Abstract
Objectives:
Saliva contains a variety of host defense factors. It influences calculus formation and periodontal disease. Different studies have been done to find exact correlation of salivary biomarkers with periodontal disease. With a multitude of biomarkers and complexities in their determination, the salivary pH may be tried to be used as a quick chairside test. The aim of this study was to analyze the pH of saliva and determine its relevance to the severity of periodontal disease.
Study Design:
The study population consisted of 300 patients. They were divided into three groups of 100 patients each: Group A had clinically healthy gingiva, Group B who had generalized chronic gingivitis and Group C who had generalized chronic periodontitis. The randomized unstimulated saliva from each patient was collected and pH was tested. Data was analyzed statistically using analysis of variance technique.
Results:
The salivary pH was more alkaline for patients with generalized chronic gingivitis as compared with the control group (P = 0.001) whereas patients with generalized chronic periodontitis had more acidic pH as compared with the control group (P = 0.001).
Conclusion:
These results indicate a significant change in the pH depending on the severity of the periodontal condition. The salivary pH shows significant changes and thus relevance to the severity of periodontal disease. Salivary pH may thus be used as a quick chairside diagnostic biomarker.
Keywords: Gingivitis/physiopathology, hydrogen-ion concentration, periodontitis, saliva/physiology
INTRODUCTION
Oral diseases such as dental caries, periodontitis and oral malodor are always initiated at the interface between microbial ecosystem and host tissue. Changes in microbial and environmental dynamics in microbial ecosystems may increase the potential for pathogenicity within a microbial ecosystem and subsequently initiate and promote oral diseases. These successional changes have recently and tentatively been referred to by Marsh as the ecological plaque hypothesis.[1]
Thus, the properties of the environment determine, which microorganisms can occupy a site while the metabolic activities of those microbial communities subsequently modify the properties of the environment.[1]
It is known that periodontal diseases in humans and other mammals are predominantly associated with Gram-negative anaerobic organisms and that, before destructive periodontal diseases are initiated, these microorganisms must colonize tooth surfaces at and just below the gingival margin.[2,3] Strong evidence exists to consider Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythia as etiologic agents. There is moderate evidence for the association of the following with periodontal disease: Campylobacter rectus, Eubacterium nodatum, Fusobacterium nucleatum, Prevotella intermedia/nigrescens, Peptostreptococcus micros, Streptococcus intermedius-complex, Treponema denticola and spirochetes. Eikenella corrodens, Staphylococcus and yeasts associated with human immunodeficiency virus periodontitis and peri-implantitis have shown weak association.[4] A study by Takahashi et al.[5,6] on the effect of pH on the growth of microorganisms showed that P. gingivalis grows at a pH of 6.5-7.0, P. intermedia grows at a pH of 5.0-7.0 and F. nucleatum grows at a pH of 5.5-7.0.
The diagnosis of active phases of periodontal disease and the identification of patients at risk for active disease represents a challenge for both clinical investigators and clinicians. In general, clinical parameters including probing depth, attachment level, bleeding on probing plaque index (PI) and radiographic loss of alveolar bone are used to assess disease severity.[7]
Occasionally, monitoring of the microbial infection[8] and analysis of the host response in gingival crevicular fluid (GCF) are utilized in an attempt to identify individuals at risk for future breakdown.[9,10]
Compelling reasons exist to use saliva as a diagnostic fluid. It meets the demands for being inexpensive, non-invasive and easy-to-use diagnostic methods.[11] As a clinical tool, saliva has many advantages over serum, including ease of collection, storing and shipping and it can be obtained at low cost in sufficient quantities for analysis.[12] For patients, the non-invasive collection techniques dramatically reduce anxiety and discomfort and simplify procurement of repeated samples for monitoring over time. Saliva also is easier to handle for diagnostic procedures because it does not clot, thus lessening the manipulations required.[13] Saliva exerts a major influence on plaque initiation, maturation and metabolism.
Saliva and crevicular fluid play a decisive role in the prevention of periodontal disease and indeed paradoxically in the induction of periodontal pathology. Thus, both of these factors have been the object of much study. There is scanty literature regarding the use of salivary pH as a diagnostic marker in periodontal disease.
