Abstract
Periodontitis is a biofilm-associated inflammatory disease of the periodontium. This disease appears to have multiple etiologies with microbial factor contributing to initiation of the disease and immunological factor of the host propagating the disease. This review is on the concept of “microbial dysbiosis” and molecular nature of periodontitis, and the scope of traditional and emerging technologies for treating this disease.
Keywords: Biofilm, dysbiosis, periodontitis
INTRODUCTION
It is estimated that over 700 bacterial species reside in the oral cavity.[1] Bacteria grow in complex polymicrobial associations known as biofilms attached to biotic or abiotic surfaces. As a surface becomes colonized with individual cells, the bacteria form microcolonies, which then secrete a sticky extracellular polymeric substance that helps the bacteria adhere to the surface, and to each other.[2] Upon secretion of the extracellular polymeric substance, the biofilm matures by becoming larger and taking on a distinctive architecture.[3] Usually, this structure includes separate regions of fast- and slow-growing cells,[4] water channels that circulate metabolites, and the establishment of nutrient gradients.[5] Such complex structural organization allows the biofilm to exhibit functional heterogeneity which allows biofilm to demonstrate tremendous metabolic and phenotypic flexibility. This confers several new characteristics and advantages of the biofilm.
One such characteristic is an increased ability to attach to surfaces, which is brought about by regulation of genes involved in attachment (e.g., pili) and by production of the extracellular polymeric substance.[3] Another advantage is metabolic cooperation,[3] wherein the waste product of one bacterial species serves as the food source for another. Additionally, and perhaps most relevant clinically, biofilms often exhibit resistance to antibiotics that easily kill bacteria growing in planktonic culture. This could be because antibiotics find it difficult to penetrate the sticky extracellular polymeric substance, or could be due to the fact that slow-growing subpopulations of bacteria found in specialized niches within the biofilm are often less susceptible to antibiotics.[5] Yet another advantage is the ability of biofilms to avoid the host immune system. Antibodies are unable to perforate the matrix, and phagocytes often have difficulty engulfing large biofilm fragments.[6] Periodontitis is considered as a biofilm-associated disease.[7] The numerous advantages that biofilms possess over planktonic bacteria make treatment of periodontal infections difficult. These complications must be kept in mind when treating periodontitis and other biofilm-associated diseases.
Microbial shift and disease-associated biofilms
Just as entire microbial communities can be associated with health; current research also indicates that entire microbial communities can be associated with disease. Since more than one bacterial species may be associated with a particular disease, the traditional concept of “one germ, one disease” can be rejected or may need modification. The idea that the lack of a beneficial organism in a biofilm may be just as important as the presence of a pathogen in the contribution to disease,[8] a hypothesis has been developed linking certain diseases to a shift in membership of the local microbiota called “Microbial Shift” hypothesis [Figure 1]. Microbial shift, more commonly known as dysbiosis, refers to the concept that some diseases are due to a decrease in the number of beneficial symbionts and an increase in the number of pathogens. The long-standing paradigm is that, as periodontitis develops, the oral microbiota shifts from one consisting primarily of gram-positive aerobes to one consisting primarily of gram-negative anaerobes.[9] Recent research has indicated that dysbiosis in the oral cavity can lead to periodontitis. The development of oral dysbiosis is likely to occur over an extended period of time, gradually changing the symbiotic host–microbe relationship to a pathogenic one. During this period, the oral health of the host deteriorates until a state of clinical disease occurs. Simultaneously, a succession of microbial complexes develops. The first such complex that has been associated with disease is the so-called orange complex, which consists of gram-negative anaerobic species such as Prevotella intermedia, P. nigrescens, P. micros, and Fusobacterium nucleatum. As the disease worsens, the microbiota shifts to the so-called red complex, which consists of the periodontal pathogens P. gingivalis, Tannerella forsythia, and Treponema denticola.
Figure 1.
