Abstract
Persistent changes in excitatory and inhibitory synaptic strengths to the ventral tegmental area (VTA) dopamine (DA) neurons in response to addictive drugs may underlie the transition from casual to compulsive drug use. While an enormous amount of work has been done in the area of glutamatergic plasticity of the VTA, little is known regarding the learning rules governing GABAergic plasticity in the VTA. Spike timing-dependent plasticity, STDP, has attracted considerable attention primarily due to its potential roles in processing and storage of information in the brain and there is emerging evidence for the existence of STDP at inhibitory synapses. We therefore used whole-cell recordings in rat midbrain slices to investigate whether near-coincident pre- and postsynaptic firing induces a lasting change in synaptic efficacy of VTA GABAergic synapses. We found that a Hebbian form of STDP including long-term potentiation (LTP) and long-term depression (LTD) can be induced at GABAergic synapses onto VTA DA neurons and relies on the precise temporal order of pre- and postsynaptic spiking. Importantly, GABAergic STDP is heterosynaptic (NMDA receptor dependent): triggered by correlated activities of the presynaptic glutamatergic input and postsynaptic DA cells. GABAergic STDP is postsynaptic and has an associative component since pre- or postsynaptic spiking per se did not induce STDP. STDP of GABAergic synapses in the VTA provides physiologically relevant forms of inhibitory plasticity that may underlie natural reinforcement of reward-related behaviours. Moreover, this form of inhibitory plasticity may mediate some of the reinforcing, aversive and addictive properties of drugs of abuse.
Key points
GABAergic synapses onto ventral tegmental area (VTA) dopamine neurons express a bidirectional spike timing-dependent plasticity (STDP; both long-term potentiation and long-term depression).
GABAergic synapses in the VTA obey the classical Hebbian learning rules of STDP.
GABAergic STDP in VTA dopamine neurons is expressed postsynaptically.
GABAergic STDP is heterosynaptic and NMDA receptor dependent.
Pairing of pre- and postsynaptic spiking is necessary for induction of GABAergic STDP.
Introduction
Dopamine (DA) neurons of the ventral tegmental area (VTA) are critically involved in the motivated learning of natural reward and addictive behaviours. Learning of natural- and drug reward-related information is probably mediated by the experience-dependent plasticity of excitatory and inhibitory synaptic components of the brain reward circuitry that includes the VTA (Luscher & Malenka, 2011). In fact, the increase of DA release from VTA DA neurons in response to addictive drugs seems to be the initial step for subsequent drug-induced synaptic changes throughout the brain reward circuitry (Creed & Luscher, 2013). The strength of excitatory and inhibitory synapses onto DA neurons would be expected to have significant influences on DA cell firing and the resulting DA release. Therefore, understanding the patterns of learning-related neuronal activity and signalling mechanisms that drive activity-dependent plasticity of VTA DA neurons seems to be essential for understanding the function of DA neurons in reward-related behaviours and subsequent remodelling of the brain reward circuits by addictive drugs.
The long-term potentiation (LTP) and long-term depression (LTD) of excitatory glutamatergic synapses have been the focus of extensive research for decades; however, there is increasing interest in the functional roles of synaptic plasticity at inhibitory GABAergic synapses in different brain circuits, including the brain reward pathway. Both LTP and LTD of GABAergic synapses (LTPGABA and LTDGABA) onto VTA DA neurons have been described (Nugent et al. 2007; Pan et al. 2008a,b; Dacher & Nugent, 2011; Dacher et al. 2013). Classical LTP and LTD protocols such as high frequency stimulation (HFS) and low frequency stimulation (LFS) paired with modest postsynaptic depolarization as used to induce LTPGABA and LTDGABA in VTA DA neurons (Nugent et al. 2007; Dacher & Nugent, 2011), respectively, are selected more for their efficiency to reveal the capacity of synapses to exhibit synaptic plasticity than for their physiological relevance. However, repetitive and coincident pre- and postsynaptic spiking within a narrow time window (several tens of milliseconds) can also result in a form of plasticity known as spike timing-dependent plasticity (STDP) in which the sequence and relative timing of pre- and postsynaptic firing are crucial variables determining the direction and extent of plasticity. In a Hebbian form of the STDP protocol, LTP is induced when presynaptic activity precedes postsynaptic spiking (pre-post spiking), whereas reversing the order induces LTD (post-pre spiking). This dependence of synaptic change and direction of plasticity on the timing between pre- and postsynaptic firing has made STDP a proved model in the characterization of physiological forms of experience-dependent plasticity in the brain (Dan & Poo, 2006; Caporale & Dan, 2008). Indeed, in STDP protocols, LFS can be used to induce both LTP and LTD and these protocols are found to be more efficient in the induction of synaptic plasticity of excitatory synapses compared to conventional induction protocols. For example, previous work by Poo's team revealed that a STDP protocol resembling the pattern of activity of VTA DA neurons of behaving rats or monkeys in response to rewarding stimuli could reliably induce LTP of excitatory synapses onto VTA DA neurons (Liu et al. 2005). Moreover, spike timing-dependent modification of excitability of neurons has been observed in vitro and in vivo and is proposed to play an important role in learning, perception and behaviour (Dan & Poo, 2006; Caporale & Dan, 2008; Lamsa et al. 2010; Feldman, 2012). STDP has been observed at both excitatory and inhibitory synapses although there is significantly less known about inhibitory STDP. Interestingly, the learning rules governing STDP induction at inhibitory synapses are more versatile than those for excitatory STDP (Caporale & Dan, 2008). Here we attempted to address a key question as to whether near-coincident pre- and postsynaptic spiking modifies synaptic efficacy of VTA GABAergic synapses in a bidirectional manner. We provide the first evidence that GABAergic synapses onto VTA DA neurons are capable of exhibiting a conventional form of STDP similar to excitatory STDP where pre-post spiking induces LTP and post-pre spiking induces LTD (we refer to these as STD-LTPGABA and STD-LTDGABA throughout the paper).
