Abstract
We reported recently that treatment of diabetic apolipoprotein E-deficient mice with the Toll-like receptor 4 (TLR4) antagonist Rs-LPS, a lipopolysaccharide isolated from Rhodobacter sphaeroides, inhibited atherosclerosis. Since it is known that Rs-LPS antagonizes TLR4 by targeting TLR4 co-receptor MD-2, this finding indicates that MD-2 is a potential target for the treatment of atherosclerosis. In this study, we determined if MD-2 is involved in the gene expression regulated by signalling pathways independent of TLR4. Given that interferon-γ (IFNγ) and hyperglycaemia play key roles in atherosclerosis, we determined if MD-2 is involved in IFN-γ and high-glucose-regulated gene expression in mononuclear cells. Results showed that IFN-γ and high glucose synergistically stimulated matrix metalloproteinase 1 (MMP-1), a proteinase essential for vascular tissue remodelling and atherosclerosis, in U937 mononuclear cells, but Rs-LPS inhibited the MMP-1 stimulation. To provide more evidence for a role of MD-2 in IFN-γ-stimulated MMP-1, studies using antibodies and small interfering RNA demonstrated that MD-2 blockade or knockdown attenuated the effect of IFN-γ on MMP-1. Furthermore, studies using PCR arrays showed that MD-2 blockade had a similar effect as IFN-γ receptor blockade on the inhibition of IFN-γ-stimulated pro-inflammatory molecules. Although these findings indicate the involvement of MD-2 in IFN-γ signalling, we also observed that MD-2 was up-regulated by IFN-γ and high glucose. We found that MD-2 up-regulation by IFN-γ played an essential role in the synergistic effect of IFN-γ and LPS on MMP-1 expression. Taken together, these findings indicate that MD-2 is involved in IFN-γ signalling and IFN-γ-augmented MMP-1 up-regulation by LPS.
Keywords: inflammation, interferon-γ, MD-2, mononuclear cells, Toll-like receptor
Introduction
Toll-like receptor 4 (TLR4), an important pattern recognizing receptor, is engaged by lipopolysaccharide (LPS) and mediates LPS-elicited innate immune responses.1,2 Accumulating studies have indicated that TLR4 plays an important role in not only infectious diseases, but also inflammation-associated diseases such as atherosclerosis and diabetes.3–6 Recently, we demonstrated that administration of the TLR4 antagonist Rs-LPS, an LPS isolated from Rhodobacter sphaeroides, attenuated atherosclerosis in diabetic apolipoprotein E-deficient (apoE−/−) mice,7 further confirming an essential role of TLR4 in diabetes-associated atherosclerosis. As it is known that Rs-LPS antagonizes TLR4 by targeting TLR4 co-receptor myeloid differentiation factor 2 (MD-2)8,9 and TLR4 activation requires MD-2,10 the finding that Rs-LPS inhibits atherosclerosis underscored the role of MD-2 in atherosclerosis.
While it has been well established that MD-2 is essential for TLR4 signalling,11 it remains unclear if MD-2 is also involved in the gene expression regulated by signalling cascades independent of TLR4. In this study, we determined if MD-2 is involved in the gene expression regulated by IFN-γ and high glucose because IFN-γ and high glucose play key roles in atherosclerosis in diabetes.12–14 We focused on the role of MD-2 in IFN-γ and high-glucose-stimulated expression of matrix metalloproteinase 1 (MMP-1), a proteinase known to play an important role in vascular tissue remodelling and atherosclerosis,15 by mononuclear cells. Interestingly, we found that Rs-LPS attenuated MMP-1 expression up-regulated by IFN-γ in U937 mononuclear cells under both normal and high glucose conditions. Our following studies to block MD-2 or knockdown MD-2 expression further demonstrated the involvement of MD-2 in IFN-γ and high-glucose-stimulated MMP-1 expression. In addition to the findings that MD-2 is involved in IFN-γ signalling, we also found that MD-2 was stimulated by IFN-γ and high glucose.
Materials and methods
Cell culture
U937 mononuclear cells16 were purchased from the American Type Culture Collection (Manassas, VA). The cells were cultured in a 5% CO2 atmosphere in RPMI-1640 medium (GIBCO; Invitrogen Corp., Carlsbad, CA) containing normal glucose (5 mm) or high glucose (25 mm), 10% fetal bovine serum, 1% minimal essential medium non-essential amino acid solution, and 0·6 g/100 ml of HEPES and treated with IFN-γ or LPS (Sigma-Aldrich, St Louis, MD). The IFN-γ solution was prepared with Tris-buffered saline (TBS), pH 7·4 and 1% of low endotoxin BSA (Sigma-Aldrich). Our studies showed that MMP-1 secretion by high glucose-treated cells in response to 100 units/ml of IFN-γ increased significantly at 24 hr (4 hr: 0·01; 8 hr: 0·06; 12 hr: 0·10; 18 hr: 0·65; 24 hr: 5·06 ng/ml) and we therefore selected 24 hr as the time for treatment with IFN-γ. Human monocytes were isolated as described previously17 from blood obtained from healthy donors and treated in the medium that was the same as that used for U937 cells. The blood donation for monocyte isolation was approved by the University Institution Review Board (IRB).
ELISA
After treatment, culture medium was collected for MMP-1 quantification using sandwich ELISA kits (R&D System, Minneapolis, MN) according to the protocol provided by the manufacturer.
Real-time PCR
Total RNA was isolated from cells using the RNeasy minikit (Qiagen, Santa Clarita, CA). First-strand cDNA was synthesized with the iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) using 20 μl of reaction mixture containing 0·25 μg of total RNA, 4 μl of 5 × iScript reaction mixture, and 1 μl of iScript reverse transcriptase. The complete reaction was cycled for 5 min at 25°, 30 min at 42° and 5 min at 85° using a PTC-200 DNA Engine (MJ Research, Waltham, MA). The reverse transcription reaction mixture was then diluted 1 : 10 with nuclease-free water and used for PCR amplification in the presence of the primers. The Beacon designer software (PREMIER Biosoft International, Palo Alto, CA) was used for primer designing (Table 1). Primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA) and real-time PCR was performed in duplicate using 25 μl of reaction mixture containing 1·0 μl of reverse transcription mixture, 0·2 μm of both primers, and 12·5 μl of iQ™ SYBR Green Supermix (Bio-Rad Laboratories). Real-time PCR was run in the iCycler™ real-time detection system (Bio-Rad Laboratories) with a two-step method. A melt-curve assay was then performed to detect the formation of primer-derived trimers and dimers. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control. Data were analysed with the iCycler iQ™ software. The average starting quantity of fluorescence units was used for analysis. Quantification was calculated using the starting quantity of targeted cDNA relative to that of GAPDH cDNA in the same sample.
Table 1.
