Abstract
Background:
The present, randomized, controlled clinical and radiographic study was undertaken to compare the effectiveness of guided tissue regeneration (GTR) by using a collagen membrane barrier with or without decalcified freeze-dried bone allograft (DFDBA) in the treatment of periodontal infrabony defects characterized by unfavorable architecture.
Materials and Methods:
Sixteen systemically healthy patients with 20 periodontal infrabony defects were selected for the study. Each patient had at least ≥ 5 mm clinical probing pocket depth (PPD) at the selected site and depth of intrabony component ≥ 3 mm as assessed by clinical and radiographic measurements. Baseline measurements included plaque index, papillary bleeding index, PPD, gingival recession, clinical attachment level and radiographic defect depth (DD). At the time of surgery, the defects were randomly assigned to either the test group (collagen membrane plus DFDBA) or the control group (collagen membrane only).
Results:
At the 6-month examination, PPPD reduction was significantly greater in the GTR + DFDBA group (4.06 ± 0.38 mm) compared with the GTR group (3.2 ± 0.74 mm). The mean gains of clinical attachment were 3.54 ± 0.36 mm in the test group and 2.50 ± 0.74 mm in the control group. Radiographic DD reduction was similarly greater in the GTR + DFDBA group (2.40 ± 0.51 mm) compared with the GTR group (1.60 ± 0.51 mm).
Conclusions:
The results of the present study indicate that the use of a GTR membrane with bone graft has significantly improved all clinical parameters tested as compared with the use of bioresorbable membrane alone in the treatment of infrabony defects characterized by unfavorable architecture.
Keywords: Bone graft, guided tissue regeneration, infrabony defect
INTRODUCTION
Filling of the intrabony defects with various types of bone grafts is one of the most widely employed techniques aimed at restoring the lost attachment apparatus.[1] The difficulties associated with bone grafts in achieving predictable regeneration have led to the development of guided tissue regeneration (GTR) procedures.
Several commercially available collagen membranes have been developed using type I collagen as their major component, and it appears to be an ideal choice for an absorbable GTR barrier.[2,3]
It has been recognized that the morphology of the osseous defect plays an important role in the healing of the defect itself.[4] This is true with all currently available regenerative technologies. It should also be emphasized that the current regenerative approaches are able to influence only the most apical portion of the defect and, in the best situations, only the three-wall component of the defect.[4] No predictable form of therapy for one- and two-wall defects has been reported. In this context, considerable research has been reported on “Combined Periodontal Regenerative Technique” (CPRT),[5] i.e., the combination of the epithelial–exclusion characteristics of membranes with the scaffold effect provided by bone grafts.
There are conflicting results reported in the literature obtained by different clinicians by using GTR in combination with bone grafts.[5,6,7] Nevertheless, it must be observed that many comparative studies[6,7] that failed to demonstrate a difference in clinical outcome between these two procedures have been carried out on three-wall intrabony defects. However, these lesions may not represent the ideal model to evaluate an advanced surgical procedure as it shows a high potential for bone fill even in the absence of GTR procedures.[8]
Therefore, the present study was undertaken to compare the effectiveness of GTR by using a collagen membrane with or without demineralized freeze-dried bone allograft (DFDBA) in the treatment of periodontal infrabony defects characterized by unfavorable architecture.
MATERIALS AND METHODS
A total of 16 patients (five males and eleven females) with mean age 35.06 ± 7.9 years (range 21–52 years) with chronic periodontitis were screened from the outpatient department of Sharad Pawar Dental College, Wardha.
The inclusion criteria were: (1) systemically healthy subjects, (2) presence of at least one radiographically detectable interproximal infrabony osseous defects with a probing depth ≥ 5 mm following initial therapy, (3) depth of the intraosseous component of the defect ≥ 3 mm as measured by bone sounding and radiographic means and (4) presence of at least 2 mm of keratinized tissue around the teeth associated with the defect.
The exclusion criteria for the study were as follows: (1) patients with unacceptable oral hygiene (plaque index [PI] >1), (2) smokers or those who used any tobacco products, (3) teeth with inadequate endodontic/restorative treatments or defects extending into furcations and around the third molars, (4) teeth with mobility exceeding Grade II and (5) patients previously treated by periodontal surgical therapy and pregnant females or lactating mothers.