Methods
Brain slice preparation and electrophysological recordings were conducted as described previously from 14- to 21-day-old Sprague–Dawley rats (Dacher et al. 2013). Briefly, animals were anaesthetized using isoflurane inhalation and quickly decapitated. The brain was rapidly dissected and placed into ice-cold artificial cerebrospinal fluid (ACSF) containing (in mm): 126 NaCl, 21.4 NaHCO3, 2.5 KCl, 1.2 NaH2PO4, 2.4 CaCl2, 1.00 MgSO4, 11.1 glucose, 0.4 ascorbic acid, saturated with 95% O2–5% CO2. Horizontal midbrain slices containing the VTA were cut using the Lieca VT1000S vibrotome (250 μm) and incubated in ACSF for at least 1 h at 34°C. Slices were then transferred into a recording chamber in ascorbic acid-free ACSF at 28°C. All experiments were carried out in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Uniformed Services University Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering, and to reduce the number of animals used.
GABAA inhibitory postsynaptic currents (IPSCs) were recorded using a patch amplifier (Multiclamp 700B) under infrared-differential interference contrast microscopy. Data acquisition and analysis were performed using DigiData 1440A and pCLAMP 10 (Molecular Devices, Union City, CA, USA). In all experiments, 6,7-dinitroquinoxaline-2,3-dione (DNQX, 10 μm) and strychnine (1 μm) obtained from Sigma were added to block AMPA- and glycine-mediated synaptic currents, respectively. Paired GABAA IPSCs were evoked using a bipolar stainless steel stimulating electrode placed 200–500 mm rostral to the recording site in the VTA at 0.1 Hz (duration 100 μs, 50 ms inter-stimulation interval) and recorded using KCl-containing electrodes and whole-cell voltage clamp in neurons held at −70 to −80 mV. Pipettes were filled with (in mm): 125 KCl, 2.8 NaCl, 2 MgCl2, 2 ATP-Na+, 0.3 GTP-Na+, 0.6 EGTA and 10 Hepes (pH adjusted to 7.28 with KOH, osmolarity adjusted to 275–280 mosmol l-1 with sucrose). For a subset of experiments, patch pipettes were filled with caesium gluconate-based internal solution (in mm): 117 caesium gluconate, 2.8 NaCl, 5 MgCl2, 2 ATP-Na+, 0.3 GTP-Na+, 0.6 EGTA and 20 Hepes (pH adjusted to 7.28 with CsOH, osmolarity adjusted to 275–280 mosmol l-1 with sucrose). In the experiments using caesium gluconate, cells were voltage-clamped at +20 mV, except during the STDP protocol. Stimulation intensity was adjusted to evoke baseline synaptic responses ranging between −200 and −800 pA (approximately 50% of maximal responses). The cell input resistance and series resistance were monitored through the experiment and if these values changed by more than 10%, data were not included. Cells were stepped from −50 mV to −100 mV during 700 ms and the appearance of an Ih current (≥50 pA, Ih(+)) was used to classify VTA DA neurons.