Primer sequences used in the real-time PCR
| Genes | Forward primer | Reverse primer |
|---|---|---|
| MMP-1 | CTGGGAAGCCATCACTTACCTTGC | GTTTCTAGAGTCGCTGGGAAGCTG |
| MD-2 | CACCATGAATCTTCCAAAGC | CTTGAAGGAGAATGATATTGTTG |
| MCP-1 | TTTAGATACAGAGACTTG | TGTATTAATGATTCTTGC |
| CXCL10 | CTTAGACATATTCTGAGCCTAC | GTTGATTACTAATGCTGATGC |
| ICAM-1 | CGTGGGGAGAAGGAGCTGAA | CAGTGCGGCACGAGAAATTG |
| TLR4 | GTCCTCAGTGTGCTTGTAG | ATCCTGGCTTGAGTAGATAAC |
| CD14 | CCGCTGCCTCTGGAAG | GGCGAGTGTGCTTGGG |
| c-Jun | GTGACGGACTGTTCTATGAC | GGTTACTGTAGCCATAAGGTC |
| c-Fos | GAGATGTCTGTGGCTTCC | ATGCTGCTGATGCTCTTG |
| GAPDH | GAATTTGGCTACAGCAACAGGGTG | TCTCTTCCTCTTGTGCTCTTGCTG |
Blocking studies
For studies in which antibodies were used to block MD-2, TLR4, CD14 or IFN-γ receptor (IFN-γR), U937 cells were treated with 100 units/ml IFN-γ or 100 ng/ml LPS in the absence or presence of 2·5–10 μg/ml of monoclonal anti-human MD-2 (Clone 9F1B1; Thermo Scientific, Rockford, IL), polyclonal goat anti-human TLR4, monoclonal anti-human CD14 or polyclonal goat anti-human IFN-γR antibodies (R&D Systems) for 24 hr. The isoform-matched control monoclonal antibody (IgG1) was purchased from R&D System. For blocking studies using pharmacological inhibitors, U937 cells were incubated with 100 units/ml of IFN-γ in the absence or presence of 5 μm of AG490 (Sigma-Aldrich), 10 μm of PD98059, SP600125, SB203580 and 2·5 μm of Bay117085 (Calbiochem, Billerica, MA) for 24 hr. The above concentrations of the inhibitors have been shown to be effective in our previous studies.18,19 After the incubation, MMP-1 in culture medium was quantified using ELISA.
Immunoblotting
U937 cells were treated with or without 100 units/ml of IFN-γ in the absence or presence of anti-MD-2 or control antibodies for 10 min and cellular proteins were extracted after the treatment. Fifty micrograms of each sample was electrophoresed in a 10% polyacrylamide gel. After transferring proteins to a nitrocellulose membrane, total and phosphorylated signal transducer and activator of transcription 1 (STAT1) were immunoblotted with anti-total and anti-phosphorylated STAT1 primary antibodies (Cell Signaling Technology, Danvers, MA), respectively, and horseradish peroxidase-conjugated secondary antibody (EMD Chemicals, Inc., Gibbstown, NJ). The STAT1 was detected by incubating the membrane with enhanced chemiluminescence (ECL) Plus Lumigen™ PS-3 detection reagent (GE Healthcare, Pittsburgh, PA) for 1 min and exposing it to X-ray film for 30–60 seconds. To detect c-Jun and c-Fos, 15 μg of cytoplasm protein for each sample was electrophoresed in a 12% polyacrylamide gel. After transferring proteins to a PVDF membrane, c-Jun and c-Fos were immunoblotted with the primary antibodies including rabbit anti-c-Jun and c-Fos (Cell Signaling Technology) and horseradish peroxidase-conjugated secondary antibody. The c-Jun or c-Fos were detected by incubating the membrane with enhanced chemiluminescence (ECL) Plus Lumigen™ PS-3 detection reagent for 1 min and exposing it to X-ray film for 1–5 min. Housekeeping gene GAPDH was also immunoblotted to ensure equal loading of all the samples.
Flow cytometry
U937 cells were washed twice and resuspended at 1 × 106 cells/ml. Non-specific staining was blocked with fetal bovine serum before cell surface staining with FITC-labelled monoclonal antibodies against MD-2, TLR4 or CD14 (Novus Biological, Littleton, CO). Extent and frequency of positively stained cells were visualized using a FACSCanto system from BD Biosciences (San Jose, CA).
MD-2 small interfering RNA (siRNA) transfection
U937 cells were transfected with 200 nm of stealth MD-2 siRNA (CGCAAAGAAGUUAUUUGCCGAGGAU) (GenBank accession No. NM 015364) or control siRNA (CGCAAGAAUUGGUUUAGCCGAAGAU) (Invitrogen Corp.) for 24–36 hr using Lipofectamine 2000 (Invitrogen) as the transfection reagent by following the manufacturer's instruction.
PCR array
First-strand cDNA was synthesized from RNA using an RT² First Strand Kit (SuperArray Bioscience Corp., Frederick, MD). Human TLR signalling pathway and matrix/adhesion molecule RT² Profiler™ PCR arrays were performed using 2× SuperArray RT² qPCR master mix and the first-strand cDNA by following the instructions from the manufacturer.
Confocal microscopy
Fluorescence immunocytochemistry for MD-2 and IFNGR2 was performed on U937 cells. U937 cells were rinsed with 10 mm PBS (pH 7·4) and fixed with 4% paraformaldehyde solution and incubated for 30 min on ice. Cells were then incubated for 1 hr at room temperature in 10 mm of PBS containing 5% normal goat serum to block non-specific binding. For dual immunofluorescence staining, cells were first incubated overnight at 4° on a shaker with the following primary antibodies: mouse anti-MD-2 (1 : 100; Thermo Scientific) and rabbit anti-IFNGR2 (1 : 100; Santa Cruz Biotechnology, Sant Cruz, CA) and then washed in 10 mm PBS at room temperature. Cells were incubated for 1 hr in the dark at room temperature with secondary goat anti-mouse Alexa Fluor 594 and goat anti-rabbit FITC (1 : 500; Abcam, Cambridge, MA). After washing, cells were mounted and stored at 4° until analyses. Imaging of fluorescence labelling for MD-2 and IFNGR2 was performed using an Olympus FV1000 confocal microscope equipped with a four-laser system (Multi AR laser, HeNe G laser, HeNe R laser, and LD405/440 laser diode) and complete integrated image analysis software system (Olympus America Inc., Melville, NY). The excitation and emission wavelengths were 590/618 nm for Alexa Fluor 594 (Red) and 495/519 nm for FITC (green).
Statistic analysis
Data were presented as mean ± SD. Student's t-tests were performed to determine the statistical significance of gene expression among different experimental groups. A value of P < 0·05 was considered significant.
Results
TLR4 antagonist Rs-LPS inhibits MMP-1 secretion stimulated by IFN-γ
Our first study was to determine the effect of IFN-γ and high glucose on MMP-1 secretion by U937 mononuclear cells. Results showed that while either 100 units/ml of IFN-γ or 25 mm of high glucose stimulated MMP-1 secretion, the combination of IFN-γ and high glucose had a synergistic effect on MMP-1 secretion (Fig. 1a,b). In addition to IFN-γ, our laboratory has shown previously that LPS also stimulates MMP-1 secretion from U937 cells.20 Therefore, we compared the inhibitory effects of Rs-LPS, a TLR4 antagonist, on the stimulation by IFN-γ and LPS of MMP-1 secretion. Since Rs-LPS is a TLR4 antagonist, it is not surprising to find that Rs-LPS effectively inhibited LPS-stimulated MMP-1 secretion under the normal glucose conditions (Fig. 1a). Interestingly, we found that Rs-LPS also inhibited IFN-γ-stimulated MMP-1 secretion in a concentration-dependent manner (Fig. 1a). Furthermore, Rs-LPS inhibited IFN-γ-stimulated MMP-1 secretion under high glucose conditions in a concentration-dependent manner (Fig. 1b). Quantitative real-time PCR showed that Rs-LPS inhibited IFN-γ-stimulated MMP-1 mRNA expression (Fig. 1c), suggesting that Rs-LPS inhibited IFN-γ-stimulated MMP-1 secretion by attenuating MMP-1 mRNA expression. Since it is known that Rs-LPS blocks TLR4 signalling by competing with LPS for the binding sites on MD-2, a TLR4 co-receptor,8,9,11 these findings indicate that MD-2 may be involved in IFN-γ-stimulated MMP-1 expression.
