Abstract
Background:
A high number of patients with periodontitis may have undiagnosed diabetes. Self-monitoring devices provide a simple method for rapid monitoring of the glucose level in the blood by utilizing a blood sample from the finger, but this method requires a needle puncture to obtain blood. It is possible that gingival crevicular blood (GCB) from routine periodontal probing may be a source of blood for glucose measurements.
Aim:
To establish whether GCB can be used as a non-invasive diagnostic aid in screening for diabetes mellitus during routine periodontal examination.
Materials and Methods:
The study involved 50 diabetics and 50 non-diabetics, with an age range of 26-66 years. Both diabetic and non-diabetic patients had moderate to severe gingivitis with at least one tooth in the maxillary anterior region showing bleeding upon probing. The Gingival Index and Oral Hygiene Index-Simplified were recorded. Blood oozing from the gingival sulcus/pocket following periodontal pocket probing was collected using a capillary tube and transferred to the test stick of a glucose self-monitoring device (Accu-Chek, Roche Diagnostic, Germany) in patients with comparable gingival and oral hygiene status. This value was compared with the peripheral fingerstick blood glucose (PFBG) value, which was obtained by pricking the finger tip at the same visit. Statistical analysis was performed using Pearson's correlation coefficient.
Result:
There was no statistically significant difference between the gingival crevicular blood glucose (GCBG) values and the PFBG values in both the diabetic (P = 0.129, NS) and the non-diabetic (P = 0.503, NS) groups. Karl Pearson's product–moment correlation coefficient was calculated, which showed a positive correlation between the two measurements in the diabetic (r = 0.943) as well as the non-diabetic (r = 0.926) groups.
Conclusion:
The results suggest that GCB can be used as a non-invasive diagnostic aid in screening for diabetes mellitus during routine periodontal examination.
Keywords: Chair-side screening for diabetes mellitus, gingival crevicular blood glucose, non-invasive, peripheral finger stick blood glucose, self-monitoring devices
INTRODUCTION
Diabetes mellitus represents one of the major chronic health problems faced by the society today. The incidence of diabetes mellitus all over the world, and especially in India, is on a steep rise. According to the diabetes atlas published by the International Diabetes Federation,[1] the estimated diabetes prevalence for 2010 rose to 285 million, representing 6.4% of the world's adult population, with a prediction that by 2030, the number of people with diabetes will have risen to 438 million. It is estimated that every fifth person with diabetes will be an Indian. The National Urban Diabetes Survey (NUDS) reported that the prevalence of impaired glucose tolerance (IGT) in the Indian subcontinent is ~8.7% in urban and ~7.9% in rural areas.[2] Because of the observation that ~35% of those with IGT will develop full-blown diabetes in ≤5 years, the sheer numbers of those with diabetes seems overwhelming.[2] Moreover, 50% of the diabetics go undiagnosed.[3] Diabetes and periodontitis seem to interact in a bidirectional manner.[4] The increased prevalence and severity of periodontitis seen in patients with diabetes, especially in those with poor metabolic control, has led to the designation of periodontal disease as the “sixth complication of diabetes,”[5,6] and successful periodontal therapy in diabetic patients entails the stabilization of blood glucose to a normal range.[5,7]
Diabetes mellitus is often asymptomatic in its early stages and can remain undiagnosed for many years.[8] As a result, by the time of diagnosis in many of these individuals, beta cell function may have declined substantially[9] and significant damage may already have occurred. Thus, there is a critical need to increase opportunities for diabetes screening and early diabetes detection, especially among those who may be at a high risk for diabetes. Expanding the range of venues to augment the conventional medical office is one strategy to increase the likelihood of identifying people with undiagnosed diabetes. Screening for type 2 diabetes mellitus would allow earlier recognition of cases, with the potential to intervene earlier in the disease course. Because of the association between dental infections and diabetes mellitus, and because the presence of one promotes the other, dentists are extremely likely to encounter an increased number of undiagnosed diabetic patients.[4,10]
Self-monitoring devices provide a simple method for rapid monitoring of the glucose level in blood by utilizing a blood sample from the finger but requiring a needle puncture of the skin to obtain a drop of blood.[11] With regard to the development of painless and non-invasive methods to measure blood glucose, considerable effort has been made in the past few years. However, until now, no such methods are in routine clinical practice. Oozing blood from the gingival crevice during periodontal probing may allow non-invasive or minimally invasive monitoring of blood glucose.[10,11,12,13] The aim of this study was to evaluate a quick, safe and non-invasive method to screen for diabetes during regular periodontal examination using Accu-Chek, self-monitoring glucometer.