We induced GABAergic STDP using a modified STDP protocol that reliably induced glutamatergic STD-LTD in striatal neurons (Shen et al. 2008). After obtaining a stable baseline, DA cells were taken to current clamp and received trains of a subthreshold presynaptic stimulation paired with back-propagating action potentials (bAPs/postsynaptic spiking) at 5 Hz. To evoke bAPs, cells were injected with direct somatic currents of 1.5 nA for 5 ms through patch pipettes. STDP protocols consisted of 30 trains of five bursts repeated at 0.1 Hz. To induce STD-LTPGABA, each burst composed of three bAPs at 50 Hz was preceded by a single presynaptic stimulation (positive timing, +15 ms, Fig. 1A). To induce STD-LTDGABA, each burst was composed of three bAPs at 50 Hz followed by a single presynaptic stimulation (negative timing, −5 ms, Fig. 2A). A complex STDP protocol was also used to induce STDP where each burst composed of three bAPs was preceded by three presynaptic stimulations at 50 Hz (the complex pairing protocol includes both positive timing, +5 ms and negative timing, −15 ms). For experiments with presynaptic stimulation only, cells were voltage clamped at −70 to −80 mV (to prevent postsynaptic spiking) and only received presynaptic stimulation of the STDP protocols (30 trains of five bursts repeated at 0.1 Hz, each burst being composed of a single presynaptic stimulation without postsynaptic spiking). For postsynaptic spiking only, cells were taken to current clamp and received 30 trains of five bursts repeated at 0.1 Hz with each burst being composed of three bAPs at 50 Hz. Values are presented as means ± SEM. Statistical significance was assessed using repeated measures ANOVA with a significance level of P < 0.05. Levels of STDP are reported as averaged IPSC amplitudes for 5 min just before STDP induction compared with averaged IPSC amplitudes during the 5 min period from 20 to 25 min after the protocol. Paired-pulse ratios (PPRs, 50 ms inter-stimulus interval) were measured over 5 min epochs of 30 IPSCs as described previously (Nugent et al. 2007). The coefficient of variation (CV) was measured by dividing the standard deviation of IPSCs recorded over 5 min epochs by the mean amplitude of these IPSCs. NMDA receptors (NMDARs) were blocked by the bath application of d-5-aminophosphonopentanoic acid (APV; 50 μm) for at least 15 min before STDP protocols. Interleaved control experiments were performed with experiments in which drugs were bath applied. Salts and all drugs were purchased from Sigma-Research Biochemicals International (st. Louis, M.O, USA) or Tocris Bioscience (Bristol, United Kingdom).
Figure 1. VTA DA neurons express STD-LTPGABA in response to a pre-post spiking (positive timing) protocol.
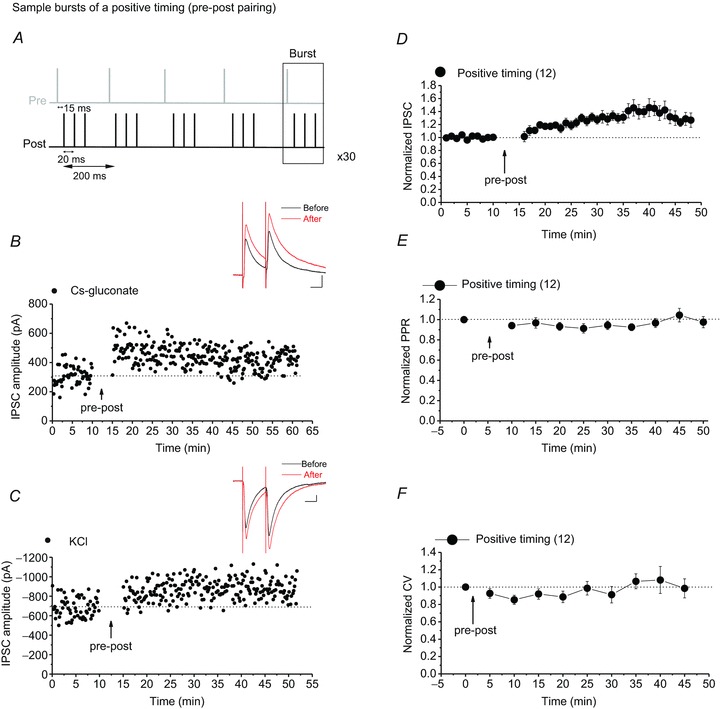
A, sample bursts of the pre-post spiking protocol (with a delay of +15 ms) for induction of STD-LTPGABA. B and C, single experiments showing induction of STD-LTPGABA recorded in Ih(+) (presumably DA) neurons using KCl or caesium gluconate-filled pipettes. At the arrow, STD-LTPGABA was induced. Insets: averaged IPSCs before and 25 min after STDP protocol. In this and all figures, ten consecutive traces from each condition were averaged for illustration as inset. Calibration: 100 pA, 25 ms. D, averaged experiments from Ih(+) neurons (filled circles) exhibiting STD-LTPGABA in response to the pre-post pairing protocol using either KCl or caesium gluconate-filled pipettes. VTA DA neurons express STD-LTPGABA (140.54 ± 5.7% of pre-STDP values, F10.45,73.19= 3.546, P < 0.001, n= 12). All Ih(+) cells recorded with either KCl or caesium gluconate that received the pre-post STDP protocol are included for the means in this graph. E, no changes in PPR were detected after induction of STD-LTPGABA (97.13 ± 4.5% of pre-STDP values, F5.66,39.23= 1.597, P= 0.177, n= 12). F, no changes in CV were detected after induction of STD-LTPGABA (101.19 ± 7.0% of pre-STDP values, F3.16,25.29= 1.522, P= 0.232, n= 12). Values shown throughout figures are the mean ± SEM.
