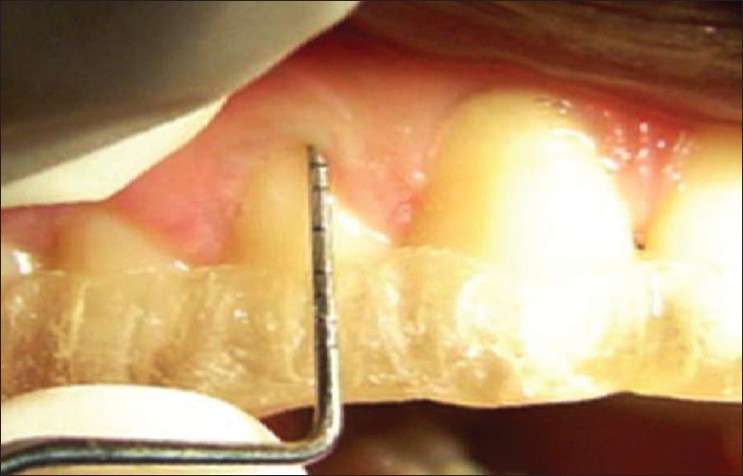
Method of measuring clinical attachment level using a stent
Figure 7.
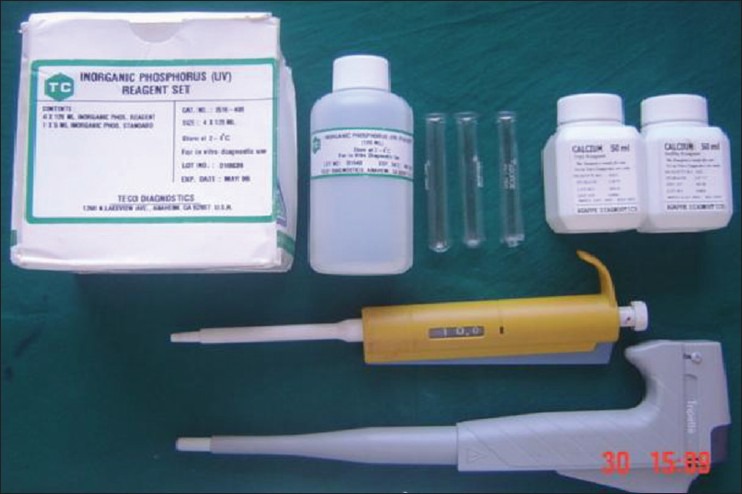
Inorganic salivary calcium and phosphate estimation kit
Screening examination included medical history, dental history, number of teeth present, number of teeth having active carious lesion, pocket depth and oral hygiene index-simplified (OHI-S). The inclusion criteria were patients with pockets more than 4 mm or an attachment loss of 1.5 mm or more from cemento-enamel junction. Patients should not have undergone oral prophylaxis for at least 6 months prior to this examination and patients with two or more active carious lesion.
Patients with lesser than 12 natural teeth with any systemic disease, using medications affecting salivary secretions and calcium level, pregnant and lactating women and smokers were excluded from this study.
The probing depth was measured around all teeth using Williams graduated periodontal probe. More than 4 mm of probing depth were considered as periodontitis. Clinical attachment level (CAL) was recorded according to the criteria followed by Armitage.[10] The attachment loss of more than 1.5 mm was recorded as periodontitis [Figures 8 and 9]. OHI-S (Greene and Vermillion),[11] was performed and scores were tabulated.
Figure 8.

Colorimeter
Figure 9.

pH meter
Collection of the salivary sample and measurement of flow rate
Patients were advised that a very small amount of saliva will ooze into their mouth in un-stimulated state and that the objective of the test was to measure the rate of flow of this secretion.[2] Saliva was collected at least 1½ h after eating. Un-stimulated whole saliva was collected by making the patient to sit in upright position at rest, bow their head and try not to move during the test. Immediately before the test begun, they were instructed to swallow any residual saliva that may be in their mouth. The saliva was allowed to accumulate for 2 min and then expectorated into the collecting vessel. If insufficient saliva was obtained then test may be conducted for a longer period of time often for 5 min. The volume was recorded in a graduated tube. Flow was expressed as ml/min.