MATERIALS AND METHODS
A total of 100 patients, 50 diabetic and 50 non-diabetic aged 26-66 years, were randomly selected from the outpatient Department of Periodontics, Pacific Dental College and Hospital, Rajasthan, India. Known diabetic cases included were on the basis of history and medical records furnished by the patients. Both the study and the control group patients had moderate to severe gingivitis, with at least one tooth in the maxillary anterior region showing bleeding upon probing. The proposed study was reviewed by the ethical committee of the institution and clearance was obtained. An informed consent was obtained from each subject before conducting the trial. Patients undergoing treatment for anemia, polycythemia, gout, dialysis or any other disorder that could cause an abnormal variation in the hematocrit and with any requirement of antibiotic premedication were excluded. In addition, subjects with a history of any systemic diseases, subjects on medication that interfered with coagulation (e.g., analgesics, anti-coagulants) or supplemental Vitamin C that could interfere with the glucose test strip oxidation reaction were also excluded.
Standard periodontal examination was carried out in subjects who befitted the selection criteria. Gingival index (Loe and Silness, 1963) and Oral Hygiene Index-Simplified (Green and Vermillion, 1964) were recorded. The gingival crevicular blood glucose (GCBG) levels were recorded using an Accu–Chek Active (Roche Diagnostics, Germany) self-monitoring device. The results from this device were compared with the peripheral finger-stick blood glucose (PFBG) values in patients with comparable gingival and oral hygiene status.
The gingiva around the upper anterior teeth was chosen to be the donor site for the gingival crevicular blood (GCB) sample as they offer ideal access. Supra- and subgingival scaling was carried out to help facilitate collection of the blood. Contamination with saliva was minimized by using gauze and air-drying. Maxillary anterior teeth were probed with a William's periodontal probe, with a force of approximately 0.2 N. Bleeding on probing was assessed during 30-60 s after probing. Sites with profuse bleeding were preferred as donor sites while sites with suppurations were avoided. To obtain a clean sample, probing was repeated, when necessary, until a sufficient quantity of blood (2-3 μL) was present to gather a sample.
The blood was collected with the help of a small glass capillary tube of 2 mm bore and transferred on to a test strip [Figure 1]. The Accu-Chek glucometer reports blood glucose measurements in mg/dL within 15-30 s [Figure 2]. If the blood sample was inadequate, the test was repeated using a new test strip. Then, the regular capillary finger stick blood was collected from one of the patient's fingers. The pad of the finger was wiped with alcohol, allowed to dry and then punctured with a sterile lancet. The blood was drawn onto the test strip preloaded in the glucometer [Figure 3]. Both samples from each individual were taken at the same visit. Results were recorded and tabulated for each patient of the diabetic and non-diabetic groups.
Figure 1.
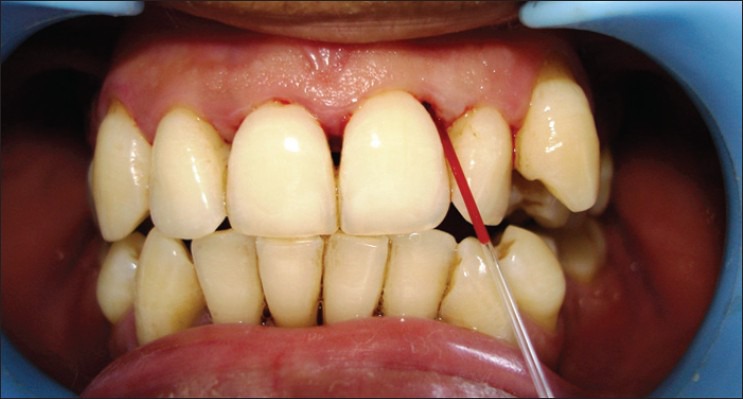
Collection of gingival crevicular blood with a glass capillary tube
Figure 2.
Blood glucose reading in the glucometer
Figure 3.
Finger tip pricked and peripheral fingerstick blood applied onto the test strip
Statistical analysis
Analyses of the obtained results were carried out by two statistical tests: (1) Student's independent t-test to test the significance of difference between the two readings and (2) Karl Pearson's product–moment correlation. The tests were performed separately for the diabetic and non-diabetic groups.