Figure 2. VTA DA neurons exhibit STD-LTDGABA in response to a post-pre spiking (negative timing) protocol.
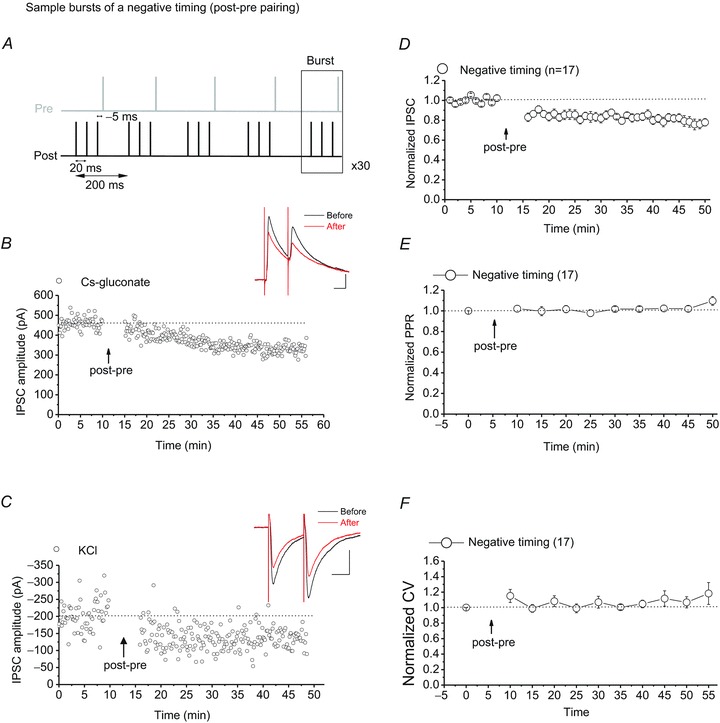
A, sample bursts of the post-pre spiking protocol (with a delay of −5 ms) for induction of STD-LTDGABA. B and C, single experiments showing induction of STD-LTDGABA recorded in Ih(+) neurons using KCl or caesium gluconate-filled pipettes. At the arrow, STD-LTDGABA was induced. Insets: averaged IPSCs before and 25 min after STDP protocol. Calibration: 100 pA, 25 ms. D, averaged experiments from Ih(+) neurons (open circles) exhibiting STD-LTDGABA in response to the post-pre pairing protocol using either KCl or caesium gluconate-filled pipettes. VTA DA neurons also express STD-LTDGABA (77.43 ± 1.5% of pre-STDP values, F4.88,48.83= 4.368, P < 0.001, n= 17). All Ih(+) cells recorded with either KCl or caesium gluconate that received the post-pre STDP protocol are included for the means in this graph. E, no changes in PPR were detected after induction of STD-LTDGABA (103.52 ± 3.4% of pre-STDP values, F2.13,17.07= 0.836, P= 0.457, n= 17). F, no changes in CV were detected after induction of STD-LTDGABA (110.39 ± 6.0% of pre-STDP values, F6.71,33.57= 1.135, P= 0.356, n= 17).