Initial therapy
After proper examination and diagnosis, initial therapy consisting of oral hygiene instructions, supragingival and subgingival scaling and root planing under local anesthesia and occlusal adjustment, if necessary, were performed. Plaque control instructions were repeated until the patients achieved a Silness and Loe[9] plaque score of ≤ 1. A re-evaluation examination was performed 6 weeks following completion of initial therapy to determine patient's response to the therapy and to confirm the need for periodontal surgery. Before initiating this study, the purpose and design of this clinical trial was explained to the patients and informed consent was signed by every patient. The study protocol was first approved by an ethical committee affiliated to the Nagpur University.
Study design
A total of 20 defects in 16 patients were found suitable after initial therapy. Before surgery, selected defects were randomly divided into test and control groups, each consisting of 10 defects, according to a randomized design. The control group was treated by an open flap debridement and a bioabsorbable collagen (type I and III) membrane of porcine origin*, while the test group was treated by a combination therapy of a bioabsorbable collagen and a demineralized freeze-dried bone allograft†.
Clinical measurements
On the day of surgery, and 3 months and 6 months post surgery, the patient's oral hygiene status was evaluated by the PI[9] as an expression of the level of full mouth plaque accumulation. Gingival inflammation was assessed by papillary bleeding index (PBI).[10]
The probing pocket depth (PPD), clinical attachment level (CAL) and gingival recession (REC) were recorded at baseline and 6 months after surgery for assessment of the results. These measurements were recorded with a computerized constant pressure probe‡ with a constant probing force of 15 gm (pressure –154 N/cm2), tip diameter of 0.45 mm, precision of 0.2 mm and a probe length of 11 mm. Custom-made occlusal acrylic stents were used to standardize the probe angulation and position [Figure 1]. The occlusal stents covered the occlusal surface of the tooth being treated and occlusal surfaces of at least one tooth in the mesial and distal directions.
Figure 1.
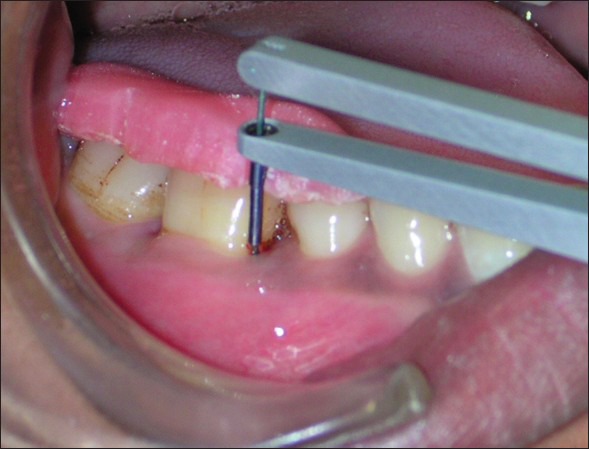
Test group - Probing shows deep pocket mesial to tooth # 46
All the measurements were recorded at six sites of the experimental tooth.
Radiographic analysis
An intra-oral periapical radiograph was taken of each selected site with the long cone (XCP Rinn, Dentsply, USA.) paralleling technique at baseline [Figure 2] and 6 months [Figure 3] after surgery to measure the defect depth and to calculate the percentage of bone fill. Radiographic measurements were obtained using a millimeter grid mount (Nix Company Ltd., Tokyo, Japan).
Figure 2.
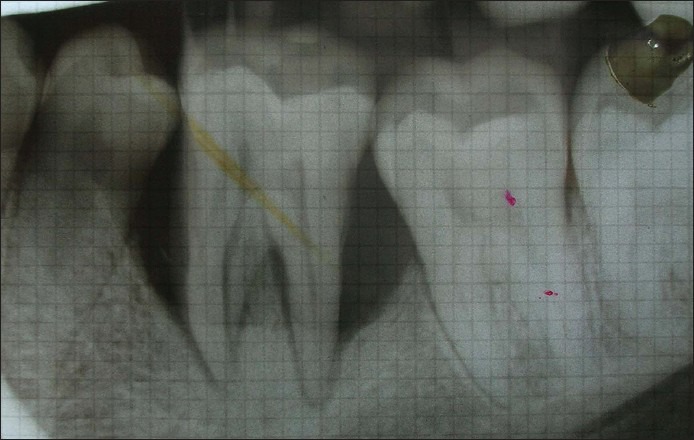
Baseline intraoral perioapical radiograph IOPA shows vertical defect mesial to tooth # 46
Figure 3.