RESULTS
The study group involved 50 diabetic patients (type I, type II): 27 males and 23 females; and the control group involved 50 non-diabetic patients: 30 males and 20 females; in the age range of 26-66 years. The sex- and age-wise distribution of the100 patients are shown in Table 1. In group 1, the prevalence of diabetes was higher for females in the 31-50 years age group, while more males had diabetes in the 51-70 years age group. Figures 4 and 5 show the comparison between GCBG levels and PFBG levels in the diabetic and non-diabetic groups, respectively. There was no statistically significant difference between the GCBG values and the PFBG levels in the diabetic (P = 0.129, NS) as well as the non-diabetic groups (P = 0.503, NS).
Table 1.
Age and sex distribution of diabetic and non-diabetic patients
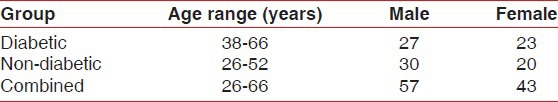
Figure 4.
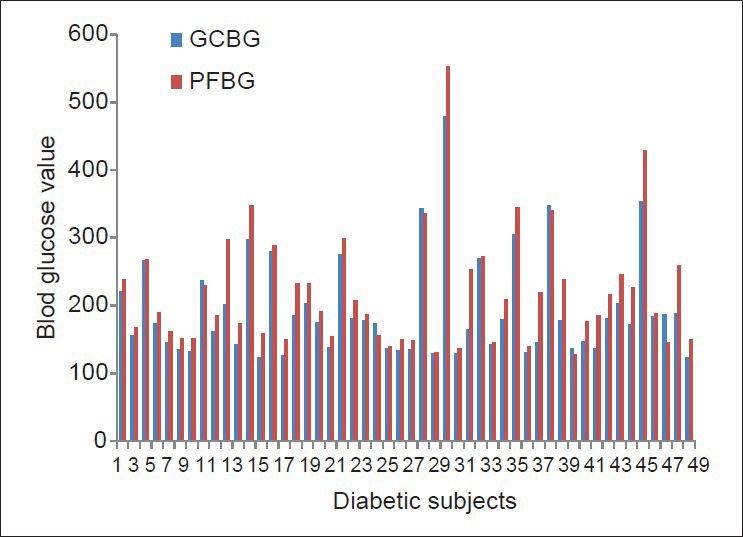
Comparison of gingival crevicular blood glucose and peripheral fingerstick blood glucose in the diabetic group
Figure 5.
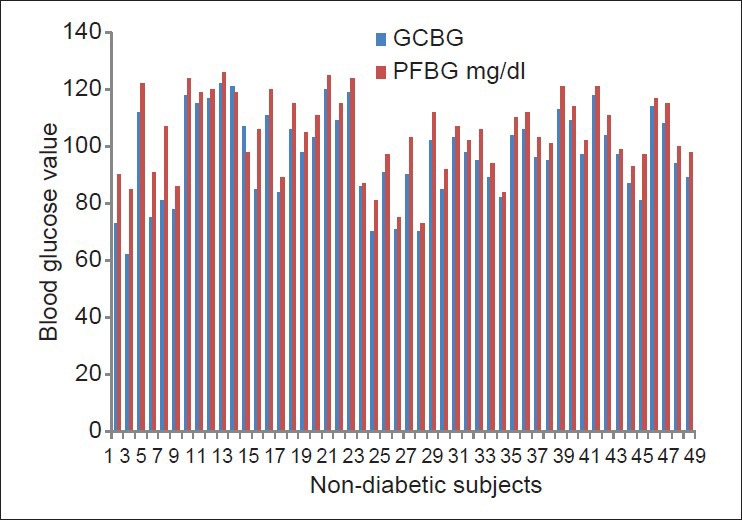
Comparison of gingival crevicular blood glucose and peripheral fingerstick blood glucose in the non-diabetic group
The mean and standard deviations values for the GCBG and PFBG in the diabetic and non-diabetic groups were calculated [Table 2]. The mean GCBG in both the diabetic and the non-diabetic (193.52 mg/dL ± 74.93 and 97.2 mg/dL ± 15.7, respectively) groups was slightly lower than the mean PFBG in both groups (218.54 mg/dL ± 84.04 and 104.48 mg/dL ± 13.84, respectively). Karl Pearson's product–moment correlation coefficient was calculated between GCBG versus peripheral finger blood glucose for the diabetic and the non-diabetic groups. There was a significantly positive correlation seen in both the groups [Table 3]. A slightly stronger positive correlation was observed between GCBG values and peripheral finger blood glucose values in the diabetic group (r = 0.943) when compared with the non-diabetic group (r = 0.926).