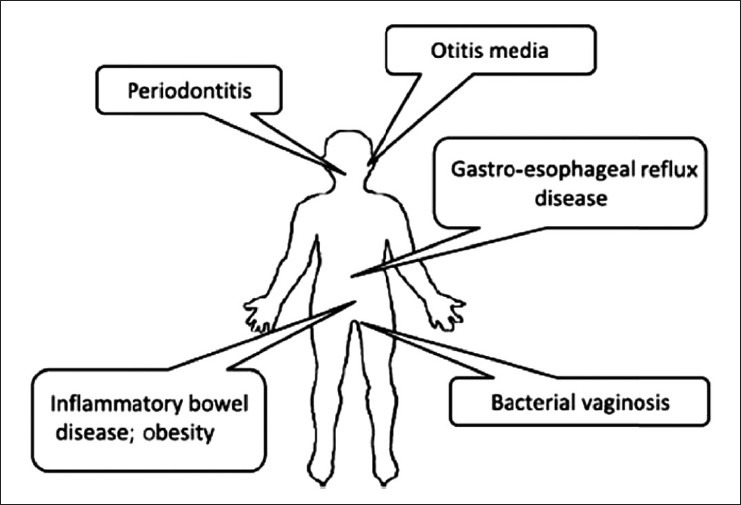
Examples of human diseases associated with shift in microbial flora
However, recent research has challenged this paradigm. For instance, Riep et al.[10] discovered that periodontal pathogens such as P. gingivalis and T. forsythia could also be frequently isolated from healthy controls. Kumar et al.[11] directly contradicted the existing pattern when they observed that the gram-negative bacterium Veillonella was associated with periodontal health, while the gram-positive anaerobe Filifactor alocis was associated with disease. To complicate matters even further, it has been proposed that two Herpes virus species, Epstein–Barr virus and Human cytomegalovirus, act synergistically with bacteria in the pathogenesis of periodontitis. These findings demonstrate that, in addition to a bacterial etiology, other factors like bacterial–viral co infection, genetic and immunological factors are also likely to contribute to periodontitis. Concomitantly, these revelations make choosing an appropriate treatment for periodontitis much more difficult.
Re-thinking Koch's postulates
Now that there is a wide consensus that periodontitis is a biofilm-associated disease, the primary goal is determining which of the 700 species or more found in the oral cavity is responsible. While Koch's postulates served medical microbiologists well for determining the causation of many human diseases, their limitations have been brought to light in the study of chronic infections. However, two concepts may help to resolve this issue.
The first is the concept of a “pathogenic microbial community”.[12] This concept was explained in a review by Siqueira and Rocas.[13] The authors suggest that since enormous variation in the composition of the oral microflora has been observed even between patients with the same disease, it is best to approach the etiology of periodontitis from a “community-as-pathogen” model, as opposed to the traditional “single-pathogen” model. This approach could be supported by the use of functional gene arrays. Environmental microbiology, just like oral microbiology, must cope with the presence of bacteria that cannot be cultivated. In the case of oral microbiology, bacterial communities from healthy and diseased periodontal samples could be screened for “pathogenic genes” using functional gene arrays, and correlations between the presence of pathogenic genes and periodontitis could be established.