Results
VTA DA neurons exhibit STD-LTPGABA
Synaptic plasticity of GABAergic synapses in the VTA has been described using conventional LTP and LTD induction protocols; i.e. HFS (Nugent et al. 2007) and LFS paired with postsynaptic depolarization (Dacher & Nugent, 2011; Dacher et al. 2013). Excitatory STD-LTP can occur at glutamatergic synapses onto VTA DA neurons (Liu et al. 2005; Luu & Malenka, 2008) but so far it was not known whether STDP occurs at GABAergic synapses onto VTA DA neurons. Here we investigated the possibility that the synaptic efficacy of GABAergic synapses onto VTA DA neurons can be bidirectionally modified by pre/post spike pairing in a narrow time window. To induce GABAergic STDP in VTA DA neurons, we adopted and modified the STDP protocol that has been shown to successfully induce STDP of excitatory synapses onto medium spiny neurons in the striatum (Shen et al. 2008). First we attempted to induce STD-LTPGABA by repeated pairing of a single subthreshold presynaptic stimulation followed by three postsynaptic spikes (positive timing/ pre-post,+15 ms, Fig. 1A). When postsynaptic spikes were evoked after presynaptic stimulation, STD-LTPGABA was reliably induced in cells loaded with a high chloride internal solution (Fig. 1C and D). While a high chloride internal solution is commonly used for the recording of GABAA IPSCs as inward currents, this solution deviates the normal intracellular chloride gradient. Therefore, activation of GABAA receptors (GABAARs) during a presynaptic stimulation of the STDP induction protocol applied under current clamp mode will be artificially depolarizing rather than hyperpolarizing (evoking EPSPs instead of IPSPs). To ensure that STD-LTPGABA can be induced with an intact chloride gradient, whole-cell recordings with STD-LTPGABA induction were repeated using a caesium gluconate-based internal solution which does not alter the internal chloride gradient and is also commonly used to evoke GABAA IPSCs as outward currents. Consistently, cells loaded with caesium gluconate also exhibited STD-LTPGABA in response to repeated pairing of an IPSP evoked by presynaptic stimulation with postsynaptic spikes 15 ms later (Fig. 1B and D). Together our data suggest that STD-LTPGABA can occur under physiological conditions. PPR and CV analyses also revealed that STD-LTPGABA is postsynaptic as these measurements did not change significantly after STDP induction (Fig. 1E and F).
VTA DA neurons also exhibit STD-LTDGABA
At excitatory synapses, STD-LTP is induced when presynaptic activity precedes postsynaptic spiking (pre-post spiking), whereas reversing the order induces STD-LTD (post-pre spiking) (Dan & Poo, 2006). On the other hand, STDP rules at GABAergic synapses seem to show more variability across brain regions and type of synapses (Caporale & Dan, 2008). We found that STD-LTPGABA is induced whenever a presynaptic stimulation precedes postsynaptic spikes within a 15 ms time window (Fig. 1). Next we examined whether repeated pairing of postsynaptic spikes followed by a single presynaptic stimulation (negative timing/post-pre, −5 ms, Fig. 2A) reverses the direction of the plasticity and induces STD-LTDGABA in VTA DA neurons. We found that DA neurons loaded with either KCl- or caesium gluconate-based internal solution exhibited STD-LTDGABA in response to the post-pre STDP protocol (Fig. 2B–D). Moreover, PPR and CV values did not change after the STDP protocol suggesting that STD-LTDGABA is also postsynaptically expressed (Fig. 2E and F). Thus, STDP rules of GABAergic synapses onto VTA DA neurons act similarly to the excitatory STDP rules.
Induction of GABAergic LTP and LTD by STDP protocols requires NMDAR activation
In the standard model of LTP and LTD in which high and modest increases in postsynaptic calcium produce LTP and LTD, respectively, NMDARs serve as coincident detectors of pre- and postsynaptic activity for STDP (Caporale & Dan, 2008). We observed that the same form of STDP could be induced regardless of the opposite polarity of GABAA receptor-mediated potentials during STDP protocols using KCl or caesium gluconate internal solution, indicating that the activity of presynaptic GABAergic inputs and subsequent activation of GABAARs themselves may not be required for induction of STDP. In our experiments, during a presynaptic stimulation of the STDP protocols, inputs from both glutamatergic and GABAergic afferents to the DA neurons are stimulated. Given that NMDARs were not blocked in the previous experiments, we hypothesized that STDP is probably heterosynaptic and NMDAR dependent. Blockade of NMDARs with APV (50 μm) prevented the induction of both STD-LTPGABA and STD-LTDGABA in response to STDP protocols suggesting that GABAergic STDP in the VTA is indeed heterosynaptic and triggered by the near-coincident activities of the presynaptic NMDAR-mediated glutamatergic inputs and DA cells (Fig. 3A–D).
Figure 3. Induction of STDP requires NMDAR activation.