Table 2.
GCBG values and PFBG values in the diabetic and non-diabetic groups
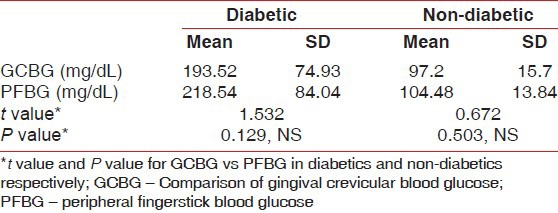
Table 3.
Karl Pearson's prodcut–moment correlation (r) for gingival crevicular blood glucose values versus peripheral fingerstick blood glucose values in the diabetic group and the non–diabetic groups

Regression line and scatter plot of the linear relationship between the glucose measurements of the gingival blood and finger puncture method were performed for both the diabetic and the non-diabetic groups, and is shown in Figures 6 and 7, respectively.
Figure 6.
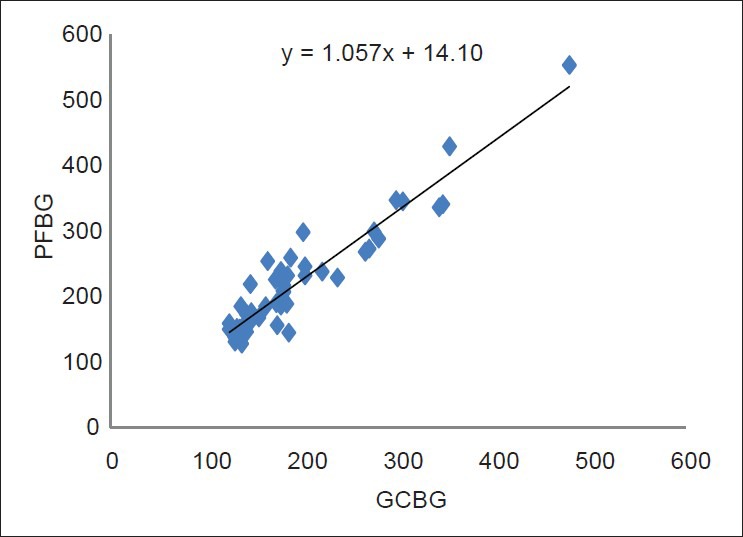
Regression line and scatter plot of the linear relationship between the glucose measurements from the gingival blood and the finger puncture method in the diabetic group
Figure 7.
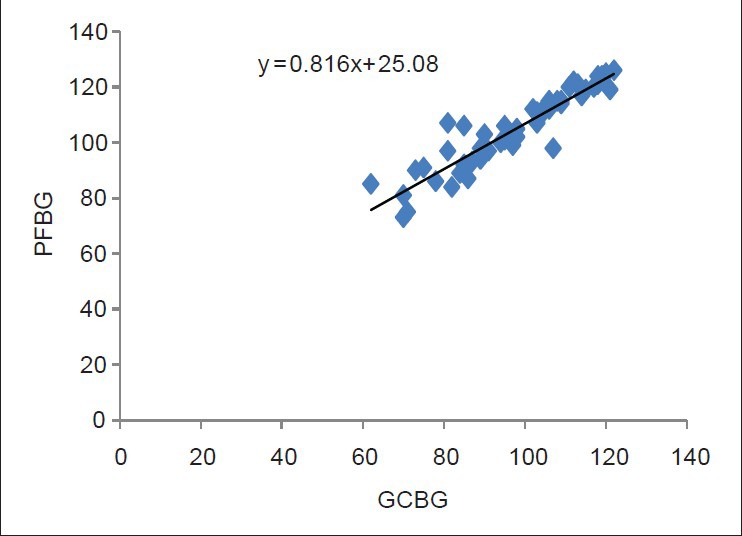
Regression line and scatter plot of the linear relationship between the glucose measurements from the gingival blood and the finger puncture method in the non-diabetic group
DISCUSSION
Diabetes mellitus and periodontitis seem to interact in a bi-directional manner.[4] Various studies prove that periodontal therapy exerts beneficial effects on diabetes mellitus control.[14] Diabetes is undiagnosed in approximately one-half of the patients with the disease.[15] The American Diabetes Association (2000) recommends that screening for diabetes should start at the age of 45 years, and be repeated every 3 years in individuals without risk factors for diabetes, and earlier and more often in individuals with risk factors.[15] Our study includes patients from the 21 to 30 years age group because of the increased prevalence of diabetes in the younger age group in India.[2,16] However, we found no incidence of diabetics in this age group in the present study.