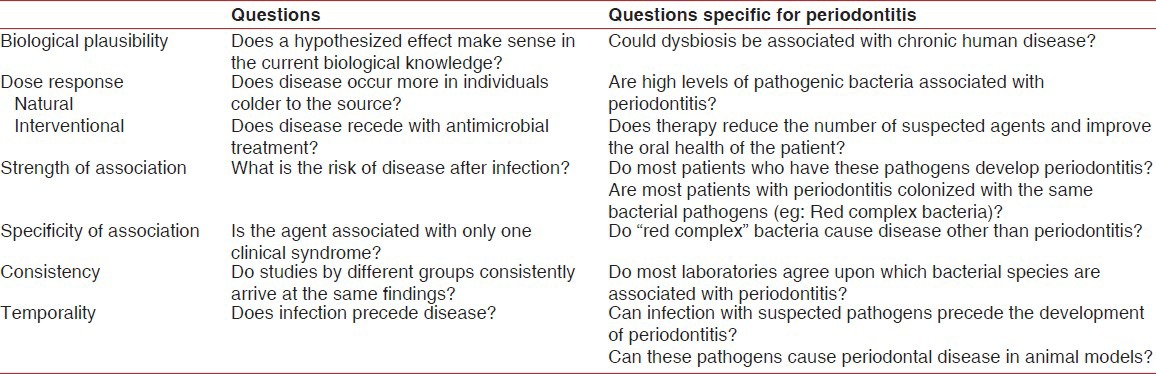
The second concept is Hill's criteria of causality [Table 1]. The rigid nature of Koch's postulates makes it difficult or impossible to satisfy them for many chronic conditions. The causal link between Helicobacter pylori infection and peptic ulcer disease is almost universally accepted, not because it fulfills Koch's postulates, but because it fulfills Hill's criteria of causality.[14] In order for causation to be established, Hill's criteria require that most of the following conditions are fulfilled: Biological plausibility, dose response, strength of association, specificity of association, consistency, and temporality.[14] Given the current obstacles, it appears that the etiology of periodontitis might be more readily established if current research combines the pathogenic microbial community concept with Hill's criteria of causality.
Table 1.
Hill's criteria of causality applied to periodontitis (Adapted from Lowe et al.[14])
Despite the difficulty in defining the precise etiology of periodontitis, one thing that is certain is the striking difference in the immune status of periodontal tissue between healthy and diseased patients. Essentially, clinically healthy periodontal tissue maintains a highly ordered, mild state of inflammation. For example, E-selectin expression[15] and an established interleukin-8 gradient[16] constantly guide neutrophils toward the junctional epithelium that borders the normal oral microflora, which is thought to provide the stimulus for this mild inflammatory response.[17] However, clinically diseased periodontal tissue exhibits a marked histopathology. For instance, expression of inflammatory molecules normally present in small amounts (such as Toll-like receptor 2) is greatly increased,[18] other inflammatory molecules (such as Toll-like receptor 4) are also expressed,[17,18] and the highly ordered state of mild inflammation is replaced by a disordered state of severe inflammation.[17] Thus, it is proposed that the shift from a symbiotic microflora to a dysbiotic pathogenic community triggers the potent host inflammatory response that contributes to the tissue destruction and alveolar bone loss that are characteristic of periodontitis.[17]
As multiple etiologies are involved in development of periodontitis [Figure 2], choosing appropriate treatment options can be quite difficult. Despite these complications, recent advances show tremendous potential to help patients suffering from periodontitis. Host modulation therapy, photodynamic therapy, and probiotic therapy may provide advantages that are not observed when antibiotics or antiseptics are used. However, much research still needs to be performed on these new alternatives. Most importantly, well-designed and large-scale randomized clinical trials are required to compare the “gold standard” of scaling and root planing to the new therapies used alone or adjunctively with scaling and root planing.
Figure 2.
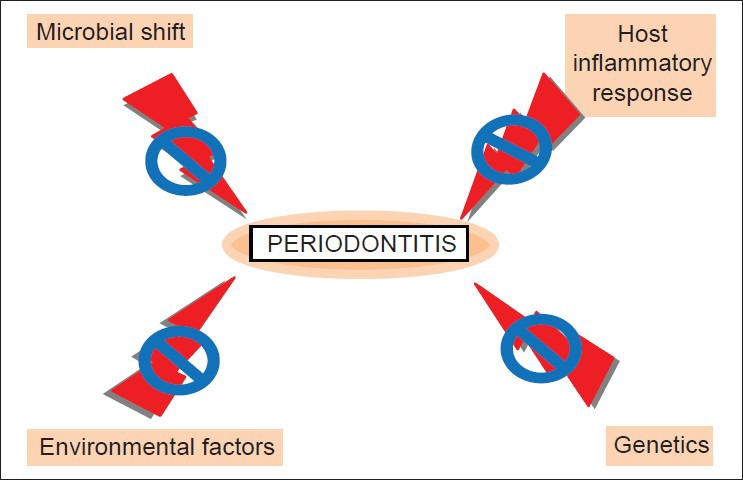
Various causes and treatment for periodontitis
CONCLUDING REMARKS
The literature as it currently stands appears to indicate that oral dysbiosis, or a shift from beneficial symbiotic bacteria to pathogenic bacteria, is at least partially responsible for the development of periodontitis. Thus, while a microbial shift is known to play a significant role in the development of periodontitis, genetic, immunological, and environmental factors must also be investigated in order for clinicians and researchers to fully understand disease progression. Because of the various risk factors that contribute to periodontitis, it is possible that there will be no “magic bullet” treatment. Indeed, the complexity of periodontitis emphasizes the necessity of a treatment that is highly tailored to the specific needs of the patient.