This study is aimed at evaluating the pH of saliva, determine its relevance to the severity of periodontal disease and thus evaluate its suitability as a diagnostic marker of disease.
MATERIALS AND METHODS
Study population
The study was conducted in the out-patient department of Department of Periodontology and Implantology, M.A. Rangoonwala College of Dental Sciences and Research Centre, Pune, India. The study population consisted of 300 patients within the age group of 20-45 years. Group A had 100 subjects of who had clinically healthy gingiva, Group B had 100 patients who had generalized chronic gingivitis and Group C had 100 subjects who had generalized chronic periodontitis.
All patients were verbally explained the nature of the study and an informed written consent was obtained (as per Helsinki declaration).
Patients with history of systemic diseases or conditions that may adversely affect periodontal health or the composition of saliva were excluded from the study. The exclusion criteria for the study were:
Patients who were completely edentulous were not selected for the study
Smoking, malocclusion, mouth breathing and local pathologic factors conducive to induction of periodontal disease
Patients with history of diabetes, kidney disease, cancer, fungal or respiratory infections
Patients giving history of hospitalization or intake of medications in a period of 6 months
Patients with current or past habit of tobacco smoking or chewing.
Gingival and periodontal findings were recorded for each patient. Control group included patients with clinically healthy gingiva with a probing depth of up to 3 mm.
The test groups included patients with generalized chronic gingivitis as evidenced with inflammation of the gingival without loss of attachment. They were selected based on the National Institute of Dental Research criteria – gingival inflammation index (bleeding index).[14]
0 = No bleeding.
1 = Bleeding after the probe is placed in the gingival sulcus up to 2 mm and drawn along the inner surface of the gingival sulcus.
The criteria for periodontitis were based on loss of attachment with pocket depth of ≥ 5 mm in at least 30% sites.
Saliva sampling
Saliva was collected as per the protocol is derived from the World Health Organization/International Agency for Research on Cancer guideline “Common Minimal Technical Standards and Protocols.”[15] Saliva samples were obtained in the morning after an overnight fast, during which subjects were requested not to drink any beverages except water. The subjects were given drinking water (bottled) and asked to rinse their mouth out well (without drinking water). 5 min after this oral rinse, the subject was asked to spit whole saliva. The subjects were asked to refrain from talking and drop down the head and let the saliva run naturally to the front of the mouth. The subjects were also asked not to cough up mucus as saliva is collected. The subjects spit into the collection tube about once a minute for up to 10 min. 5 ml of saliva was collected in sterile 10 ml beakers. The salivary sample was collected between 9:00 am and 11:00 am.
The pH of the saliva was immediately measured in order to prevent any deterioration of the sample.
Salivary analysis
Salivary pH was measured with the help of a single electrode digital pH meter (Electronics India, Model 111E) as shown in Figure 1. The pH meter was calibrated every day. The electrode was dipped in hydrochloric acid of 0.1 N overnight. The pH meter was then calibrated using freshly prepared buffers of pH 7 and pH 4. The latter was used for finer adjustment to the pH. Following this the electrode was kept dipped in double distilled water. Prior to dipping the electrode in the sample, it was gently dried completely using fresh sterile filter papers each time. After analyzing the pH, the electrode tip was again washed with a gentle stream of distilled water and then dipped in the double distilled water. The liquids and chemicals were freshly prepared every day.
Figure 1.

Single electrode digital pH meter (Electronics India, Model 111 E)
Statistical analysis
The mean and standard pH for all the three groups was calculated. The P values were calculated by one-way analysis of variance using Tukey's correction for multiple group comparisons and was considered statistically significant if P value was < 0.05.
RESULTS
As shown in Table 1, the average pH for the population with clinically healthy gingiva was 7.06 ± 0.04. The average pH of the group having chronic generalized gingivitis was 7.24 ± 0.10 while average pH of those having chronic generalized periodontitis was 6.85 ± 0.11.
Table 1.
Average pH values for the three groups

The mean gingival index and mean PI for the three groups is shown in Table 2.
Table 2.