The periodontist frequently manages diabetic patients using limited information about their blood glucose control.[17] Often, the only information available is from a single laboratory test that may not reflect their current blood glucose status. Monitoring their blood glucose during the office visit may be a better alternative. Periodontal inflammation is known to produce ample extravasated blood during diagnostic procedures. It is possible that GCB from probing may be an excellent source of blood for glucometric analysis using the technology of portable glucose monitors. The American Diabetic Association recommended that the prediction error of blood glucose monitoring devices falls within 15% of the laboratory standard.[18] Consequently, studies evaluating the predictability of test strip glucose monitors have shown to fall within this range.[10] The test strip reaction is time dependent and begins as soon as blood is applied. Reagent strips were also shown to be fairly reliable and accurate for clinical use.[19]
In this study, the GCBG value was compared with the PFBG value to ascertain whether the former relates to the latter, and thus whether it could serve as an alternative to measure the blood glucose. Estimations of sulcular blood glucose levels were previously conducted and showed correlations with capillary blood glucose levels, thereby suggesting that testing sulcular blood may be a valuable tool in identifying potential patients with diabetes.[10,11,20] However, the correlation between the two measures can be influenced by a variety of factors such as site of sample collection, sampling methodology, type of instrument used and duplicate sampling. Regarding site of sample collection, a previous study by Strauss et al.,[21] reported that GCB samples were suitable to screen for diabetes in persons with sufficient bleeding on probing to obtain a sample without touching the tooth or the gingival margin[21] (i.e., in patients having the basic clinical signs of gingivitis or periodontal disease). Also, the method of collection of sulcular blood is critical because the resultant glucose values may be altered if there is any contamination of the collected sample by the oral tissues or tissue products. Past intraoral blood glucose studies[10,12,22] have transferred blood onto the test strip by wiping blood directly from the hemorrhagic gingival tissue with the test strip itself or by rubbing blood onto the test strip from a blood-laden dental curette. Rubbing or direct wiping of intraoral blood onto the strip will not produce a uniformly timed reaction and may damage the strip's chemical indicator surface. Significant contamination may occur from saliva and oral debris present at the wiped gingival area or from plaque and crevicular fluid on the dental curette. In the present study, isolating the bleeding gingival site with a gauze after scaling and then rapidly sampling blood with the capillary tube was an improvement over the past studies. Using the capillary tube might not eliminate contamination. However, it does reduce saliva, plaque and debris by collecting free-flowing blood just inside the gingival crevice. As early as 1969, Stein and Nebbia[22] used the interdental gingival papilla prick method with test strips to screen patients with high gingival blood glucose and, more recently, Shetty and Kohad (2011) et al.,[23] studied a previously unsuspecting periodontal population for diabetes using the same method. However, because majority of the patients are usually apprehensive whenever invasive techniques are used, we have incorporated the non-invasive method where the blood oozing out during routine periodontal examination is checked for diabetes.
Regression line and scatter diagram of the linear relationship between fingerstick capillary and GCB were plotted for the diabetic as well as the non-diabetic groups [Figures 6 and 7, respectively]. With the help of this chart, the capillary fingerstick blood glucose values can be predicted by knowing the GCBG value. Additionally, Student's independent “t” test was calculated in this study, which showed no significant difference between the GCBG levels and the PFBG levels in both the groups. Although the sample collection from both the sites would involve capillary blood, still, a variance was observed with a general trend of finger stick blood glucose values being slightly higher than the crevicular blood glucose values. The reason for this could be dilution of gingival blood with gingival crevicular fluid and other contaminants. Interestingly, however, it should be noted that as the blood travels from the capillary to the venous circulation, the blood glucose values come down (by approximately 3.5 mg/dL) due to the normal glycolysis that takes place. Hence, true laboratory glucose values obtained by measuring venous blood would be closer to the capillary blood glucose values taken from the gingival sulcus rather than the finger.[11]
The clinical application of these findings involves the interrelationship of diabetes mellitus and periodontal disease. Consulting with the diabetic patient's physician routinely yields the result of a single blood glucose test. Because periodontal disease requires long-term treatment that often continues for years, a single blood glucose test would definitely be insufficient for periodontal management. Using the method described in this study, the practitioner can rapidly measure blood glucose many times using the GCB. Multiple measurements of the diabetic patient's blood glucose allows the practitioner to better assess the patient's diabetic control as the treatment progresses. Apart from this, suspected diabetics can also be screened in the dental office itself and then referred for further investigations if required. Because the measurement of glucose through GCB involves a quick and simple intraoral procedure with minimal cost, dental professionals may be motivated to implement diabetes screening and feel comfortable and confident in doing so.