Estimation of inorganic salivary calcium by colorimetric method
The kit used for this study was manufactured by Agappe Diagnostics, USA. Three clean and dry disposable test tubes or cuvettes were taken and labeled as blank (B), standard (S) and test (T). Distilled water of 0.01 ml is pipetted out into the test tube B, 0.01 ml of standard solution into the test tube S and 0.01 ml of test solution into the test tube T. Then, 0.01 ml of reagent is added into all the three test tubes and the contents were mixed thoroughly. The optical density of standard (S) and test (T) were measured against blank (B) on colorimeter at 570 nm. Salivary calcium was estimated by using the formula:
![]()
Estimation of inorganic salivary phosphate by colorimetric method
Three cleaned dry disposable cuvettes were taken and labeled as blank (B), standard (S) and test (T). In cuvette labeled blank (B), 1.0 ml of reagent and 0.01 ml of distilled water was added. In cuvette labeled standard (S), 1.0 ml of reagent and 0.01 ml of phosphate was added. In cuvette labeled test (T), 1.0 ml of reagent and 0.01 ml of test solution was added.[12] The reagents were mixed thoroughly. The absorbance of standard and the test was read and recorded at 340 nm versus blank as a reference. Salivary phosphate was estimated by using the formula mentioned before. The kit used for the study is manufactured by Teco Diagnostics, USA.
Criteria for diagnosing and differentiating between active and inactive carious lesions
The following tables distinguishing between active and inactive caries sites based on visual (color and glossy vs. non-glossy surface) and tactile information (rough vs. smooth surface) obtained through limited and careful use of the explorer.[13]
Estimation of pH of saliva using pH meter
Most reliable method for measuring pH is by pH meter. Scale of pH meter extends from 0 to 14 (acidic pH < 7, basic pH > 7, neutral = 7). A change of pH by one unit corresponds to 10 folds change in (H+) concentration of the solution. Variation of pH with E may be recorded directly on the potentiometer scale graduated to read pH directly.[14]
The values obtained were subjected to statistical analysis. The mean salivary calcium, phosphate, salivary flow rate and salivary pH of un-stimulated saliva and OHI-S of the three groups of were statistically analyzed using analysis of variance. Inter-comparison between the groups was done using “Tukey's honestly significance difference” test.
RESULTS
In this study, 30 patients affected with periodontitis, 30 patients affected by dental caries and 30 subjects as control group were compared. In all the three groups, un-stimulated saliva was collected and analyzed for inorganic calcium, phosphate and pH and flow rate. Periodontal status was assessed by OHI-S, probing depth and CAL. Number of teeth present and number of intact teeth was also recorded.
The comparison of mean of salivary inorganic calcium and phosphate of periodontitis, dental caries and control group [Table 1, Graph 1] revealed that mean of salivary inorganic calcium is 1.6957 ± 0.11209 for periodontitis group, 1.3477 ± 0.9269 for dental caries group and 1.5550 ± 1.0686 for the control group (P > 0.001). The observed mean between the three groups was statistically very highly significant. Mean of salivary inorganic phosphate is 5.7147 ± 0.71330 for periodontitis group, 3.9600 ± 0.55373 for dental caries group and 4.8767 ± 0.35006 for the control group (P > 0.001). The observed mean between the three groups was statistically very highly significant.
Table 1.
Comparison of scores of salivary inorganic calcium and phosphate between periodontitis, dental caries and control group
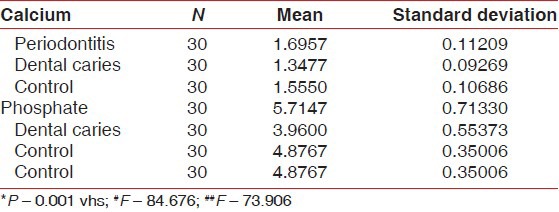
Graph 1.