Figure 1.
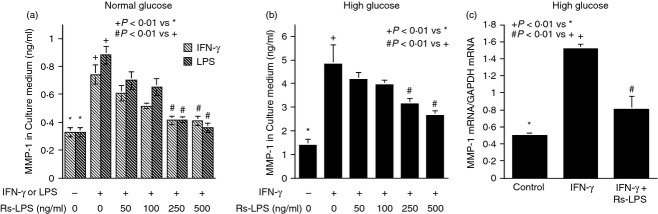
Inhibition of interferon-γ (IFN-γ) -stimulated matrix metalloproteinase 1 (MMP-1) secretion and expression by Rhodobacter sphaeroides lipopolysaccharide (Rs-LPS). (a) Inhibition of IFN-γ- or LPS-stimulated MMP-1 secretion by Rs-LPS in cells cultured with normal glucose (5 mm). U937 cells cultured in normal glucose-containing medium were treated with 100 units/ml of IFN-γ or 100 ng/ml of LPS in the absence or presence of different concentrations of Rs-LPS (50–500 ng/ml) for 24 hr. After the treatment, MMP-1 in culture medium was quantified using ELISA. (b) Inhibition of IFN-γ-stimulated MMP-1 secretion and expression by Rs-LPS in cells cultured with high glucose (25 mm). U937 cells cultured in high glucose-containing medium were treated with 100 units/ml of IFN-γ in the absence or presence of different concentrations of Rs-LPS (50–500 ng/ml) for 24 hr. After the treatment, MMP-1 in culture medium was quantified using ELISA. (c) U937 cells cultured in high glucose-containing medium were treated with 100 units/ml of IFN-γ in the absence or presence of 500 ng/ml of Rs-LPS for 24 hr. After the treatment, cellular MMP-1 mRNA was quantified using real-time PCR and normalized to GAPDH mRNA. The data (mean ± SD) were from one of three experiments with similar results.
Blockade of MD-2, but not TLR4 and CD14, inhibits IFN-γ-stimulated MMP-1 secretion
We further determined the role of MD-2 in IFN-γ-stimulated MMP-1 expression by performing blocking studies using anti-MD-2 antibodies. To validate the effectiveness of the MD-2 antibodies in MD-2 blockade, we conducted control studies in which the effect of the MD-2 antibodies on LPS-stimulated MMP-1 secretion was determined. Results showed that the anti-MD-2 antibodies inhibited MMP-1 secretion stimulated by LPS under both normal and high glucose conditions in a concentration-dependent fashion (Fig. 2a,b). Interestingly, the anti-MD-2 antibodies also inhibited IFN-γ-stimulated MMP-1 secretion in a concentration-dependent manner (Fig. 2c,d). In contrast, the isoform-matched control antibodies did not have an inhibitory effect (Fig. 2a–d). The blockade of MD-2 also inhibited IFN-γ-stimulated MMP-1 secretion from human normal monocytes (Fig. 2e), indicating that the involvement of MD-2 in IFN-γ signalling is not U937 cell-specific.
Figure 2.
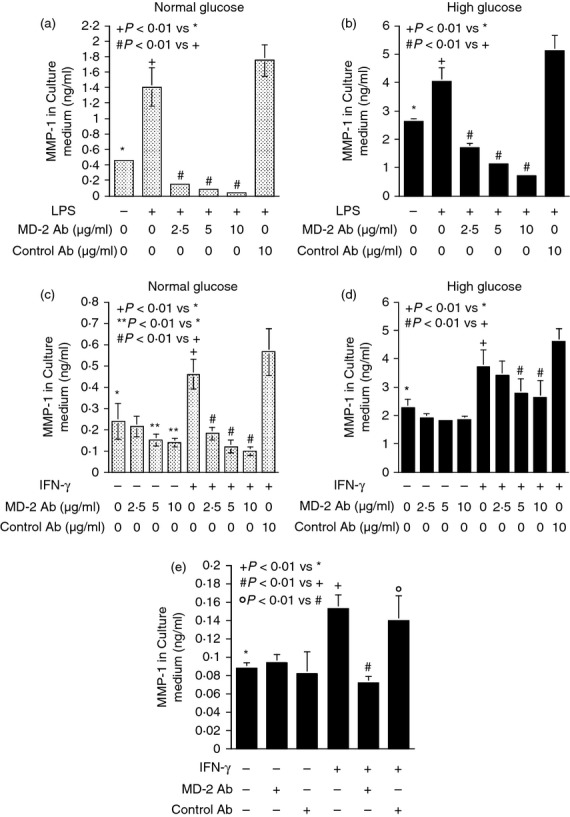
The effect of MD-2 blockade on lipopolysaccharide- (LPS) or interferon-γ (IFNγ) -stimulated matrix metalloproteinase 1 (MMP-1) secretion. (a, b) U937 cells cultured in normal (a) or high glucose (b)-containing medium were treated with 100 ng/ml of LPS in the absence or presence of different concentrations (2·5–10 μg/ml) of anti-MD-2 antibodies or 10 μg/ml of control antibodies for 24 hr. After the treatment, MMP-1 in culture medium was quantified using ELISA. (c, d) U937 cells cultured in normal (c) or high glucose (d) -containing medium were treated with 100 units/ml of IFN-γ in the absence or presence of different concentrations (2·5–10 μg/ml) of anti-MD-2 antibodies or 10 μg/ml of control antibodies for 24 hr. After the treatment, MMP-1 secretion was quantified using ELISA. (e) Human monocytes were treated similarly as U937 cells with IFN-γ as described above in the absence or presence of 5 μg/ml of anti-MD-2 antibodies or control antibodies. After the treatment, MMP-1 secretion was quantified using ELISA.
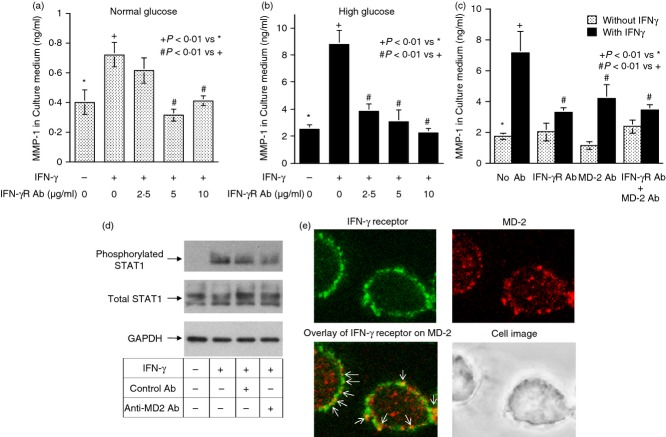
Contrary to MD-2 blockade, TLR4 or CD14 blockade failed to inhibit IFN-γ-stimulated MMP-1 secretion whereas TLR4 or CD14 blockade did inhibit LPS-stimulated MMP-1 secretion (Figs 3 and 4). To ensure that the stimulation of MMP-1 secretion by IFN-γ is mediated by IFNGR, we determined the effect of IFNGR blockade on IFN-γ-stimulated MMP-1 secretion. Results showed that the anti-IFNGR antibodies inhibited IFN-γ-stimulated MMP-1 secretion under both normal and high glucose conditions (Fig. 5a,b). Furthermore, when both MD-2 and IFNGR were blocked simultaneously, the extent of the inhibition on MMP-1 secretion was not further increased compared with the inhibition by IFNGR blockade alone (Fig. 5c), suggesting that MD-2 and IFNGR share the same pathway in stimulating MMP-1 secretion. This notion was further supported by observations that blockade of MD-2 reduced the phosphorylation of STAT1, which is considered as a major signalling pathway for IFN-γ13 (Fig. 5d) and MD-2 was found to be potentially co-localized with IFNGR on the surface of U937 cells by confocal microscopy (Fig. 5e).