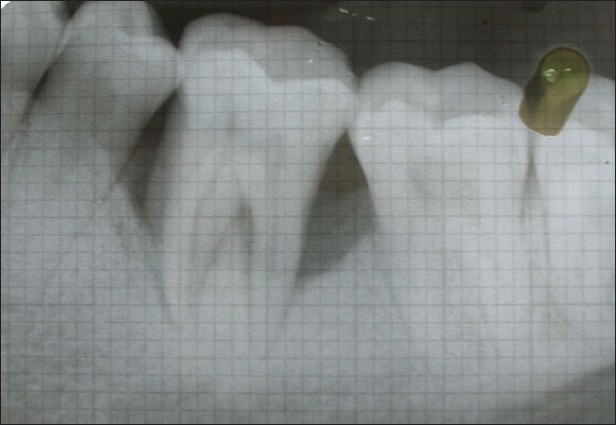
Six months post-operative IOPA shows defect depth reduction
Surgical procedure
Following administration of local anesthesia, incision and debridement were carried out [Figure 4]. At this stage, the depth of the osseous defect was measured and classified depending on the number of bony walls present.
Figure 4.
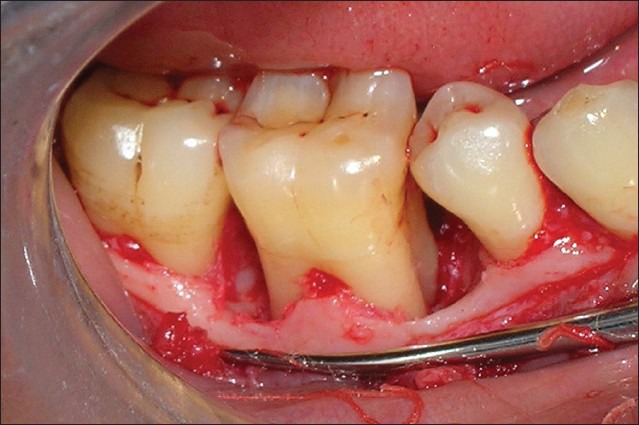
Debridement reveals configuration of infrabony defect
The control sites were treated according to the principles of the GTR, with the application of the resorbable bilayer membrane [Figure 5]. In test sites, in addition to the placement of the GTR membrane, DFDBA was filled in the osseous defect by raising the membrane on one side [Figures 6 and 7]. Before placement in the defect, the DFDBA was previously mixed with an adequate amount of sterile saline solution. The flaps were sutured using interdental interrupted sutures and primary, tension-free wound closure was attempted [Figure 8].
Figure 5.
Guided tissue regeneration membrane trimmed to size
Figure 6.
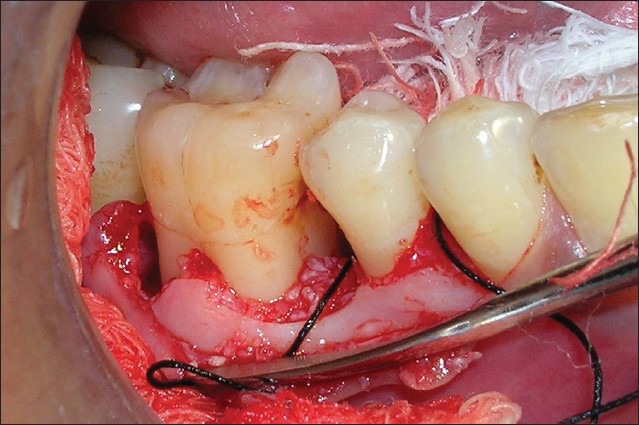
Decalcified freeze-dried bone allograft filled in defect
Figure 7.
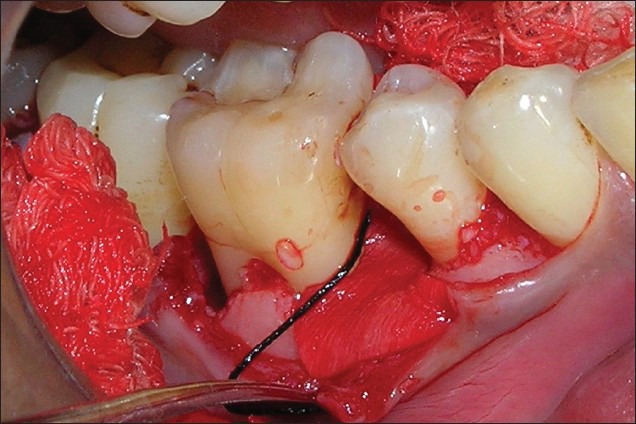
Guided tissue regeneration membrane in position
Figure 8.