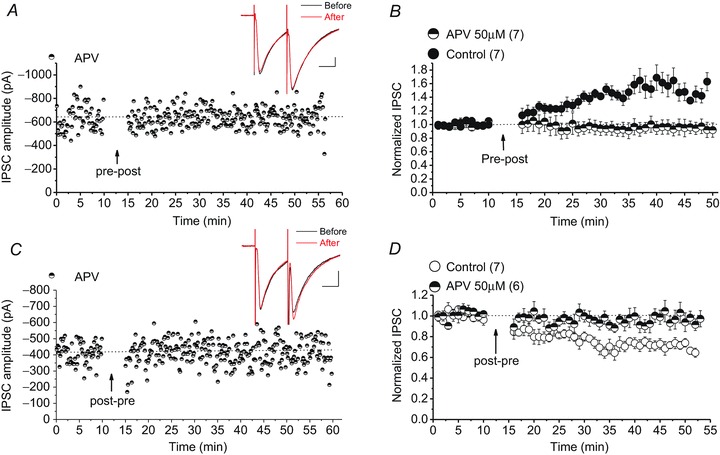
A and C, single experiments showing induction of STD-LTPGABA and STD-LTDGABA in DA cells in the presence of 50 μm APV (half-filled symbols). Insets: averaged IPSCs before and 25 min after STDP protocol. Calibration: 100 pA, 25 ms. B, averaged control STD-LTPGABA experiments (filled symbols) or APV experiments (half-filled symbols). Bath-applied APV blocks the induction of STD-LTPGABA whereas control cells exhibit STD-LTPGABA (control cells, 146.61 ± 10% of pre-STDP values, F8.18,40.93= 5.608, P < 0.0001; APV cells, 94.26 ± 2% of pre-STDP values, F3.16,18.99= 0.377, P= 0.781). D, averaged control STD-LTDGABA experiments (open symbols) or APV experiments (half-filled symbols). Bath-applied APV also blocks the induction of STD-LTDGABA whereas control cells exhibit STD-LTDGABA (control cells, 70.5 ± 4% of pre-STDP values, F4.1,74.2= 6.012, P < 0.0001; APV cells, 96.85 ± 4% of pre-STDP values, F13.01,52.04= 0.725, P= 0.731).
Postsynaptic spiking or presynaptic stimulation alone is not sufficient to induce STDP
The near-coincident activity of presynaptic and postsynaptic cells seems to be a key factor for induction of STDP. To test whether this rule also applies to STDP of GABAergic synapses in the VTA, DA cells only received a short burst of postsynaptic spiking or presynaptic stimulation from the STDP protocol. Presynaptic spiking or postsynaptic spiking alone did not yield any form of plasticity suggesting that coincident pre- and postsynaptic activity at GABAergic synapses in the VTA is also required for successful induction of STDP (Fig. 4A–D).
Figure 4. Pre-/postsynaptic spiking alone does not induce STDP at GABAergic synapses in VTA DA neurons.
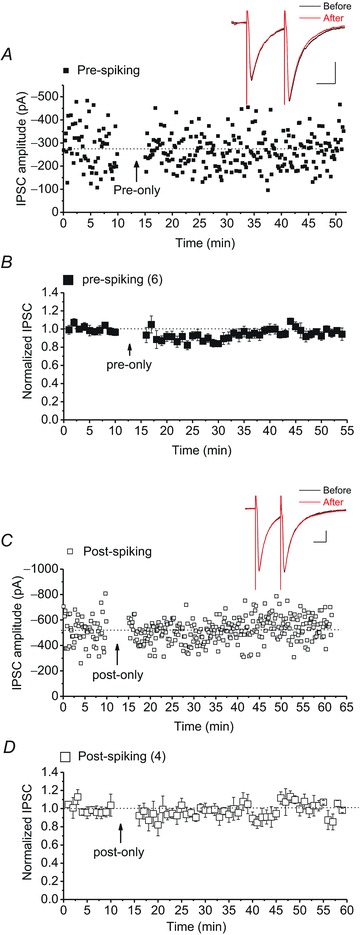
A and C, sample experiments of IPSCs recorded from DA neurons that only received bursts of presynaptic stimulations of the STDP protocol (pre-only, filled square symbols) or bursts of postsynaptic depolarizing steps of the STDP protocol (post-only, open square symbols). Insets: averaged IPSCs before and 25 min after pre-/post-spiking protocol. Calibration: 100 pA, 25 ms. B and D, averaged experiments illustrating the absence of GABAergic STDP in response to pre-/postsynaptic spiking (pre-only cells, filled square symbols, 93 ± 3% of baseline values before pre-spiking protocol, F16.46,50.28= 1.309, P= 0.227; post-only cells, open square symbols, 96.7 ± 1% of baseline values before post-spiking protocol, F23.06,46.13= 0.809, P= 0.703). Pairing of pre- and postsynaptic spiking is necessary for successful induction of GABAergic STDP.