Figure 3.
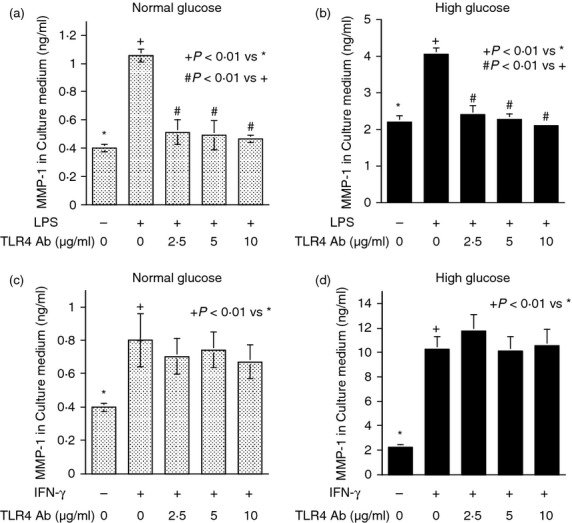
The effect of Toll-like receptor 4 (TLR4) blockade on lipopolysaccharide- (LPS) or interferon-γ (IFN-γ) -stimulated matrix metalloproteinase 1 (MMP-1) secretion. (a, b) U937 cells cultured in normal (a) or high glucose (b) -containing medium were treated with 100 ng/ml of LPS in the absence or presence of different concentrations (2·5–10 μg/ml) of anti-TLR4 antibodies for 24 hr. After the treatment, MMP-1 in culture medium was quantified using ELISA. (c, d) U937 cells cultured in normal (c) or high glucose (d) -containing medium were treated with 100 units/ml of IFN-γ in the absence or presence of different concentrations (2·5–10 μg/ml) of anti-TLR4 antibodies for 24 hr. After the treatment, MMP-1 secretion was quantified using ELISA.
Figure 4.
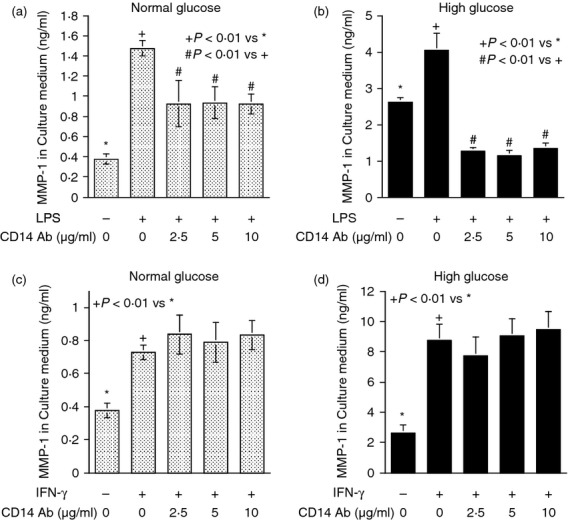
The effect of CD14 blockade on lipopolysaccharide (LPS) or interferon-γ (IFN-γ) -stimulated matrix metalloproteinase 1 (MMP-1) secretion. (a, b) U937 cells cultured in normal (a) or high glucose (b) -containing medium were treated with 100 ng/ml of LPS in the absence or presence of different concentrations (2·5–10 μg/ml) of anti-CD14 antibodies for 24 hr. After the treatment, MMP-1 in culture medium was quantified using ELISA. (c, d) U937 cells cultured in normal (c) or high glucose (d) -containing medium were treated with 100 units/ml of IFN-γ in the absence or presence of different concentrations (2·5–10 μg/ml) of anti-CD14 antibodies for 24 hr. After the treatment, MMP-1 secretion was quantified using ELISA.
Figure 5.
The effect of IFNGR blockade on interferon-γ (IFN-γ) -stimulated matrix metalloproteinase 1 (MMP-1) secretion. (a, b) U937 cells cultured in normal (a) or high glucose (b) -containing medium were treated with 100 units/ml of IFN-γ in the absence or presence of different concentrations (2·5–10 μg/ml) of anti-IFNGR antibodies for 24 hr. After the treatment, MMP-1 secretion was quantified using ELISA. (c) The effect of blockade of both IFNGR and MD-2 on IFN-γ-stimulated MMP-1 secretion. U937 cells cultured in high glucose-containing medium were treated with 100 units/ml of IFN-γ in the absence or presence of 10 μg/ml of anti-IFNGR, anti-MD-2 or both antibodies for 24 hr. After the treatment, MMP-1 secretion was quantified using ELISA. (d) The effect of MD-2 blockade on IFN-γ-induced signal transducer and activator of transcription 1 (STAT1) phosphorylation. U937 cells were treated with100 units/ml of IFN-γ in the absence or presence of 10 μg/ml of anti-MD-2 or isoform-matched control antibodies for 10 min. After the treatment, cells were lysed and subjected to immunoblotting of phosphorylated or total STAT1 as well as GAPDH. (e) Co-localization of MD-2 and IFNGR on the surface of U937 cells. U937 cells were treated with or without 100 units/ml of IFN-γ for 24 hr and then incubated with primary anti-MD-2 and IFNGR antibody and fluorescence-labelled secondary antibodies as described in the Materials and methods. After the incubation, confocal microscopy was performed to localize the expression of MD-2 and IFNGR. MD-2 was labelled with Alexa 594 (red colour) while IFNGR was labelled with FITC (green colour). The image for IFNGR was merged with that for MD-2 and the arrows indicate the possible co-localization of MD-2 and IFNGR. Magnification 63 ×.
Studies using MD-2 siRNA showed that MD-2 knockdown (Fig. 6a) effectively inhibited IFN-γ-stimulated MMP-1 secretion (Fig. 6b). Taken together, these studies provide more evidence to support a role of MD-2 in IFN-γ-stimulated MMP-1 expression.
Figure 6.
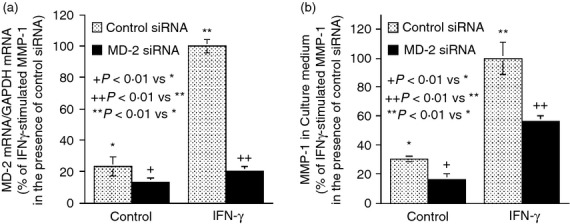
The effect of MD-2 mRNA knockdown by small interfering (si) RNA on interferon-γ (IFN-γ) -stimulated matrix metalloproteinase 1 (MMP-1) secretion. U937 cells were transfected with 200 nm of control or MD-2 siRNA for 24 hr. After the transfection, the cells were treated with or without 100 units/ml of IFN-γ for 24 hr. At the end of the experiment, the cells were harvested for MD-2 mRNA quantification (a) and the medium was collected for MMP-1 quantification (b). The MD-2 and MMP-1 mRNA expression by cells transfected with control siRNA and treated with IFN-γ was designated as 100%.
To further assess the effect of MD-2 blockade on IFN-γ signalling, we employed the PCR arrays and compared the effect of MD-2 blockade with that of IFNGR blockade on IFN-γ-stimulated gene expression. Results showed that IFN-γ increased expression of a number of important pro-inflammatory molecules such as MMP-8, monocyte chemotactic protein 1 (MCP-1), C-X-C motif ligand (CXCL)10, intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), interferon regulatory factor 1 (IRF-1), MD-2 and TLR10 (Table 2). The stimulation on many of these molecules by IFN-γ has been reported previously.21 Interestingly, results showed that MD-2 blockade exerted a similar inhibition to IFNGR blockade on IFN-γ-stimulated expression of these molecules (Table 2). The findings from the PCR arrays were confirmed by the quantitative real-time PCR of MCP-1, CXCL-10 and ICAM-1 mRNA (Fig. 7a–c).