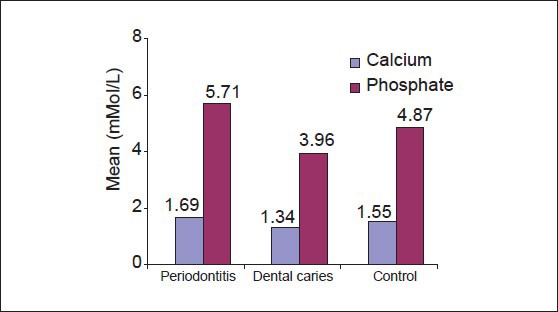
Comparison of salivary calcium and phosphate scores
Multiple comparisons and mean difference of salivary inorganic calcium and phosphate of periodontitis, dental caries and control group [Table 2, Graph 2]. The mean difference for salivary inorganic calcium between periodontitis and dental caries is 0.3480 (P < 0.001) and between periodontitis and controls was 0.1407 (P < 0.001). The observed mean between the three groups was statistically very highly significant. The mean difference for salivary inorganic phosphate between periodontitis and dental caries was 1.7547 (P < 0.001) and between periodontitis and controls was 0.8380 (P < 0.001). The observed mean difference between the three groups was statistically very highly significant.
Table 2.
Multiple comparisons and mean difference of salivary inorganic calcium and phosphate between periodontitis, dental caries and control group
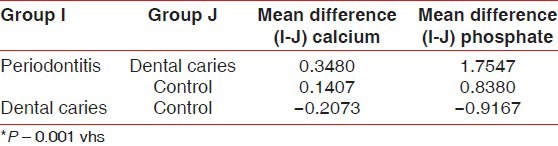
Graph 2.
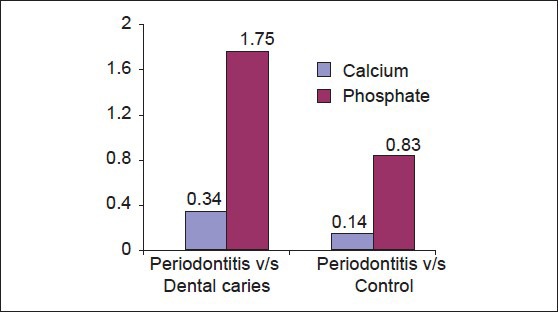
Multiple comparisons and mean difference of salivary calcium and phosphate scores
Oral hygiene status as assessed by OHI-S was statistically very highly significant in periodontitis group when compared with dental caries and control group, showing that periodontitis group had poor oral hygiene when compared with dental caries and control group who had good oral hygiene.
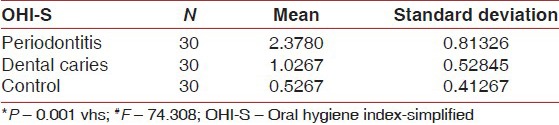
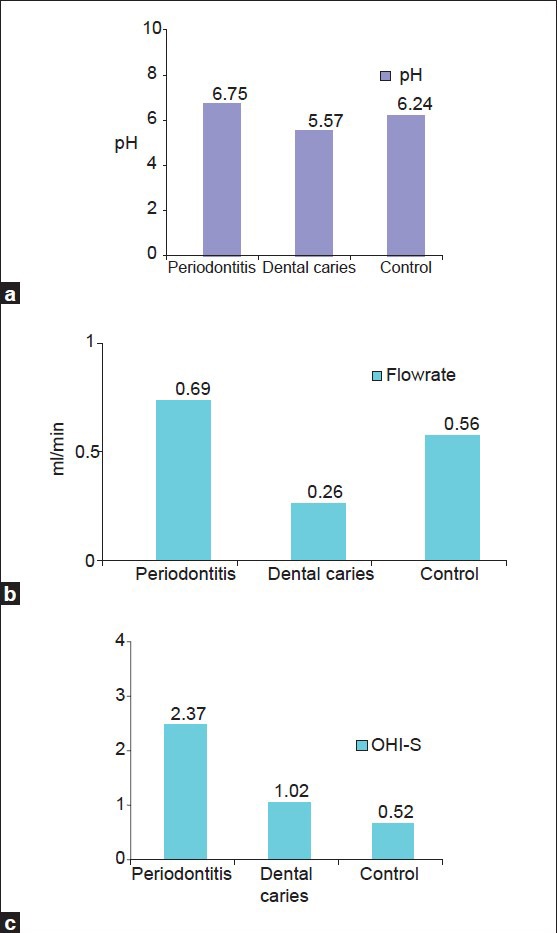
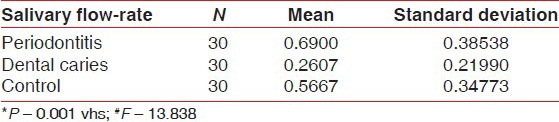
A comparison of mean of salivary pH, flow rate and OHI-S between periodontitis, dental caries and control group [Table 3a–c, Graph 3a–c] showed that mean of salivary pH is 6.7533 ± 0.12243 for periodontitis group, 5.5733 ± 0.30050 for dental caries group and 6.2410 ± 0.56661 for the control group. The observed mean between the three groups was statistically very highly significant. The mean of salivary flow rate is 0.6900 ± 0.2607 for periodontitis group, 0.2607 ± 