Mean Plaque index and Gingival index of the three groups

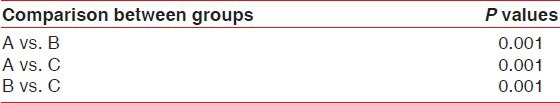
It was found that the pH of saliva from population having chronic generalized gingivitis was alkaline as compared with that of the population having clinically healthy gingiva (P = 0.001), whereas the population having chronic generalized periodontitis comparatively had a more acidic pH of saliva than the clinically healthy group (P = 0.001) [Table 3].
Table 3.
P values by one-way analysis of variance using Tukey's correction for multiple group comparisons
DISCUSSION
Saliva is a dilute fluid, over 99% being made up of water. Whole saliva collected from the mouth is a complex mixture. Apart from secretions of all the glands it also contains desquamated oral epithelial cells, microorganisms and their products, leucocytes, serum constituents, fluid from gingival crevice and food remnants. The concentrations of dissolved solids (organic and inorganic) are characterized by wide variation, both between individuals and within a single individual. Of the approximately 750 ml of saliva secreted daily, submandibular glands[1] account for 60%, parotid for about 30% and sublingual glands for 5% or less. About 7% of saliva is derived from minor salivary glands.[16] The saliva that basically forms the environment of the oral cavity is the resting or pooled saliva.
Saliva has a pH normal range of 6.2-7.6 with 6.7 being the average pH. Resting pH of mouth does not fall below 6.3. In the oral cavity, the pH is maintained near neutrality (6.7-7.3) by saliva. The saliva contributes to maintenance of the pH by two mechanisms. First, the flow of saliva eliminates carbohydrates that could be metabolized by bacteria and removes acids produced by bacteria. Second, acidity from drinks and foods, as well as from bacterial activity, is neutralized by the buffering activity of saliva.
The present study included 300 patients from the Department of Periodontology an Implantology at M. A. Rangoonwala Dental College, Pune, India. Participants below 20 years were excluded from the study due to the possible effect of physiological metabolic growth changes on Ca and PO4 levels of saliva consequently affecting its pH.[17]
Inflammation of the gingival tissue results in gingivitis, which if not resolved leads to inflammation of the periodontium called as periodontitis.[18]
The induction and progression of periodontal tissue destruction is a complex process involving plaque accumulation, release of bacterial substances and host inflammatory response. It is characterized by pocket formation and bone loss. Pockets are caused by microorganisms and their products, which produce pathologic tissue changes that lead to deepening of the gingival sulcus.
The saliva that basically forms the environment of the oral cavity is the resting or pooled saliva. Henskens et al.[19] evaluated the effect of periodontal treatment on the protein composition of whole and parotid saliva. Significant changes in salivary protein composition including that of albumin occurred only in whole saliva, after treatment. Concentrations of parotid cystatin S were unchanged during the periodontal treatment process.[20] Salivary levels of alpha 2-macroglobulin, C-reactive protein, cathepsin G and elastase levels are directly related to an individual's periodontal status.[21]
The gingival sulcus contains a fluid that seeps into it from the gingival connective tissue through the thin sulcular epithelium and is called as GCF. Thus, it is contributory to the pH of saliva. For this reason, unstimulated whole saliva was collected from the subjects.
A saliva pH of 7.0 usually indicates a healthy dental and periodontal situation. At this pH, there is a low incidence of dental decay combined and little or no calculus. Therefore, stable conditions should basically be found in this environment.
A saliva pH below 7.0 usually indicates acidemia (abnormal acidity of the blood). If a chronic condition exists, the mouth is more susceptible to dental decay, halitosis and periodontitis. Chronic acidemia can be a causative factor for a multitude of diseases affecting the whole body.
A saliva pH above 7.0 usually indicates alkalinity. Excessive alkalinity can bring about the same anaerobic conditions as acidemia, but it is much rarer condition.
Plaque bacteria take calcium compounds in the environment and use the minerals to protect them from the high pH. The two key factors to plaque formation are first there must be oral bacteria to attack food particles and elevate the pH. Second the pH must elevate above 7.6 to grow dental plaque crystals that cause periodontal disease.
Thus, alkaline pH is essential for plaque growth suggesting the mildly alkaline pH of the saliva obtained from the subjects with generalized chronic gingivitis.