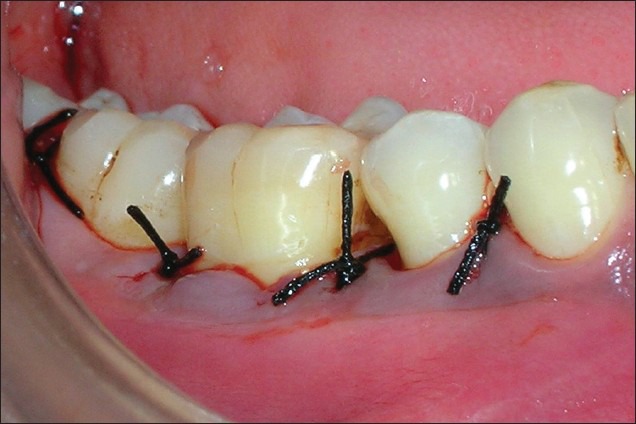
Post-operative view
The surgical site was covered with a non-eugenol periodontal dressing on the buccal and lingual aspects.
Antibiotics (Amoxicillin 500 mg tid) and analgesics (Ibuprofen 325 mg and Paracetamol 400 mg tid) were prescribed for the 5-day post-surgical period.
Maintenance care (Months 1, 3 and 6)
The patients were recalled at 1, 3 and 6 months post surgery. No probing was performed during the first 6 months of the post-surgical period.
A complete post-operative evaluation was performed at the 6-month follow-up visit.
Statistical analysis
Student's paired t-test was used to compare the data from baseline with the data at 6 months for each treatment group. Comparison between treatment groups at 6 months post surgery was accomplished with the Student unpaired t-test.[11]
RESULTS
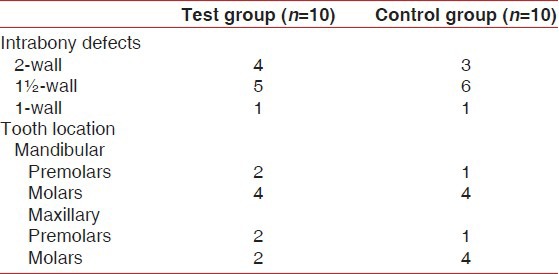
Table 1 shows the distribution and configuration of treated infrabony defects and their location.
Table 1.
Distribution and configuration of treated infrabony defects and tooth location
During the course of the study, wound healing was uneventful. No membrane had to be removed nor was any site to be eliminated from the study. In general, patients showed good oral hygiene throughout the study. The mean PI and PBI scores during the 6-month period remained below score 1 [Table 2].
Table 2.
Plaque and Papillary bleeding index scores at baseline, at 3 months and at 6 months after surgery (mean±SD)

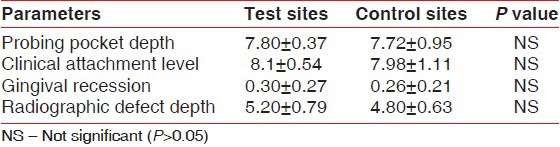
At baseline, no statistically significant differences in any of the investigated parameters were observed between the test and the control groups [Table 3].
Table 3.
Baseline defect characteristics for the test and control groups (mean±SD; in mm)
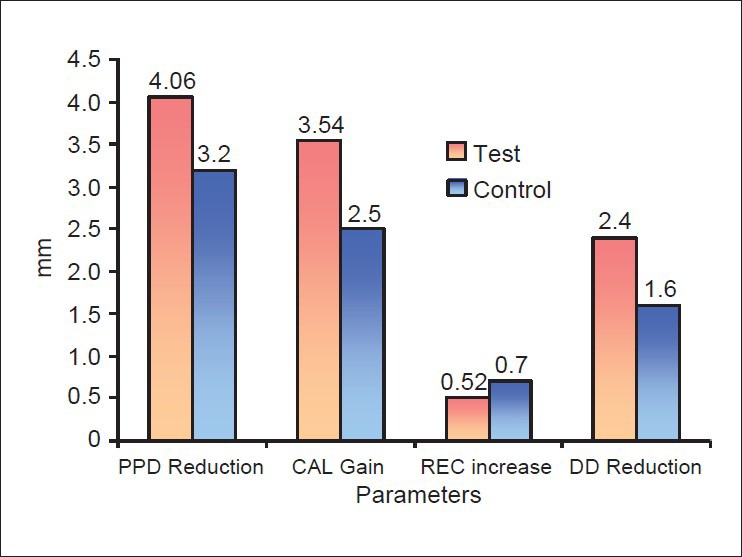
At 6 months, the mean PPD reduction was 4.06 ± 0.38 mm for the test group and 3.2 ± 0.74 mm for the control group. A significantly greater reduction in mean PPD was demonstrated in the test group as compared with the control group [Figure 9]. A mean CAL gain of 3.54 ± 0.36 mm was observed in the test group, while the control group displayed a mean CAL gain of 2.50 ± 0.74 mm [Figure 10]. The observed differences between baseline CAL and 6-month CAL were analyzed by Student's paired t-test, and were found to be statistically significant in both the groups |(P < 0.05).