0.21990 for dental caries group and 0.5667 ± 0.34773 for the control group. The observed mean between the three groups was statistically very highly significant. Mean of OHI-S is 2.3780 ± 0.81326 for periodontitis group, 1.0267 ± 0.52845 for dental caries group and 0.5267 ± 0.41267 for the control group. The observed mean between the three groups was statistically very highly significant.
Table 3a.
Comparison of scores of salivary pH between periodontitis, dental caries and control group
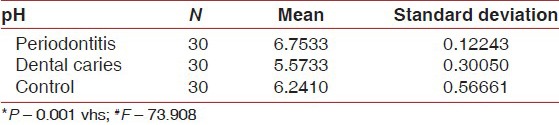
Table 3c.
Comparison of scores of OHI-S between periodontitis, dental caries and control group
Graph 3.
Comparison of mean scores for (a) salivary pH, (b) salivary flow-rate, (c) OHI-S
Table 3b.
Comparison of scores of flow rate between periodontitis, dental caries and control group
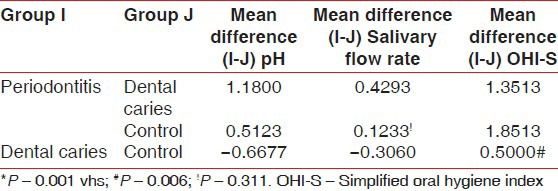
Multiple comparisons and mean difference of salivary pH, flow rate and OHI-S between periodontitis, dental caries and control group [Table 4, Graph 4]. The mean difference of pH between periodontitis and dental caries was 1.1800 (P < 0.001) and between periodontitis and controls was 0.5123 (P < 0.001). The observed mean difference between the three groups was statistically very highly significant. The mean difference of flow rate between periodontitis and dental caries was 0.4293 (P < 0.001) and between periodontitis and controls was 0.1233 (P > 0.05). The observed mean difference between periodontitis and dental caries, but the observed mean difference between periodontitis and controls was statistically insignificant. The mean difference of OHI-S between periodontitis and dental caries was 1.3513 (P < 0.001) and between periodontitis and controls was 1.8513 (P < 0.001). The observed mean difference between the three groups was statistically very highly significant.
Table 4.
Multiple comparisons and mean difference of salivary pH, flow rate and OHI-S between periodontitis, dental caries and control group
Graph 4.