Table 2.
Comparison of the inhibition on interferon-γ (IFN-γ) -stimulated gene expression by anti-MD-2 and anti-IFNγR antibodies
| Ct | Fold change compared with control | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene name | Control | IFN-γ | IFN-γ in the presence of anti-IFN-γR | IFN-γ in the presence of anti-MD-2 | By IFN-γ | By IFN-γ in the presence of anti-IFN-γR | By IFN-γ in the presence of anti-MD-2 | |
| MMP-1 | 27·33 | 25·88 | 26·58 | 27·62 | 2·73 | 1·68 | 0·82 | |
| MMP-8 | 26·98 | 25·90 | 26·29 | 27·05 | 2·11 | 1·61 | 0·95 | |
| MCP-1 | 24·95 | 22·25 | 24·23 | 25·88 | 6·50 | 1·65 | 0·52 | |
| CXCL10 | 27·84 | 25·31 | 27·00 | 27·23 | 5·78 | 1·79 | 1·53 | |
| ICAM-1 | 25·99 | 24·70 | 25·89 | 25·94 | 2·43 | 1·07 | 1·04 | |
| VCAM-1 | 27·96 | 26·97 | 27·36 | 28·60 | 1·99 | 1·52 | 0·64 | |
| Interferon regulatory factor-1 | 28·76 | 26·59 | 27·70 | 28·22 | 4·5 | 2·08 | 1·45 | |
| MD-2 | 27·14 | 26·04 | 26·84 | 27·58 | 2·14 | 1·23 | 0·74 | |
| TLR10 | 27·73 | 26·62 | 27·04 | 28·56 | 2·17 | 1·61 | 0·56 | |
U937 cells were treated with 100 units/ml of IFN-γ in the absence or presence of 10 μg/ml of anti-IFN-γR or anti-MD-2 antibodies for 24 hr. After the treatment, RNA was isolated and PCR array was performed as described in Materials and methods. The threshold cycle (Ct) is the cycle at which the amplification plot crosses the threshold. Smaller Ct means higher gene expression. The Ct of the all gene expression was normalized to that of GAPDH.
Figure 7.
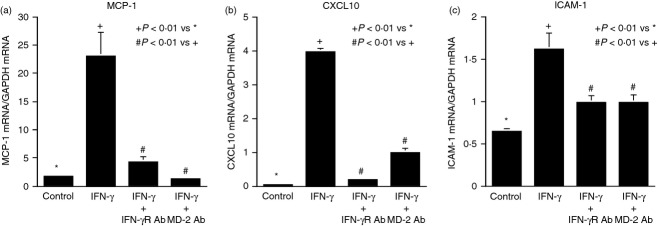
The effect of MD-2 or IFNGR blockade on interfeorn-γ (IFN-γ) -stimulated expression of pro-inflammatory molecules. The RNA samples used for the PCR array as shown in Table 2 were used in real-time PCR to quantify MCP-1 (a), CXCL10 (b), and ICAM-1 mRNA (c).
MD-2 is specifically stimulated by IFN-γ
From the results of MD-2 knockdown (Fig. 6a) and PCR array (Table 2), we found that IFN-γ stimulated MD-2 mRNA expression. Interestingly, the PCR array showed that IFN-γ had no effect on TLR4 and CD14 mRNA expression (data not shown). To confirm these findings, quantitative real-time PCR was performed. Results showed that IFN-γ and high glucose had a synergy on MD-2 expression (Fig. 8a). In contrast, IFN-γ and high glucose had no effect on TLR4 and CD14 (Fig. 8b,c). Time–course study showed that the peak stimulation of MD-2 mRNA expression by IFN-γ after 20 hr of IFN-γ treatment (Fig. 8d). Furthermore, flow cytometry showed that IFN-γ stimulated MD-2 surface protein expression (Fig. 8e) but had no effect on TLR4 and CD14 surface expression (data not shown). Taking these findings together, it is indicated that among TLR4, CD14 and MD-2, three major proteins in TLR4 receptor complex, MD-2 is specifically up-regulated by IFN-γ.
Figure 8.
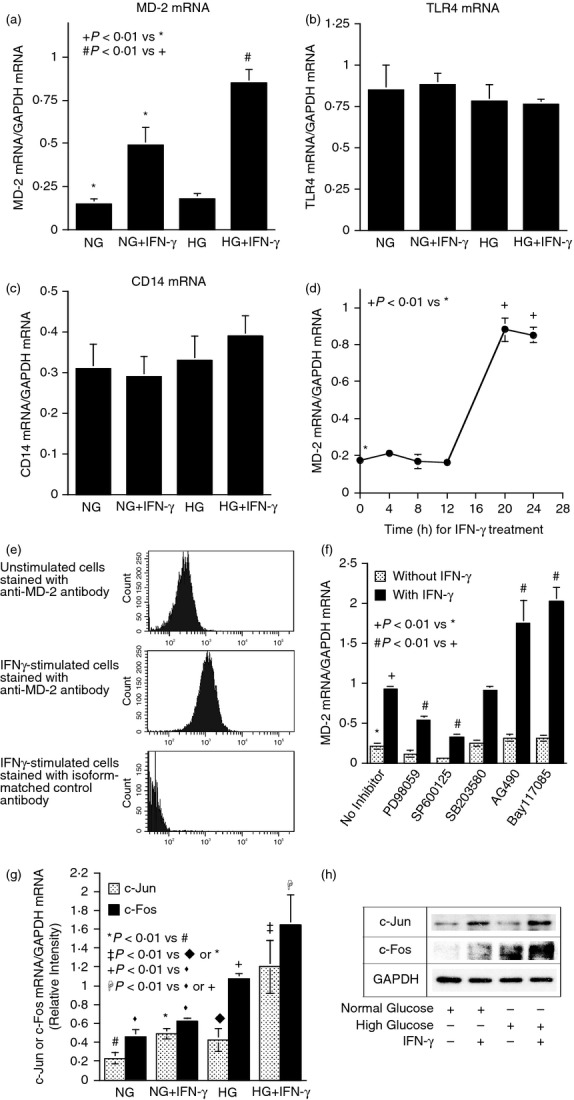
Interferon-γ (IFN-γ) and high glucose stimulate MD-2 expression. (a–c) U937 cells cultured with normal or high glucose were treated with 100 units/ml of IFN-γ for 24 hr. After the treatment, MD-2 (a), Toll-like receptor 4 (TLR4) (b) or CD14 mRNA (c) was quantified using real-time PCR. (d) Time course of MD-2 mRNA expression in response to IFN-γ in U937 cells. U937 cells cultured in high glucose were treated with 100 units/ml of IFN-γ for different times as indicated. At each time-point, cells were harvested and RNA was isolated from the cells. MD-2 mRNA was then quantified using real-time PCR. All the tested mRNA was normalized to GAPDH mRNA. The data (mean ± SD) were from one of two experiments with similar results. (e) Surface expression of MD-2 detected by flow cytometry. U937 cells cultured with high glucose were treated with 100 units/ml of IFN-γ for 24 hr. After the treatment, cells were incubated with fluorescence-labelled anti-MD-2 antibodies for 30 min and flow cytometry was performed to detect surface expression of MD-2. (f) The effects of pharmacological inhibitors on IFN-γ-stimulated MD-2 expression. U937 cells cultured with high glucose were treated with 100 units/ml of IFN-γ in the absence or presence of 10 μm of PD98059, 10 μm of SP600125, 10 μm of SB203580, 5 μm of AG490 or 2·5 μm of Bay117085 for 24 hr. After the treatment, MD-2 mRNA was quantified using real-time PCR and normalized to GAPDH mRNA. (g) The effect of IFN-γ and high glucose on c-Jun and c-Fos mRNA expression. U937 cells cultured with normal or high glucose-containing medium were treated with 100 units/ml of IFN-γ for 24 hr. After the treatment, c-Jun and c-Fos mRNA were quantified using real-time PCR and normalized to GAPDH mRNA. The data (mean ± SD) were from one of two experiments with similar results. (h) The effect of IFN-γ and high glucose on c-Jun and c-Fos protein expression. U937 cells were treated as described above and c-Jun and c-Fos proteins in cytoplasm were detected using immunoblotting.