Figure 9.
Comparison of parameters between Test (GTR+DFDBA) and Control (GTR) groups at 6 months
Figure 10.
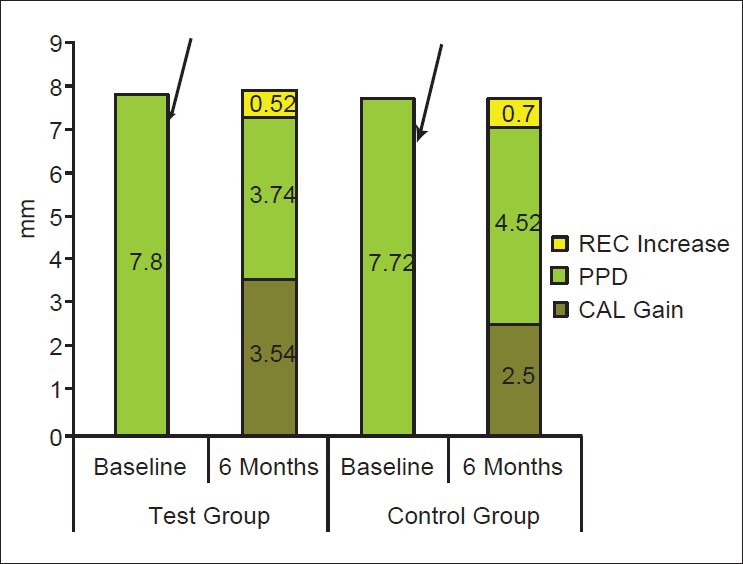
Mean baseline and post surgical PPD, CAL gains and Increase in REC in test and control groups
The mean CAL gain observed in the test group was significantly greater than that observed in the control group. The magnitude of the observed additional benefit was 1.04 ± 0.78 mm. A mean reduction in radiographic DD of 2.40 ± 0.51 mm was observed in the test group, while the control group displayed a mean reduction of radiographic DD of 1.60 ± 0.51 mm. Statistically significant reductions of radiographic DD were recorded for the test group as well as for the control group (P < 0.05) [Figure 9].
DISCUSSION
The present study was designed to compare the clinical regenerative capacity of GTR in combination with bone graft (DFDBA) and GTR alone using bioabsorbable collagen membranes in the treatment of periodontal intrabony defects. To limit the patient- and defect-based confounding factors, the study was carried out in non-smoking, compliant subjects with comparable defect and subject characteristics.
In the present study, a statistically significant greater amount of PPD reduction (0.86 ± 0.76 mm) was demonstrated in the GTR combined with DFDBA group compared with the GTR alone group. Orsini[12] et al. in 2001 reported a PPD reduction of 4.33 mm by using a bioabsorbable collagen membrane with autologous bone. Batista et al.[13] also reported PPD reduction values of 4.46 mm by using bioabsorbable membrane and bovine bone. Vouros et al.[14] in 2004 reported a PPD reduction of 4.72 mm by the implantation of bovine bone with a –polylactic acid bioabsorbable barrier. The mean PPD reductions reported in these studies are comparable to the mean PPD reduction in the present study. McClain and Schallhorn,[15] Paolantonio et al.,[5,16] Blumenthal and Steinberg[17] and Tonetti et al.[18] also found significantly improved PPD reductions in GTR combined with bone graft group as compared with the GTR alone group.
A statistically significant greater amount of mean CAL gain (1.04 ± 0.78 mm) was observed in the GTR combined with DFDBA group compared with the GTR alone group. Tonetti et al.,[18] in their recent study, also reported a mean CAL gain of 3.3 mm in a group treated by collagen membrane combined with bovine bone. The present findings also compare well with other studies that have reported greater CAL gains with combination therapy compared with GTR alone.[5,15,16,17,18]
From a clinical standpoint, it is even more significant to observe that 90% of the sites treated with combination therapy experienced a CAL gain of 3 mm or more, while only 30% of the GTR-treated sites showed a CAL gain of 3 mm or more.