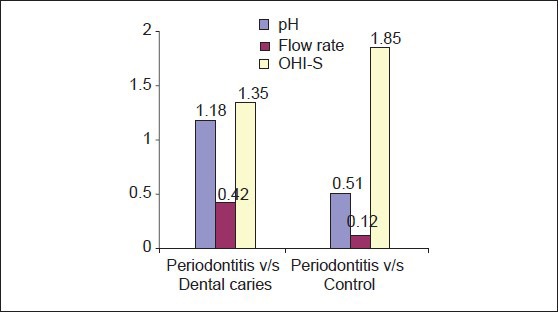
Multiple comparisons and mean difference for salivary pH, flow-rate and OHI-S
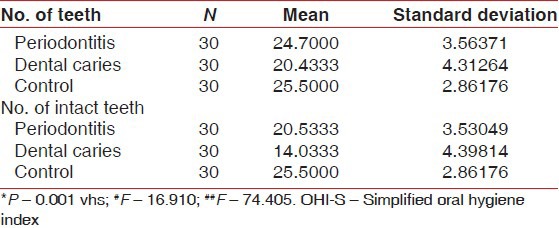
Comparison of mean between the number of teeth present and number of intact teeth among periodontitis, dental caries and control group was performed Table 5, Graph 5]. Mean of number of teeth present is 24.7000 ± 3.56371 for periodontitis group, 20.433 ± 4.31264 for dental caries group and 25.5000 ± 2.86176 for the control group (P > 0.001). The observed mean between the three groups was statistically very highly significant. Mean of number of intact teeth is 20.5333 ± 3.530489 for periodontitis group, 14.0333 ± 4.39814 for dental caries group and 25.5000 ± 2.86176 for the control group (P > 0.001). The observed mean between the three groups was statistically very highly significant.
Table 5.
Comparison of scores of number of intact teeth between periodontitis, dental caries and control group
Graph 5.
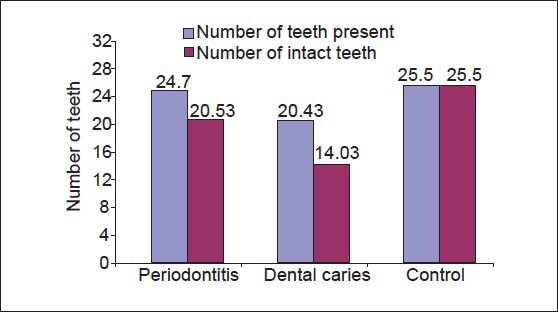
Comparison of mean of number of teeth present and number of intact teeth between periodontitis, dental caries and control group
Multiple comparisons and mean difference of number of teeth present and number of intact teeth between periodontitis, dental caries and control group is shown in [Table 6, Graph 6]. The mean difference in number of teeth present between periodontitis and dental caries group was 4.2667 (P < 0.001) and between periodontitis and controls was 0.8000 (P > 0.05). The observed mean between the periodontitis and dental caries was statistically very highly significant, but the observed mean difference between periodontitis and controls was statistically not significant. The mean difference for a number of intact teeth between periodontitis and dental caries was 6.5000 (P < 0.001) and between periodontitis and controls was 1.012. The observed mean difference between periodontitis and dental caries was statistically very highly significant, but the observed mean difference between periodontitis and controls was statistically not significant.
Table 6.
Multiple comparisons and mean difference of number of teeth present and number of intact teeth between periodontitis, dental caries and control group
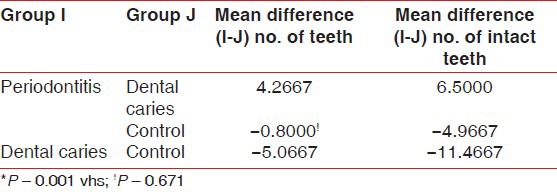
Graph 6.