We acknowledge the limitations in the present research. First, as was true of the studies by Beikler et al.,[10] Khader et al.[12] and Müller and Behbehani,[24] we did not collect venous blood samples, the gold standard with which to measure glucose in the laboratory, nor did we collect duplicate GCB and capillary finger-stick bloodsamples, as was done in the study by Parker et al.,[11] However, when using the mean of the duplicate measures of the GCB and CFB glucose readings, Parker et al.,[11] determined that it was not better than a single measurement in determining the correlation, bias, precision and prediction error of the GCB and CFB glucose measures. Also, as was the case with the studies by Beikler et al.,[10] and Müller and Behbehani,[24] our participants were not fasting, nor were the results adjusted based on the time since the participants last ate. Notably, although some research[25,26] found that the concurrence of glucose measures from CFB and from alternate testing sites is diminished in the postprandial period, other studies[27,28] failed to find that the length of time since last food intake affected this concurrence.
CONCLUSION
The results of the present study indicate that GCB collected during diagnostic periodontal examination may be an excellent source of blood for glucometric analysis. In addition, the technique described is safe, easy to perform and comfortable for the patient and might therefore help to increase the frequency of diabetes screening in dental offices. Although not a test to diagnose diabetes, such screening is an important aid in identifying those for whom follow-up tests regarding possible diabetes are warranted. Furthermore, the costs associated with the purchase of a readily available glucometer and individual test strips are extremely modest. Thus, with minimal cost and a limited investment of time for patients and clinicians, dental professionals can play a critical role in supporting their patients’ overall health.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, et al. Diabetes Epidemiology Study Group in India (DESI). High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia. 2001;44:1094–101. doi: 10.1007/s001250100627. [DOI] [PubMed] [Google Scholar]
- 3.Harris MI, Eastman RC. Early detection of undiagnosed diabetes Mellitus: A US perspective. Diabetes Metab Res Rev. 2000;16:230–6. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr122>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 4.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: A two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 5.Loe H. Periodontal disease: The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 6.Ainamo J, Lahtinen A, Vitto VJ. Rapid periodontal destruction in adult humans with poorly-controlled diabetes. A report of 2 cases. J Periodontol. 1990;17:22–8. doi: 10.1111/j.1600-051x.1990.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 7.Sastrowijoto SH, van der Velden U, van Steenbergen TJ, Hillemans P, Hart AA, de Graaff J, et al. Improved metabolic control, clinical periodontal status and subgingival microbiology in insulin-dependent diabetes mellitus. A prospective study. J Clin Periodontol. 1990;17:233–42. doi: 10.1111/j.1600-051x.1990.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 8.Harris MI, Klein R, Welborn TA. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care. 1992;15:815–9. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 9.U.K. Prospective Diabetes Study Group. U.K Prospective Diabetes Study 16: Overview of 6 years’ therapy of type II diabetes: A progressive disease. Diabetes. 1995;44:1249–58. [PubMed] [Google Scholar]
- 10.Beikler T, Kuczek A, Petersilka G, Flemmig TF. In-Dental-Office Screening for diabetes mellitus using gingival crevicular blood. J Clin Periodontol. 2002;29:216–8. doi: 10.1034/j.1600-051x.2002.290306.x. [DOI] [PubMed] [Google Scholar]