In the present study, increase in REC was observed to a limited extent in both the test (0.52 ± 0.25 mm) and the control (0.70 ± 0.17 mm) groups. Blumenthal et al.,[17] Lekovic et al.[19] and Paolantonio et al.[16] have reported less REC at the sites treated with combination therapy as compared with sites treated with membrane alone. The maintenance of the membranes in a coronal position may explain the lower REC increase observed in the test group.
Also, the bone graft beneath the GTR membrane sustained the membrane in a relatively more coronal portion of the defect as the architecture of the defect could not guarantee an adequate support to the bioabsorbable membrane if used alone.[5] In the present study, we used collagen membranes that are characterized by a lack of stiffness when they are dampened by biological fluids.[20] The presence of a physical support under such a material allows the membrane to maintain its position when the flaps are sutured over the defect, exerting pressure on to the membrane itself. Thus, results confirm the principle that the coronal limit to periodontal regeneration is set by the membrane position.[5,16] The use of titanium-reinforced membranes that allow for maintenance of the original position of the barrier without the use of a filling material could probably have produced different results in the GTR group; however, the use of a metallic frame[21] to enhance the space-making characteristics of the membrane is associated with non-bioresorbable barriers and cannot eliminate the need for a second surgical procedure, resulting in an additional burden to both the clinician and the patient.
A statistically significant greater amount of radiographic DD reduction (0.80 mm) was observed in the GTR plus DFDBA group compared with the GTR group. Aichelman-Riedy et al.[22] found a gain in radiographic bone level of 2.7 mm in the membrane with bone graft group. Also, Batista et al.[13] reported a gain of bone height of 2.69 mm, which is comparable to the results obtained in the present study. McClain and Schallhorn[4] (1993), Paolantonio[5,16] (2002 and 2010), Blumenthal and Steinberg[17] (1990) and Tonetti et al.[18] also found significantly improved radiographic defect resolution in the GTR combined with bone graft group as compared with the GTR alone group.
Many studies carried out on combined therapy have used DFDBA as a filling material. This grafting material is reported to be characterized by osteoinductive properties due to its contents of a protein substrate that stimulates bone formation.[23] In a histological study by Blumenthal et al.,[17] combined bone graft and membrane therapy using DFDBA addressed many of the regenerative challenges offered by non-contained defects. In addition to the potential osteoinduction–osteoconduction effect, the graft provides a scaffold for clot and granulation tissue maturation and wound stabilization[24] and also supports the membrane and flap from collapsing into the defect.
On the contrary, other researchers[25,26,27] have found that addition of a filler material to GTR did not significantly improve the clinical outcomes. It must be noted that in most studies, three-wall defects were the object of the study. In this regard, Schallhorn and McClain[4] (1993) observed that three-walled defects offered a high predictability in the outcome of regenerative procedures while, on the other hand, defects characterized by one-wall or one- and two-wall combinations or furcation involvement had a lower degree of predictability. Therefore, the defects characterized by one-wall or one- and two-wall combination defects or furcation involvements may represent a better experimental model to test differences between two advanced regenerative procedures. This may avoid the mistaken conclusion that both procedures are equivalent when, in reality, a superiority of a particular procedure may exist.[28] In clinical practice, pure contained three-wall defects are uncommon. Chodroff and Ammons[29] (1984) found that only 16 of 130 defects had a pure three-wall morphology; the rest were combinations. Selvig et al.[30] found that in combination defects, the more occlusal portion was a combination of one- and two-wall configurations. The regenerative potential of such a more superficial component is compromised due to biological limitations of non-contained defect and/or the increased susceptibility to oral environmental factors, leading to incomplete fill.[31] In order to provide the most predictive therapy, it has been suggested to eliminate the coronal one- or two-wall component of the defect with osteotomy–osteoplasty and the remaining three-wall component can then be treated predictably with GTR or other procedures.[32] However, the present study showed that the regenerative capacity of the non-contained one- to two-wall component may be enhanced with a combined graft–membrane procedure. While not as definitive as a resective procedure, the increase in potential new periodontal support may be worth the minor compromise in predictability.
In conclusion, results from the present study indicate that GTR in combination with DFDBA resulted in statistically significant improvements in terms of CAL gains, PPD reduction and radiographic defect resolution when used to treat infrabony defects characterized by unfavorable architecture. Further controlled clinical trials with long-term evaluation on a large data base are needed to support these conclusions.