Complex patterns of spiking induce STD-LTDGABA
In a previous study describing striatal excitatory STDP, a STDP protocol consisting of a triplet of pre-post pairs separated by 15 ms intervals induced STD-LTP (Shen et al. 2008). Interestingly, it has been shown that more complex patterns of spiking such as the one used in this study can change the direction of STDP (Froemke & Dan, 2002; Wang et al. 2005). Using the same STDP protocol as above, we found that the addition of preceding presynaptic spikes, which represents a combination of both positive and negative timing, resulted in STD-LTDGABA (Fig. 5A–C). The expression site for STD-LTDGABA induced in response to this complex STDP protocol seems to be also postsynaptic because the PPR and CV values did not alter after the STDP protocol (Fig. 5D and E).
Figure 5. VTA DA neurons exhibit STD-LTDGABA in response to a complex spike-pairing protocol.
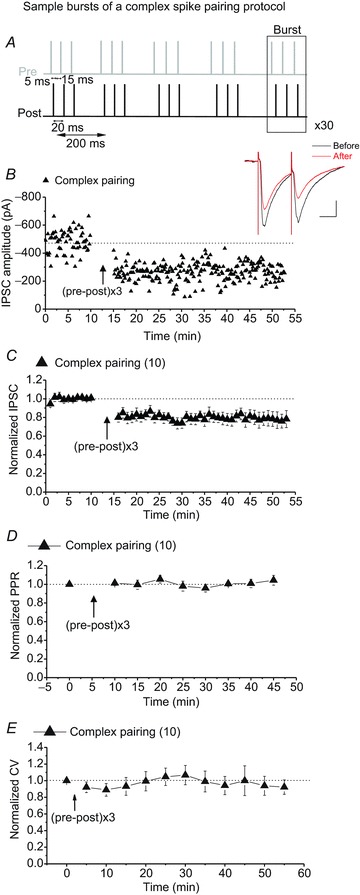
A, sample bursts of the complex STDP protocol (triplets of pre-post pairs with +5 ms delay separated by 15 ms intervals). B, single experiment showing induction of STD-LTDGABA recorded in Ih(+) neuron using KCl-filled pipette. At the arrow, STD-LTDGABA was induced. Insets: averaged IPSCs before and 25 min after STDP protocol. Calibration: 100 pA, 25 ms. C, averaged experiments from Ih(+) neurons (filled triangle symbols) exhibiting STD-LTDGABA in response to the complex spike pairing protocol using KCl-filled pipettes. VTA DA neurons also express STD-LTDGABA in response to a complex spiking protocol (80.03 ± 1% of pre-STDP values, F3.073,18.440= 7.754, P= 0.0014, n= 10). D, no changes in PPR were detected after induction of STD-LTDGABA (99.93 ± 3.1% of pre-STDP values, F4.788,28.727= 1.337, P= 0.278, n= 10). E, no changes in CV were detected after induction of STD-LTDGABA (95.056 ± 3.0% of pre-STDP values, F4.94,19.79= 1.522, P= 0.754, n= 10).
Discussion
The transient drug-evoked increase in VTA DA release and subsequent triggering of synaptic modifications by DA inside the VTA as well as its projection areas is thought to contribute to the shaping of powerful drug-related memories and development of addiction (Creed & Luscher, 2013). In fact, it has been shown that cocaine-induced AMPA receptor redistribution in VTA DA neurons (a form of excitatory drug-evoked synaptic plasticity) can be mimicked by optogenetic activation of VTA DA neurons and local DA release highlighting the importance of VTA DA neurons and DA signalling as the converging point at which addictive drugs act to reorganize the brain reward circuitry (Brown et al. 2010). DA signalling within the VTA also plays an important role in induction of GABAergic plasticity (Pan et al. 2008a; Dacher & Nugent, 2011; Dacher et al. 2013), so it is likely that selective activation of DA neurons by such in vivo manipulations produce parallel drug-induced GABAergic plasticity as appeared after exposures to morphine and cocaine (Liu et al. 2005; Dacher & Nugent, 2011). Because of the importance of GABAergic transmission and plasticity in VTA DA cell firing and the shaping of reward- and drug-related learning (Parker et al. 2011; Tan et al. 2012; van Zessen et al. 2012; Tolu et al. 2013; Graziane et al. 2013), the study of GABAergic synaptic plasticity in the VTA is of great interest. Here we describe for the first time a Hebbian form of NMDAR-dependent inhibitory plasticity that is triggered at GABAergic synapses onto VTA DA neurons in both forms of LTP and LTD and relies on the precise temporal order of pre- and postsynaptic spiking.