The ERK and JNK pathways mediate MD-2 expression stimulated by IFN-γ and high glucose
Since it is known that IFN-γ regulates gene expression via the Janus kinase/signal transducers and activators of transcription (JAK/STAT1) pathway,21 mitogen-activated protein kinase (MAPK) pathways,22 and nuclear factor-κB (NF-κB) cascade,23 we determined which signalling pathway is involved in IFN-γ-stimulated MD-2 mRNA expression. Interestingly, results showed that while SB203580 (p38 MAPK pathway inhibitor) had no effect, SP600125 (JNK pathway inhibitor) and PD98059 (ERK pathway inhibitor) inhibited IFN-γ-stimulated MD-2 mRNA expression by 84% and 54%, respectively (Fig. 8f), indicating the involvement of JNK and ERK pathways in IFN-γ-stimulated MD-2 mRNA expression. In contrast, AG490 (JAK/STAT1 pathway inhibitor) and Bay117085 (NF-κB pathway inhibitor) increased IFN-γ-stimulated MD-2 mRNA expression, suggesting a negative regulation by the JAK/STAT1 and NF-κB pathways on IFN-γ-stimulated MD-2 expression.
Since activation of the JNK and ERK pathways leads to an increased AP-1 transcriptional activity,24,25 we further examined the effect of IFN-γ and high glucose on the expression of c-Jun and c-Fos, two major subunits of AP-1. Results showed that IFN-γ and high glucose had a synergistic effect on c-Jun and c-Fos mRNA and protein expression (Fig. 8g,h).
MD-2 plays an essential role in the synergistic stimulation of MMP-1 by LPS and IFN-γ
Previous studies have shown that LPS and IFN-γ have a synergy on the up-regulation of pro-inflammatory molecules.19,26,27 As it is known that MD-2 plays a crucial role in TLR4 signalling,11 we determined if MD-2 up-regulation by IFN-γ is essential for the augmentation of MMP-1 secretion by IFN-γ and LPS. In this study, we knocked down MD-2 expression using siRNA (Fig. 9a) and then determined the effect of MD-2 mRNA knockdown on MMP-1 expression stimulated by LPS alone or IFN-γ plus LPS. Results showed that MD-2 knockdown significantly attenuated MMP-1 secretion stimulated by not only LPS alone, but also the combination of IFN-γ and LPS (Fig. 9b).
Figure 9.
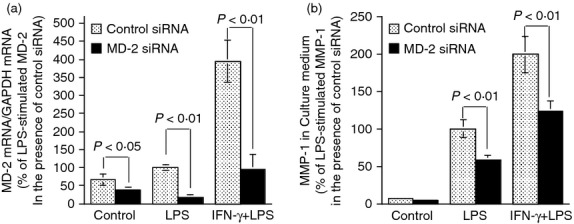
The effect of MD-2 mRNA knockdown on matrix metalloproteinase 1 (MMP-1) secretion from cells treated with lipopolysaccharide (LPS) or interferon-γ (IFN-γ) plus LPS. U937 cells were transfected with 200 nm of control or MD-2 small interfering (si) RNA for 24 hr. After the transfection, the cells were treated with or without 100 units/ml of IFN-γ for 24 hr, followed by treatment with 100 ng/ml of LPS for another 24 hr. At the end of the experiment, the cells were harvested for MD-2 mRNA quantification (a) and the medium was collected for MMP-1 quantification (b). The MD-2 and MMP-1 mRNA expression by cells transfected with control siRNA and treated with LPS was designated as 100%.
Discussion
It was surprising to find that Rs-LPS inhibited IFN-γ- and high-glucose-stimulated MMP-1 expression in U937 mononuclear cells given that Rs-LPS is a TLR4 antagonist. The following studies using anti-MD-2 antibodies and MD-2 siRNA support the notion that MD-2 is involved in IFN-γ-stimulated expression of MMP-1. As IFN-γ is a potent cytokine involved in both innate and adaptive immunities,21 these findings suggest that MD-2 may play a more important role in immunity and inflammation than was previously thought.
In fact, the previous studies have shown that MD-2 is not exclusively involved in TLR4 signalling. For instance, MD-2 binds to TLR2 besides TLR428–30 and soluble MD-2 is an acute-phase protein that binds to Gram-negative bacteria as opsonin to enhance phagocytosis.31 Therefore, it appears that MD-2 has both TLR4-dependent and TLR4-independent functions. It is likely that while MD-2 primarily conveys TLR4 signalling, it may be also involved in the signalling pathways mediated by other receptors such as IFNGR to render a strong and complex responsiveness to stimuli such as IFN-γ and LPS, which are present simultaneously.
Interferon-γ is a potent stimulator of host inflammatory responses.32,33 It has been well documented that IFN-γ enhances inflammation by stimulating a large number of pro-inflammatory molecules such as MCP-1, CXCL10, ICAM-1, VCAM-1 and IRF-1.21 Our findings from the PCR array (Table 2) and real-time PCR (Fig. 4) confirmed the stimulatory effect of IFN-γ on these genes in U937 mononuclear cells. Using the anti-MD-2 antibody, we showed that the MD-2 blockade inhibited the stimulatory effect of IFN-γ in a similar way to the IFNGR blockade. For example, IFN-γ increased the expression of CXCL10, a chemokine also called IFN-γ-induced protein 10 (IP-10), to a high level that is nearly sixfold of the control, but anti-MD-2 and anti-IFNGR antibodies reduced the stimulatory effect of IFN-γ by 73% and 69%, respectively. By comparing the effect of the MD-2 blockade with that of the IFNGR blockade on IFN-γ-stimulated gene expression, we further demonstrated a role of MD-2 in IFN-γ signalling.
An interesting finding from this study is that while MD-2 was involved in IFN-γ signalling, it was also up-regulated by IFN-γ. As shown in Fig. 8, IFN-γ specifically stimulates MD-2 among TLR4, CD14 and MD-2. As MD-2 is a crucial co-receptor for TLR4 signalling, the up-regulation of MD-2 by IFN-γ is likely to enhance TLR4 signalling in response to LPS. Furthermore, as illustrated by Fig. 10, it is known that LPS stimulates IFN-γ expression by mononuclear cells34 and released IFN-γ up-regulates MD-2 expression. Clearly, MD-2 mediates the cross-talk between IFN-γ and LPS signalling pathways for cellular inflammatory responses. In addition to IFN-γ, our previous studies have shown that LPS and lactate also stimulate MD-2 expression in U937 cells by 2·95-fold and 2·88-fold, respectively, and the combination of LPS and lactate led to a 10·46-fold increase in MD-2 expression.35 Collectively, all these findings indicate that MD-2 is highly regulated by inflammatory stimuli and so likely to play an important role in controlling inflammatory response.