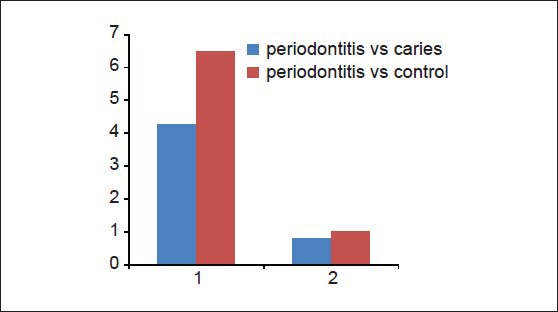
Multiple comparison and mean difference of number of teeth present and number of intact teeth between periodontitis, dental caries and control group
DISCUSSION
Whether the two oral diseases, dental caries and periodontitis are interdependent has been a matter of interest for at least 50 years. Epidemiologic data showing high periodontal index scores in caries free population suggest an inverse relation between these diseases. Some authors however, have presented a synergistic association, claiming that both diseases are caused by same dental plaque,[15] whereas other authors have found a correlation between caries and periodontitis among caries-resistant and caries susceptible people.[16] Since 1990, Sewσn et al. with their series of studies,[4,5,6,7,17,18] have shown that oral mineralization potential of saliva plays an important role in periodontal health and disease. Mineralization favoring factors are known to maintain the integrity of enamel surfaces and intraoral mineralization capacity has been a matter of scientific interest for decades. In the presence of low plaque pH, demineralization of enamel and dental decay may occur.[18]
In our present study when salivary inorganic calcium and phosphate levels were compared in patients with periodontitis, dental caries and control group, the results were statistically very highly significant showing high levels of calcium and phosphate in periodontitis patients when compared to healthy controls and dental caries group. This is in accordance with a study conducted by Sewón et al. who showed positive correlations between high salivary calcium content and periodontitis.[4] Patients with dental caries showed least values among all the three groups. They also found higher calcium concentration of plaque is associated with low caries experience.[8,19]
In our study, we have also compared the levels of salivary pH, flow rate and oral hygiene status in patients with periodontitis, dental caries and control group. The results are statistically very highly significant showing high pH value in periodontitis patients when compared with healthy controls and dental caries group. Dental caries group showed lower values reflecting acidic pH favorable for enamel demineralization. This is in accordance to a study by Dong et al. whose results support the view that factors such as the frequency of acidogenic episodes may be more important in caries progression than the degree of acidogenicity during any one episode.[20]
In our study, we found that periodontitis patients had poor oral hygiene when compared to dental caries group and control group. This is in accordance to Axelsson and Lindhe who studied the effect of controlled oral hygiene procedures on caries and periodontal disease in adults and concluded that the appearance of dental caries and periodontal disease is a result of neglected oral hygiene.[15]
In our present study when number of teeth present and number of intact teeth was compared between patients with periodontitis, dental caries and control group, we found that patients with periodontitis and control group had more number of teeth, which were intact when compared with dental caries group. This is in accordance to studies by Sewón et al. that subjects with periodontitis have more intact teeth and more intact molars than subjects who are free of the disease.[6]
Therefore, the present concept is that periodontitis affected subjects have higher intraoral mineralization potential. Sewón et al. 1990, have also demonstrated that several mineralization favoring factors are prominent in periodontitis-affected subjects when compared with those who are periodontitis-free.[4,7]
The results of this study provide evidence that subjects who have increased salivary mineralization parameters, especially inorganic salivary calcium and phosphate, high salivary pH, increased salivary flow rate and poor oral hygiene are at a higher risk of developing periodontitis as calcification of plaque occurs more readily in such patients. Furthermore, these individuals are resistant to caries and have increased number of intact teeth due to increased remineralization potential of their saliva. Salivary calcium increases the level of plaque calcium and is therefore readily available for remineralization.[21]
On the contrary, subjects with decreased salivary mineralization parameters, especially inorganic salivary calcium and phosphate, low salivary pH, reduced salivary flow rate are at a higher risk of developing dental caries as their plaque is more acidogenic and demineralization of enamel occurs more readily; therefore, these individuals have less number of intact teeth.
Hence, our data indicate that patients who develop periodontitis are resistant to dental caries and vice-versa, or in other words periodontitis and dental caries have an inverse relationship.