As the gingival crevice deepens; the environmental factors in the subgingival site become more stable, i.e., neutral pH and anaerobic. Under these conditions, asaccharolytic and anaerobic and/or proteolytic bacteria such as Fusobacterium, Campylobacter, Prevotella and Porphyromonas are found. Proteolytic bacteria can degrade nitrogenous compounds into small peptides and amino acids by cell membrane-bound and/or extracellularly secreted proteases for subsequent use as metabolic substrates.[22]
Fusobacterium species also utilize glutamic acid as a nutrient and produce acetic and butyric acids. P. gingivalis, P. intermedia and C. rectus metabolizes aspartic acid to succinic acid but requires formic acid as a reducing agent.[22] Oral Treponemal species are strictly dependent on isobutyric acid for growth. Thus, we can see that, with a few exceptions, most of the subgingival microbiota responsible for chronic periodontitis either utilize or create end products that are mild to moderately acidic in nature.
Takahashi et al.[5,6] concluded in their study that the periodontopathogens grow in a mildly acidic pH. This is in accordance to our result for pH of chronic periodontitis.
Fujikawa et al.[23] studied the correlation between the pH level and the microflora in periodontal pockets in the various stages of periodontal disease. A change in pH level was seen in deep pockets or severe gingival inflammation. A close correlation was seen between salivary and crevicular pH. The pH level was significantly positively related with the proportion of coccoid forms, but was negatively correlated with the proportion of motile organisms that are reported to be related with periodontal disease.
Galgut[24] conducted a study to investigate any possible correlations between pH and gingivitis and periodontal pockets. Correlations between pH and gingivitis were not identified, but significant correlations between pH and periodontal pockets were evident.
CONCLUSION
Saliva is a fluid that can be easily collected, contains locally-derived and systemically derived markers of periodontal disease and hence may offer the basis for a patient specific diagnostic test for periodontitis.
Saliva can be used as an indicator of prognosis during periodontal treatment. Within the limitations of this study, it has been observed that there is a correlation between pH of saliva and periodontal diseases when compared with healthy groups. Salivary pH in patients with chronic generalized gingivitis was more alkaline than that in patients with clinically healthy gingiva. In patients with chronic generalized periodontitis, the salivary pH was more acidic than the control group. This may be of diagnostic value in the future, but further elaborate studies with larger sample size, microbiological analysis and ions in the salivary sample are needed to draw definite conclusions.
A study closer to the seat of periodontal disease such as within plaque is necessary to establish the nature of the exact role in the induction and the progression of periodontal disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263–71. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 2.Loesche LV. The bacterial etiology of periodontal disease: The specific plaque hypothesis. In: Clark JW, editor. Clinical Dentistry. Philadelphia: Harper and Row; 1987. [Google Scholar]
- 3.Moore WE, Moore LH, Ranney RR, Smibert RM, Burmeister JA, Schenkein HA. The microflora of periodontal sites showing active destructive progression. J Clin Periodontol. 1991;18:729–39. doi: 10.1111/j.1600-051x.1991.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 4.Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: Current concepts. J Periodontol. 1992;63:322–31. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi N, Schachtele CF. Effect of pH on the growth and proteolytic activity of Porphyromonas gingivalis and Bacteroides intermedius. J Dent Res. 1990;69:1266–9. doi: 10.1177/00220345900690060801. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi N, Saito K, Schachtele CF, Yamada T. Acid tolerance and acid-neutralizing activity of Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum. Oral Microbiol Immunol. 1997;12:323–8. doi: 10.1111/j.1399-302x.1997.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 7.Polson AM, Goodson JM. Periodontal diagnosis. Current status and future needs. J Periodontol. 1985;56:25–34. doi: 10.1902/jop.1985.56.1.25. [DOI] [PubMed] [Google Scholar]