Figure 10.
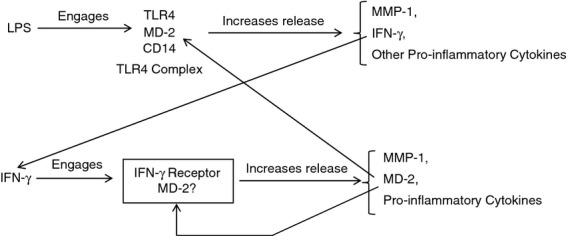
The flow chart revealing a potential MD-2-mediated interaction between lipopolysaccharide (LPS) and interferon-γ (IFN-γ) signalling pathways in mononuclear cells: LPS engages Toll-like receptor 4 (TLR4)/MD-2/CD14 complex to stimulate the secretion of IFN-γ, matrix metalloproteinase 1 (MMP-1) and other pro-inflammatory cytokines. The secreted IFN-γ engages IFN-γ receptor to stimulate MD-2, MMP-1 and pro-inflammatory cytokine expression. The secreted MD-2 is involved in both LPS and IFN-γ-stimulated gene expression.
Roy et al. 26 reported that IFN-γ induced MD-2 expression on human primary corneal epithelial cells and corneal epithelial cell lines via the STAT1 pathway, which is different from our observations that IFN-γ stimulated MD-2 expression in human mononuclear cells via MAPK pathways (Fig. 8f). To propose the possible reasons for the difference, we believed that the different types of cells might use different signalling pathways to control the expression of certain genes. Indeed, we have shown recently that interleukin-6 expression was regulated by LPS in mononuclear cells and fibroblasts via different signalling pathways.36 Interestingly, Li et al. have reported that MAPK pathways including ERK and JNK cascades are required for enhancement of MD-2 gene expression during differentiation of HL-60 cells, a myeloid cell line,37 which supports a role of MAPK pathways in the regulation of MD-2 expression.
The interaction of MD-2 with LPS and TLR4 has been well characterized.11,28,30 MD-2 is a glycoprotein co-expressed with TLR4 at the surface of various cell types.38,39 Recent studies have shown that LPS first binds to CD14 and then transfers to a hydrophobic pocket of MD-2. The binding of LPS with MD-2 leads to the formation of TLR4 receptor complex comprising TLR4, MD-2, CD14, LPS and several additional passively recruited components, resulting in TLR4-dependent signalling activation.40,41 Clearly, MD-2 plays a key role in the formation of the TLR4 receptor complex and subsequent LPS signalling activation. Based on these studies, it is plausible that targeting MD-2 would disrupt the formation of the TLR4 receptor complex and so reduce LPS signalling.
In contrast to the well-illustrated interaction of MD-2 with LPS and TLR4, the interaction of MD-2 with IFNGR remains unclear. However, studies have suggested that IFNGR may interact with other accessory factors33 and MD-2 could be one of them. The IFNGR complex comprises the heterodimer of two IFNGR1 chains and two IFNGR2 chains.33 In the absence of IFN-γ, IFNGR1 and IFNGR2 are unassociated and only bind to inactivated JAK1 and JAK2, respectively. Binding of IFN-γ to IFNGR1 induces the dimerization of IFN-γR1, which forms a site for binding of IFNGR2. The IFN-γ-induced formation of the complete receptor complex leads to activation of JAK1 and JAK2, and phosphorylation of IFNGR1. The phosphorylation of two IFNGR1 chains provides a paired set of STAT1 docking sites for two STAT1 molecules. Activated STAT1 in pairs then move to the nucleus and activate gene transcription by binding to the IFN-γ activation site (GAS) in the promoter region.
Interestingly, studies on the IFNGR structure and function relationship found that the complete IFNGR complex including two pairs of IFNGR1 and IFNGR2 is not sufficient to generate all IFN-γ-induced responses.33 Furthermore, studies have shown that IFN-γ stimulates gene expression via not only JAK/STAT pathway, but also MAPK and NF-κB cascades.22,23 These observations support the hypothesis by Garotta and co-workers that other accessory factors necessary for some functions of IFNGR may exist.33 Obviously, further investigations are warranted to determine if MD-2 is one of the proposed accessory factors for IFN-γ signalling.
In conclusion, this study has demonstrated that MD-2 is involved in IFN-γ signalling and also up-regulated by IFN-γ to enhance inflammatory response. The findings suggest that MD-2 is a potential target for reducing the expression of pro-inflammatory molecules by mononuclear cells in response to not only LPS, but also IFN-γ, and hindering the progression of atherosclerosis in both non-diabetic and diabetic patients.
Acknowledgments
This work was supported by the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs and by NIH grant DE016353 (to Y.H.). This study was presented as an abstract at the 72nd American Diabetes Association Scientific Sessions, in Philadelphia, PA, 8–12 June 2012.
Disclosure
The authors have no financial conflicts of interest.
References
- 1.Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–20. doi: 10.1007/978-3-642-59430-4_7. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, Poltorak A. The sole gateway to endotoxin response: how LPS was identified as Tlr4, and its role in innate immunity. Drug Metab Dispos. 2001;29(4 Pt 2):474–8. [PubMed] [Google Scholar]
- 3.den Dekker WK, Cheng C, Pasterkamp G, Duckers HJ. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis. 2010;209:314–20. doi: 10.1016/j.atherosclerosis.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 4.Miller YI, Choi SH, Fang L, Harkewicz R. Toll-like receptor-4 and lipoprotein accumulation in macrophages. Trends Cardiovasc Med. 2009;19:227–32. doi: 10.1016/j.tcm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkanani AK, Rewers M, Dong F, Waugh K, Gottlieb PA, Zipris D. Dysregulated toll-like receptor-induced interleukin-1β and interleukin-6 responses in subjects at risk for the development of type 1 diabetes. Diabetes. 2012;61:2525–33. doi: 10.2337/db12-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57:3090–8. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Z, Zhang X, Li Y, Jin J, Huang Y. TLR4 antagonist reduces early-stage atherosclerosis in diabetic apolipoprotein E-deficient mice. J Endocrinol. 2013;216:61–71. doi: 10.1530/JOE-12-0338. [DOI] [PubMed] [Google Scholar]
- 8.Lohmann KL, Vandenplas ML, Barton MH, Bryant CE, Moore JN. The equine TLR4/MD-2 complex mediates recognition of lipopolysaccharide from Rhodobacter sphaeroides as an agonist. J Endotoxin Res. 2007;13:235–42. doi: 10.1177/0968051907083193. [DOI] [PubMed] [Google Scholar]
- 9.Rallabhandi P, Phillips RL, Boukhvalova MS, et al. Respiratory syncytial virus fusion protein-induced toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2. MBio. 2012;3 doi: 10.1128/mBio.00218-12. pii: e00218–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visintin A, Iliev DB, Monks BG, Halmen KA, Golenbock DT. MD-2. Immunobiology. 2006;211:437–47. doi: 10.1016/j.imbio.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Leon ML, Zuckerman SH. Gamma interferon: a central mediator in atherosclerosis. Inflamm Res. 2005;54:395–411. doi: 10.1007/s00011-005-1377-2. [DOI] [PubMed] [Google Scholar]