Footnotes
BIO-GIDE®, Geistlich Biomaterials, Geistlich Pharma AG, Wolhusen, Switzerland.
Dembone®, Pacific Coast Tissue Bank, California, USA.
Florida probe, Florida Probe Corporation, Gaineswille, FL, USA.
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Brunsvold M, Mellonig J. Bone grafts and periodontal regeneration. Periodontol 2000. 1993;1:80–91. [PubMed] [Google Scholar]
- 2.Pitaru S, Tal H, Soldinger M, Grosskopf A, Noff M. Partial regeneration of periodontal tissues using collagen barriers-Initial observations in the canine. J Periodontol. 1988;59:380–6. doi: 10.1902/jop.1988.59.6.380. [DOI] [PubMed] [Google Scholar]
- 3.Bunyaratavej P, Hom-Lay W. Collagen membranes: A Review. J Periodontol. 2001;72:215–29. doi: 10.1902/jop.2001.72.2.215. [DOI] [PubMed] [Google Scholar]
- 4.Schallhorn RG, McClain PK. Periodontal regeneration using combined techniques. Periodontol 2000. 1993;1:109–17. [PubMed] [Google Scholar]
- 5.Paolantonio M. Combined periodontal regenerative technique in intrabony defects by collagen membranes and anorganic bovine bone: A controlled clinical study. J Periodontol. 2002;73:158–66. doi: 10.1902/jop.2002.73.2.158. [DOI] [PubMed] [Google Scholar]
- 6.Parashis A, Andronikaki-Faldami A, Tsiklakis K. Clinical and radiographic comparison of three regenerative procedures in the treatment of intrabony defects. Int J Periodontics Restorative Dent. 2004;24:81–90. [PubMed] [Google Scholar]
- 7.Camelo M, Nevins ML, Schenk RK, Simion M, Rasperini G, Lynch SE, et al. Clinical, radiographic and histologic evaluation of human periodontal defects treated with Bio- Oss and Bio-Gide. Int J Periodont Restorative Dent. 1998;18:321–31. [PubMed] [Google Scholar]
- 8.Ellegard B, Löe H. New attachment of periodontal tissue after treatment of intrabony lesions. J Periodontol. 1971;42:648–53. doi: 10.1902/jop.1971.42.10.648. [DOI] [PubMed] [Google Scholar]
- 9.Silness J, Loe H. Periodontal disease in pregnancy. II correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 10.Muhlemann HR. Psychological and chemical mediators of gingival health. J Prev Dent. 1977;4:6–16. [PubMed] [Google Scholar]
- 11.Mahajan BK. 5th Ed. New Delhi: Jaypee Brothers; 1991. Methods in Bio-statistics; pp. 128–53. [Google Scholar]
- 12.Orsini M, Orsini G, Benlloch D, Aranda JJ, Lazaro P, Sanz M, et al. Comparison of calcium sulfate and autogenous bone graft to bioabsorbable membranes plus autogenous bone graft in the treatment of intrabony periodontal defects: A split-mouth study. J Periodontol. 2001;72:296–302. doi: 10.1902/jop.2001.72.3.296. [DOI] [PubMed] [Google Scholar]
- 13.Batista EL, Novaes AB, Simonpietri CJ, Batista FC. Use of bovine-derived anorganic bone associated with guided tissue regeneration in intrabony defects. Six-month evaluation at re-entry. J Periodontol. 1999;70:1000–7. doi: 10.1902/jop.1999.70.9.1000. [DOI] [PubMed] [Google Scholar]
- 14.Vouros I, Aristodimou E, Konstantinidis A. Guided tissue regeneration in intrabony periodontal defects following treatment with two bioabsorbable membranes in combination with bovine bone mineral graft. A clinical and radiographic study. J Clin Periodontol. 2004;31:908–17. doi: 10.1111/j.1600-051X.2004.00583.x. [DOI] [PubMed] [Google Scholar]
- 15.Schallhorn RG, McClain PK. Combined osseous composite grafting, root conditioning and guided tissue regeneration. Int J Periodontics Restorative Dent. 1988;8:8–31. [PubMed] [Google Scholar]
- 16.Paolantonio M, Femminella B, Coppolino E, Sammartino G, D’Arcangelo C, Perfetti G, et al. Autogenous periosteal barrier membranes and bone grafts in the treatment of periodontal intrabony defects of single-rooted teeth: A 12-month reentry randomized controlled clinical trial. J Periodontol. 2010;81:1587–95. doi: 10.1902/jop.2010.100094. [DOI] [PubMed] [Google Scholar]