- 8.Listgarten MA. Microbiological testing in the diagnosis of periodontal disease. J Periodontol. 1992;63:332–7. doi: 10.1902/jop.1992.63.4s.332. [DOI] [PubMed] [Google Scholar]
- 9.Curtis MA, Gillett IR, Griffiths GS, Maiden MF, Sterne JA, Wilson DT, et al. Detection of high-risk groups and individuals for periodontal diseases: Laboratory markers from analysis of gingival crevicular fluid. J Clin Periodontol. 1989;16:1–11. doi: 10.1111/j.1600-051x.1989.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 10.Lamster IB. Evaluation of components of gingival crevicular fluid as diagnostic tests. Ann Periodontol. 1997;2:123–37. doi: 10.1902/annals.1997.2.1.123. [DOI] [PubMed] [Google Scholar]
- 11.Erdemir EO, Erdemir A. The detection of salivary minerals in smokers and non-smokers with chronic periodontitis by the inductively coupled plasma-atomic emission spectrophotometry technique. J Periodontol. 2006;77:990–5. doi: 10.1902/jop.2006.050202. [DOI] [PubMed] [Google Scholar]
- 12.Zuabi O, Machtei EE, Ben-Aryeh H, Ardekian L, Peled M, Laufer D. The effect of smoking and periodontal treatment on salivary composition in patients with established periodontitis. J Periodontol. 1999;70:1240–6. doi: 10.1902/jop.1999.70.10.1240. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths GS, Sterne JA, Wilton JM, Eaton KA, Johnson NW. Associations between volume and flow rate of gingival crevicular fluid and clinical assessments of gingival inflammation in a population of British male adolescents. J Clin Periodontol. 1992;19:464–70. doi: 10.1111/j.1600-051x.1992.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 14.National Institute of Dental Research. Washington D.C: U.S. Government Printing Office; 1987. Oral Health of United States Adults-the National Survey of Oral Health in U.S. Employed Adults and Seniors 1985-1986: National Findings; pp. 167–8. NIH Publication No. 87-2868. [Google Scholar]
- 15.International Agency for Research on Cancer. Selected protocols. [Accessed on 2010 Oct 6]. Available from: http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk2/standardsBRC-8.pdf .
- 16.Provenza DV. 2nd ed. Philadelphia: Lea and Gebinger; 1986. Textbook of Oral Histology, Inheritance and Development. [Google Scholar]
- 17.Waerhaug J. The gingival pocket; anatomy, pathology, deepening and elimination. Odontol Tidskr. 1952;60:1–186. [PubMed] [Google Scholar]
- 18.Carranza FA., Jr . 8th ed. Philadelphia: W. B. Saunders Company; 1998. Textbook of Clinical Periodontology. [Google Scholar]
- 19.Henskens YM, van den Keijbus PA, Veerman EC, Van der Weijden GA, Timmerman MF, Snoek CM, et al. Protein composition of whole and parotid saliva in healthy and periodontitis subjects. Determination of cystatins, albumin, amylase and IgA. J Periodontal Res. 1996;31:57–65. doi: 10.1111/j.1600-0765.1996.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 20.Henskens YM, van der Weijden FA, van den Keijbus PA, Veerman EC, Timmerman MF, van der Velden U, et al. Effect of periodontal treatment on the protein composition of whole and parotid saliva. J Periodontol. 1996;67:205–12. doi: 10.1902/jop.1996.67.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Pederson ED, Stanke SR, Whitener SJ, Sebastiani PT, Lamberts BL, Turner DW. Salivary levels of alpha 2-macroglobulin, alpha 1-antitrypsin, C-reactive protein, cathepsin G and elastase in humans with or without destructive periodontal disease. Arch Oral Biol. 1995;40:1151–5. doi: 10.1016/0003-9969(95)00089-5. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi N. Microbial ecosystem in the oral cavity: Metabolic diversity in an ecological niche and its relationship with oral diseases. Int Congr Ser. 2005;1284:103–12. [Google Scholar]
- 23.Fujikawa K, Numasaki H, Kobayashi M, Sugano N, Tomura S, Murai S. pH determination in human crevicular fluids. Examination of the pH meter and evaluation of the correlation between pH level and clinical findings or the microflora in each periodontal pocket. Nihon Shishubyo Gakkai Kaishi. 1989;31:241–8. doi: 10.2329/perio.31.241. [DOI] [PubMed] [Google Scholar]
- 24.Galgut PN. The relevance of pH to gingivitis and periodontitis. J Int Acad Periodontol. 2001;3:61–7. [PubMed] [Google Scholar]