- 13.McLaren JE, Ramji DP. Interferon gamma: a master regulator of atherosclerosis. Cytokine Growth Factor Rev. 2009;20:125–35. doi: 10.1016/j.cytogfr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Schuyler CA, Ta NN, Li Y, Lopes-Virella MF, Huang Y. Insulin treatment attenuates diabetes-increased atherosclerotic intimal lesions and matrix metalloproteinase 9 expression in apolipoprotein E-deficient mice. J Endocrinol. 2011;210:37–46. doi: 10.1530/JOE-10-0420. [DOI] [PubMed] [Google Scholar]
- 15.Libby P, Aikawa M. New insights into plaque stabilisation by lipid lowering. Drugs. 1998;56(Suppl. 1):9–13. doi: 10.2165/00003495-199856001-00002. discussion 33. [DOI] [PubMed] [Google Scholar]
- 16.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–77. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 17.Seager DJ, Lutz M, Hama S, et al. Method for large scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays, macrophage and dendritic cell differentiation. J Immunol Methods. 2004;288:123–34. doi: 10.1016/j.jim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Nareika A, He L, Game BA, Slate EH, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. Sodium lactate increases LPS-stimulated MMP and cytokine expression in U937 histiocytes by enhancing AP-1 and NF-κB transcriptional activities. Am J Physiol Endocrinol Metab. 2005;289:E534–42. doi: 10.1152/ajpendo.00462.2004. [DOI] [PubMed] [Google Scholar]
- 19.Nareika A, Sundararaj KP, Im YB, Game BA, Lopes-Virella MF, Huang Y. High glucose and interferon γ synergistically stimulate MMP-1 expression in U937 macrophages by increasing transcription factor STAT1 activity. Atherosclerosis. 2009;202:363–71. doi: 10.1016/j.atherosclerosis.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundararaj KP, Samuvel DJ, Li Y, Nareika A, Slate EH, Sanders JJ, Lopes-Virella MF, Huang Y. Simvastatin suppresses LPS-induced MMP-1 expression in U937 mononuclear cells by inhibiting protein isoprenylation-mediated ERK activation. J Leukoc Biol. 2008;84:1120–9. doi: 10.1189/jlb.0108064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saha B, Jyothi Prasanna S, Chandrasekar B, Nandi D. Gene modulation and immunoregulatory roles of interferon γ. Cytokine. 2010;50:1–14. doi: 10.1016/j.cyto.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-α, interleukin-1β, and interferon-γ stimulate γ-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem. 2004;279:49523–32. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- 23.Lombardi A, Cantini G, Piscitelli E, et al. A new mechanism involving ERK contributes to rosiglitazone inhibition of tumor necrosis factor-α and interferon-γ inflammatory effects in human endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:718–24. doi: 10.1161/ATVBAHA.107.160713. [DOI] [PubMed] [Google Scholar]
- 24.Gomez dAP, Martinez-Martinez S, Calvo V, Armesilla AL, Redondo JM. Antioxidants and AP-1 activation: a brief overview. Immunobiology. 1997;198:273–8. doi: 10.1016/S0171-2985(97)80047-2. [DOI] [PubMed] [Google Scholar]
- 25.Kyriakis JM. Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family. Gene Expr. 1999;7:217–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Roy S, Sun Y, Pearlman E. Interferon-γ-induced MD-2 protein expression and lipopolysaccharide (LPS) responsiveness in corneal epithelial cells is mediated by Janus tyrosine kinase-2 activation and direct binding of STAT1 protein to the MD-2 promoter. J Biol Chem. 2011;286:23753–62. doi: 10.1074/jbc.M111.219345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamana J, Santos L, Morand E. Enhanced induction of LPS-induced fibroblast MCP-1 by interferon-γ: involvement of JNK and MAPK phosphatase-1. Cell Immunol. 2009;255:26–32. doi: 10.1016/j.cellimm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Dziarski R, Gupta D. Role of MD-2 in TLR2- and TLR4-mediated recognition of Gram-negative and Gram-positive bacteria and activation of chemokine genes. J Endotoxin Res. 2000;6:401–5. doi: 10.1179/096805100101532243. [DOI] [PubMed] [Google Scholar]
- 29.Zughaier SM. Neisseria meningitidis capsular polysaccharides induce inflammatory responses via TLR2 and TLR4-MD-2. J Leukoc Biol. 2011;89:469–80. doi: 10.1189/jlb.0610369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dziarski R, Wang Q, Miyake K, Kirschning CJ, Gupta D. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol. 2001;166:1938–44. doi: 10.4049/jimmunol.166.3.1938. [DOI] [PubMed] [Google Scholar]
- 31.Tissieres P, Dunn-Siegrist I, Schappi M, Elson G, Comte R, Nobre V, Pugin J. Soluble MD-2 is an acute-phase protein and an opsonin for Gram-negative bacteria. Blood. 2008;111:2122–31. doi: 10.1182/blood-2007-06-097782. [DOI] [PubMed] [Google Scholar]
- 32.Bernabei P, Allione A, Rigamonti L, Bosticardo M, Losana G, Borghi I, Forni G, Novelli F. Regulation of interferon-γ receptor (INF-γR) chains: a peculiar way to rule the life and death of human lymphocytes. Eur Cytokine Netw. 2001;12:6–14. [PubMed] [Google Scholar]
- 33.Pestka S, Kotenko SV, Muthukumaran G, Izotova LS, Cook JR, Garotta G. The interferon γ (IFN-γ) receptor: a paradigm for the multichain cytokine receptor. Cytokine Growth Factor Rev. 1997;8:189–206. doi: 10.1016/s1359-6101(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 34.Fultz MJ, Barber SA, Dieffenbach CW, Vogel SN. Induction of IFN-γ in macrophages by lipopolysaccharide. Int Immunol. 1993;5:1383–92. doi: 10.1093/intimm/5.11.1383. [DOI] [PubMed] [Google Scholar]
- 35.Samuvel DJ, Sundararaj KP, Nareika A, Lopes-Virella MF, Huang Y. Lactate boosts TLR4 signaling and NF-κB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J Immunol. 2009;182:2476–84. doi: 10.4049/jimmunol.0802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin J, Sundararaj KP, Samuvel DJ, Zhang X, Li Y, Lu Z, Lopes-Virella MF, Huang Y. Different signaling mechanisms regulating IL-6 expression by LPS between gingival fibroblasts and mononuclear cells: seeking the common target. Clin Immunol. 2012;143:188–99. doi: 10.1016/j.clim.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallagher R, Collins S, Trujillo J, et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–33. [PubMed] [Google Scholar]
- 38.Gruber A, Mancek M, Wagner H, Kirschning CJ, Jerala R. Structural model of MD-2 and functional role of its basic amino acid clusters involved in cellular lipopolysaccharide recognition. J Biol Chem. 2004;279:28475–82. doi: 10.1074/jbc.M400993200. [DOI] [PubMed] [Google Scholar]
- 39.Miyake K. Innate recognition of lipopolysaccharide by CD14 and toll-like receptor 4-MD-2: unique roles for MD-2. Int Immunopharmacol. 2003;3:119–28. doi: 10.1016/s1567-5769(02)00258-8. [DOI] [PubMed] [Google Scholar]
- 40.Ohto U, Satow Y. Crystal twinning of human MD-2 recognizing endotoxin cores of lipopolysaccharide. J Synchrotron Radiat. 2008;15(Pt 3):262–5. doi: 10.1107/S0909049507056531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohnishi T, Muroi M, Tanamoto K. The lipopolysaccharide-recognition mechanism in cells expressing TLR4 and CD14 but lacking MD-2. FEMS Immunol Med Microbiol. 2007;51:84–91. doi: 10.1111/j.1574-695X.2007.00281.x. [DOI] [PubMed] [Google Scholar]