- 17.Blumenthal NM, Alves ME, Al-Huwais S, Hofbauer AM, Koperski RD. Defect-determined regenerative options for treating periodontal intrabony defects in baboons. J Periodontol. 2003;74:10–24. doi: 10.1902/jop.2003.74.1.10. [DOI] [PubMed] [Google Scholar]
- 18.Tonetti MS, Cortellini P, Lang NP, Suvan JE, Adriaens P, Dubravec D, et al. Clinical outcomes following treatment of human intrabony defects with GTR/bone replacement material or access flap alone. A multicenter randomized controlled clinical trial. J Clin Periodontol. 2004;31:770–76. doi: 10.1111/j.1600-051X.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 19.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Kenney EB. Comparison of platelet-rich plasma, bovine porous bone mineral, and GTR versus platelet-rich plasma and bovine porous bone mineral in the treatment of intrabony defects: A reentry study. J Periodontol. 2002;73:198–205. doi: 10.1902/jop.2002.73.2.198. [DOI] [PubMed] [Google Scholar]
- 20.Wang HL, MacNeil RL. Guided tissue regeneration: Absorbable barriers. Dent Clin North Am. 1998;42:505–22. [PubMed] [Google Scholar]
- 21.Cortellini P, Pini-Prato G, Tonetti M. Periodontal regeneration of human infrabony defects with titanium reinforced membranes. A controlled clinical trial. J Periodontol. 1995;66:797–803. doi: 10.1902/jop.1995.66.9.797. [DOI] [PubMed] [Google Scholar]
- 22.Aichelmann-Reidy, Carlette D, Reynolds H. Clinical evaluation of calcium sulfate in combination with DFDBA for the treatment of human intraosseous defects. J Periodontol. 2004;75:340–47. doi: 10.1902/jop.2004.75.3.340. [DOI] [PubMed] [Google Scholar]
- 23.Urist MR, DeLange RJ, Finerman GA. Bone cell differentiation and growth factors. Science. 1983;20:680–6. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- 24.Wikesjö UM, Selvig KA. Periodontal wound healing and regeneration. Periodontol 2000. 1999;19:21–39. doi: 10.1111/j.1600-0757.1999.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen CC, Wang HL, Smith F, Glickman GN, Shyr Y, O’Neal RB. Evaluation of a collagen membrane with and without bone grafts in treating periodontal infrabony defects. J Periodontol. 1995;66:838–47. doi: 10.1902/jop.1995.66.10.838. [DOI] [PubMed] [Google Scholar]
- 26.Guillemin MR, Mellonig JT, Brunsvold MA. Healing in periodontal defects treated by decalcified freeze-dried bone allografts in combination with ePTFE membranes (I). Clinical and scanning electron microscope analysis. J Clin Periodontol. 1993;20:528–36. doi: 10.1111/j.1600-051x.1993.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 27.Garrett S, Loos B, Chamberbin D, Egelberg J. Treatment of intraosseous periodontal defects with a combined therapy of citric acid conditioning, bone grafting and placement of collageneous membranes. J Clin Periodontol. 1998;15:383–9. doi: 10.1111/j.1600-051x.1988.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 28.Gunsolley JC, Elswick RK, Davenport JM. Equivalence and Superiority Testing in Regeneration Clinical Trials. J Periodontol. 1998;69:521–7. doi: 10.1902/jop.1998.69.5.521. [DOI] [PubMed] [Google Scholar]
- 29.Chodroff RE, Ammons WF. Periodontal repair after surgical debridement with and without cartilage allografts. J Clin Periodontol. 1984;115:295–312. doi: 10.1111/j.1600-051x.1984.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 30.Selvig K, Kersten B, Wikesjo UM. Surgical treatment of intrabony periodontal defects using ePTFE barrier membranes: Influence of defect configuration on healing response. J Periodontol. 1993;64:730–3. doi: 10.1902/jop.1993.64.8.730. [DOI] [PubMed] [Google Scholar]
- 31.Tonetti M, Pini-Prato G, Cortellini P. Factors affecting the healing response of infrabony defects following GTR and access flap surgery. J Clin Periodontol. 1996;23:548–56. doi: 10.1111/j.1600-051x.1996.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 32.Ochsenbein C. Combined approach to the management of intrabony defects. Int J Periodontics Restorative Dent. 1995;15:328–43. [PubMed] [Google Scholar]