- 11.Parker RC, Rapley JW, Ishley W, Spencer P, Killoy WJ. Gingival crevicular blood for assessment of blood glucose in diabetic patient. J Periodontol. 1993;64:666–72. doi: 10.1902/jop.1993.64.7.666. [DOI] [PubMed] [Google Scholar]
- 12.Khader YS, Al-Zu’bi BN, Judeh A, Rayyan M. Screening for type 2 diabetes mellitus using gingival crevicular blood. Int J Dent Hygiene. 2006;4:179–82. doi: 10.1111/j.1601-5037.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- 13.Müller HP, Behbehani E. Methods for measuring agreement: Glucose levels in gingival crevice blood. Clin Oral Investig. 2005;9:65–9. doi: 10.1007/s00784-004-0290-3. [DOI] [PubMed] [Google Scholar]
- 14.Mealey B. Position paper. Diabetes and periodontal diseases. J Periodontol. 2000;71:664–78. doi: 10.1902/jop.2000.71.4.664. [DOI] [PubMed] [Google Scholar]
- 15.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran A, Snehalatha C, Latha E, Vijay V, Viswanathan M. Rising prevalence of NIDDM in an urban population in India. Diabetologia. 1997;40:232–7. doi: 10.1007/s001250050668. [DOI] [PubMed] [Google Scholar]
- 17.Rees TD. Periodontal management of the patients with diabetes mellitus. Periodontology 2000. 2000;23:63–72. doi: 10.1034/j.1600-0757.2000.2230105.x. [DOI] [PubMed] [Google Scholar]
- 18.Consensus statement on self-monitoring of blood glucose. Diabetes Care. 1987;10:95–9. [PubMed] [Google Scholar]
- 19.Thai AC, Wang KW, Lui KF, Yeo PP. Home blood glucose monitoring without a meter (Haemo-Glukotest 20-800 test strip): The Singapore experience. Singapore Med J. 1983;24:67–70. [PubMed] [Google Scholar]
- 20.Ardakani MR, Moeintaghavi A, Haerian A, Ardakani MA, Hashemzadeh M. Correlation between levels of sulcular and capillary blood glucose. J Contemp Dent Pract. 2009;10:10–7. [PubMed] [Google Scholar]
- 21.Strauss SM, Wheeler AJ, Russell SL, Brodsky A, Davidson RM, Gluzman R, et al. The potential use of gingival crevicular blood for measuring glucose to screen for diabetes: An examination based on characteristics of the blood collection site. J Periodontol. 2009;80:907–14. doi: 10.1902/jop.2009.080542. [DOI] [PubMed] [Google Scholar]
- 22.Stein GM, Nebbia AA. Chairside method of diabetic screening with gingival blood. Oral Surg Oral Med Oral Pathol. 1969;27:607–12. doi: 10.1016/0030-4220(69)90092-9. [DOI] [PubMed] [Google Scholar]
- 23.Shetty S, Kohad R, Ramreddy Y, Shetty K. Gingival blood glucose estimation with reagent test strips: A method to detect diabetes in a periodontal population. J Periodontol. 2011;82:1548–55. doi: 10.1902/jop.2011.110009. [DOI] [PubMed] [Google Scholar]
- 24.Müller HP, Behbehani E. Screening of elevated glucose levels in gingival crevice blood using a novel, sensitive self-monitoring device. Med Princ Pract. 2004;13:361–5. doi: 10.1159/000080474. [DOI] [PubMed] [Google Scholar]
- 25.Jungheim K, Koschinsky T. Glucose monitoring at the arm: Risky delays of hypoglycemia and hyperglycemia detection. Diabetes Care. 2002;25:956–60. doi: 10.2337/diacare.25.6.956. [DOI] [PubMed] [Google Scholar]
- 26.Ellison JM, Stegmann JM, Colner SL, Michael RH, Sharma MK, Ervin KR, et al. Rapid changes in postprandial blood glucose produce concentration differences at finger, forearm, and thigh sampling sites. Diabetes Care. 2002;25:961–4. doi: 10.2337/diacare.25.6.961. [DOI] [PubMed] [Google Scholar]
- 27.Kempe KC, Budd D, Stern M, Ellison JM, Saari LA, Adiletto CA, et al. Palm glucose readings compared with fingertip readings under steady and dynamic glycemic conditions, using the OneTouch Ultra Blood Glucose Monitoring System. Diabetes Technol Ther. 2005;7:916–26. doi: 10.1089/dia.2005.7.916. [DOI] [PubMed] [Google Scholar]
- 28.Lock JP, Szuts EZ, Malomo KJ, Anagnostopoulos A. Whole-blood glucose testing at alternate sites: Glucose values and hematocrit of capillary blood drawn from fingertip and forearm. Diabetes Care. 2002;25:337–41. doi: 10.2337/diacare.25.2.337. [DOI] [PubMed] [Google Scholar]