Overall, the goal for both researchers and clinicians is to find the best treatment. From a biological perspective, the most successful treatments need to attack the integrity of the periodontal biofilm and suppress the destructive host inflammatory response. From a clinical perspective, the best treatments are those that are simple, affordable, and able to confer a clinically relevant benefit to the patient.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu YQ, Liu Y, Tay JH. The effects of extracellular polymeric substances on the formation and stability of biogranules. Appl Microbiol Biotechnol. 2004;65:143–8. doi: 10.1007/s00253-004-1657-8. [DOI] [PubMed] [Google Scholar]
- 3.Davey ME, O’Toole GA. Microbial biofilms: From ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64:847–67. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An D, Parsek MR. The promise and peril of transcriptional profiling in biofilm communities. Curr Opin Microbiol. 2007;10:292–6. doi: 10.1016/j.mib.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11:1034–43. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 6.Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Schaudinn C, Gorur A, Keller D, Sedghizadeh PP, Costerton JW. Periodontitis: An archetypical biofilm disease. J Am Dent Assoc. 2009;140:978–86. doi: 10.14219/jada.archive.2009.0307. [DOI] [PubMed] [Google Scholar]
- 8.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–87. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 9.Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263–71. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 10.Riep B, Edesi-Neuss L, Claessen F, Skarabis H, Ehmke B, Flemmig TF, et al. Are putative periodontal pathogens reliable diagnostic markers? J Clin Microbiol. 2009;47:1705–11. doi: 10.1128/JCM.01387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar PS, Leys EJ, Bryk JM, Martinez FJ, Moeschberger ML, Griffen AL. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol. 2006;44:3665–73. doi: 10.1128/JCM.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–97. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siqueira JF, Jr, Rocas IN. Community as the unit of pathogenicity: An emerging concept as to the microbial pathogenesis of apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:870–8. doi: 10.1016/j.tripleo.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 14.Lowe AM, Yansouni CP, Behr MA. Causality and gastrointestinal infections: Koch, Hill, and Crohn's. Lancet Infect Dis. 2008;8:720–6. doi: 10.1016/S1473-3099(08)70257-3. [DOI] [PubMed] [Google Scholar]
- 15.Moughal NA, Adonogianaki E, Thornhill MH, Kinane DF. Endothelial cell leukocyte adhesion molecule-1 (ELAM-1) and intercellular adhesion molecule-1 (ICAM-1) expression in gingival tissue during health and experimentally-induced gingivitis. J Periodontal Res. 1992;27:623–30. doi: 10.1111/j.1600-0765.1992.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 16.Tonetti MS, Imboden MA, Lang NP. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol. 1998;69:1139–47. doi: 10.1902/jop.1998.69.10.1139. [DOI] [PubMed] [Google Scholar]
- 17.Darveau RP. The oral microbial consortium's interaction with the periodontal innate defense system. DNA Cell Biol. 2009;28:389–95. doi: 10.1089/dna.2009.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren L, Leung WK, Darveau RP, Jin L. The expression profile of lipopolysaccharide-binding protein, membrane-bound CD14, and toll-like receptors 2 and 4 in chronic periodontitis. J Periodontol. 2005;76:1950–9. doi: 10.1902/jop.2005.76.11.1950. [DOI] [PubMed] [Google Scholar]