CONCLUSION
The present study highlights the potential for a relationship between levels of salivary inorganic calcium, phosphate, pH, flow rate and oral hygiene and diseases like periodontitis and dental caries, which commonly affect humans and needs further detailed investigation. Conclusions that can be drawn from this study are that individuals who have increased salivary inorganic calcium, phosphate, pH, flow rate and who maintain poor oral hygiene could be at a higher risk for developing periodontitis and individuals who have higher salivary calcium may have more number of intact teeth.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19:119–25. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 2.Edgar M, O’Mullane D, Dawes C. Saliva and Oral Health. 2nd ed. London: British Dental Journal; 1996. [Google Scholar]
- 3.Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–87. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 4.Sewón L, Söderling E, Karjalainen S. Comparative study on mineralization-related intraoral parameters in periodontitis-affected and periodontitis-free adults. Scand J Dent Res. 1990;98:305–12. doi: 10.1111/j.1600-0722.1990.tb00977.x. [DOI] [PubMed] [Google Scholar]
- 5.Sewón L, Mäkelä M. A study of the possible correlation of high salivary calcium levels with periodontal and dental conditions in young adults. Arch Oral Biol. 1990;35(Suppl):211S–2. doi: 10.1016/0003-9969(90)90160-c. [DOI] [PubMed] [Google Scholar]
- 6.Sewón LA, Parvinen TH, Sinisalo TV, Larmas MA, Alanen PJ. Dental status of adults with and without periodontitis. J Periodontol. 1988;59:595–8. doi: 10.1902/jop.1988.59.9.595. [DOI] [PubMed] [Google Scholar]
- 7.Sewón L, Söderling E, Karjalainen S. A mineral-related feature of young plaque characteristic to periodontitis-affected adults. J Periodontol. 1990;61:42–4. doi: 10.1902/jop.1990.61.1.42. [DOI] [PubMed] [Google Scholar]
- 8.Ashley FP. Calcium and phosphorus concentrations of dental plaque related to dental caries in 11-to 14-year-old male subjects. Caries Res. 1975;9:351–62. doi: 10.1159/000260168. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder HE. Formation and inhibition of dental calculus. J Periodontol. 1969;40:643–6. doi: 10.1902/jop.1969.40.11.643. [DOI] [PubMed] [Google Scholar]
- 10.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 12.Toora BD, Rajagopal G. 2nd ed. India: Ahuja Book Publisher and Distributor; 2002. Practical Biochemistry for Dental, Medical and Allied Courses. [Google Scholar]
- 13.Nyvad B, Machiulskiene V, Baelum V. Reliability of a new caries diagnostic system differentiating between active and inactive caries lesions. Caries Res. 1999;33:252–60. doi: 10.1159/000016526. [DOI] [PubMed] [Google Scholar]
- 14.Vasudevan DM, Sreekumari S. 4th ed. New Dehli: Jaypee Brothers Medical Publishers; 2005. Text Book of Biochemistry for Dental Students. [Google Scholar]
- 15.Axelsson P, Lindhe J. Effect of controlled oral hygiene procedures on caries and periodontal disease in adults. J Clin Periodontol. 1978;5:133–51. doi: 10.1111/j.1600-051x.1978.tb01914.x. [DOI] [PubMed] [Google Scholar]
- 16.Skier J, Mandel ID. Comparative periodontal status of caries resistant versus susceptible adults. J Periodontol. 1980;51:614–6. doi: 10.1902/jop.1980.51.10.614. [DOI] [PubMed] [Google Scholar]
- 17.Sewón LA, Karjalainen SM, Sainio M, Seppä O. Calcium and other salivary factors in periodontitis-affected subjects prior to treatment. J Clin Periodontol. 1995;22:267–70. doi: 10.1111/j.1600-051x.1995.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 18.Sewón LA, Karjalainen SM, Söderling E, Lapinleimu H, Simell O. Associations between salivary calcium and oral health. J Clin Periodontol. 1998;25:915–9. doi: 10.1111/j.1600-051x.1998.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 19.Sewon L, Hyyppa T, Paunio K, Talonpoika J. Calcium concentration of dental plaque in women with and without periodontitis. J Dent Res. 1988;67:397–9. [Google Scholar]
- 20.Dong YM, Pearce EI, Yue L, Larsen MJ, Gao XJ, Wang JD. Plaque pH and associated parameters in relation to caries. Caries Res. 1999;33:428–36. doi: 10.1159/000016547. [DOI] [PubMed] [Google Scholar]
- 21.Ashley FP, Coward PY, Jalil RA, Wilson RF. Relationship between calcium and inorganic phosphorus concentrations of both resting and stimulated saliva and dental plaque in children and young adults. Arch Oral Biol. 1991;36:431–4. doi: 10.1016/0003-9969(91)90133-f. [DOI] [PubMed] [Google Scholar]