Classical LTP and LTD protocols that are frequency dependent have been successfully used to induce synaptic plasticity in different brain areas, but many synapses are also capable of showing a form of plasticity where timing matters (i.e. STDP). In STDP, the temporal order of pre- and postsynaptic spiking determines whether synapses are potentiated or weakened. Based on Hebb's postulate, if presynaptic spikes repetitively occur before postsynaptic spikes within a narrow time window, LTP is induced and reversing the order elicits LTD. However, there are other forms of STDP where the classical temporal rules of Hebbian STDP are not obeyed (Caporale & Dan, 2008). Specifically, inhibitory synapses show a variety of long-term plasticity in response to the STDP protocols (Holmgren & Zilberter, 2001; Woodin et al. 2003; Haas et al. 2006; Lien et al. 2006; Sivakumaran et al. 2009).
GABAA synapses onto VTA DA neurons are believed to mostly arise from GABAergic interneurons of the VTA, and activation of these interneurons and GABAAR-mediated signalling in the VTA have a significant impact on DA cell activity and DA release in VTA projection areas (Omelchenko & Sesack, 2009; Lobb et al. 2010; Parker et al. 2011; van Zessen et al. 2012). Dopamine cells fire in pacemaker, irregular and bursting modes while under the influence of tonic GABAergic inhibition. The glutamatergic afferents and activation of NMDARs seem to increase the firing rates of DA neurons and promote burst firing of these neurons in vivo (Johnson et al. 1992; Chergui et al. 1993; Murase et al. 1993; Tong et al. 1996). Given that addictive drugs can influence the firing mode and rate of VTA DA neurons by altering the strength of GABAergic and glutamatergic inputs, it is curious how different patterns of correlated activities of DA neurons and their inputs affects the spike-timing plasticity of DA neurons. Glutamatergic synapses onto VTA DA neurons express glutamatergic STD-LTP in response to a STDP protocol where EPSPs evoked by presynaptic stimulations precede the postsynaptic spikes by ∼5 ms and this STD-LTP is facilitated by repeated exposure to cocaine (Liu et al. 2005). Interestingly, reversing the order of spiking (using a post-pre spiking with 3–5 ms delays) did not induce STD-LTD in control VTA slices (Luu & Malenka, 2008) though different post-pre spiking delays might be needed to uncover such plasticity. Here, we showed that by applying a ‘pre-post’ STDP protocol (postsynaptic spikes were evoked 15 ms after a presynaptic stimulation) in DA cells recorded with either KCl or caesium gluconate-filled pipettes, STD-LTPGABA was reliably elicited. By reversing the order of spiking (postsynaptic spikes were evoked 5 ms prior to a presynaptic stimulation), STD-LTDGABA was induced. STD-LTPGABA and STD-LTPGABA were both postsynaptically expressed since PPR and CV values did not change after STDP protocols. Regardless of the opposite polarity of GABAAR-mediated potentials during STDP protocols in DA cells loaded with KCl or caesium gluconate internal solution (depolarizing versus hyperpolarizing, respectively), the same forms of GABAergic STDP were elicited. These data suggest that under physiological conditions STDP can occur at these inhibitory synapses. Moreover, the firing of GABAergic inputs and subsequent activation of GABAARs leading to hyperpolarization of postsynaptic DA cells is not necessary for successful induction of STDP. Rather the presynaptic stimulation of NMDAR-mediated glutamatergic inputs during the STDP protocols seems to be important for induction of STDP as both STD-LTPGABA and STD-LTDGABA were blocked in the presence of the NMDAR antagonist. Based on these findings, we propose an NMDAR-based model for induction of bidirectional GABAergic STDP in the VTA where NMDARs serve as coincident detectors for this heterosynaptic plasticity. We assume that in the case of STD-LTPGABA, a presynaptic release of glutamate during the STDP protocol followed by subsequent depolarization caused by postsynaptic spikes will open NMDARs and allow for a large influx of Ca2+ through NMDARs. As proposed by standard mechanistic models of STDP (Shouval et al. 2002; Caporale & Dan, 2008), glutamate remains bound to the NMDARs for tens of milliseconds and postsynaptic spikes produce a voltage-dependent removal of Mg2+ which leads to maximal opening of NMDARs. In the case of STD-LTDGABA, glutamate binds to the NMDARs after postsynaptic spikes, which instead leads to a moderate increase in Ca2+ influx through NMDARs promoting LTD.
It should be noted that the direction of synaptic modification induced in response to more complex patterns of spike trains may also differ from those in response to simple STDP induction protocols (Froemke & Dan, 2002; Wang et al. 2005). The commonly used STDP protocols, including those in this study, consist of repetitive pairing of pre- and postsynaptic spikes at fixed intervals. We also observed that increasing the number of preceding presynaptic spikes followed by postsynaptic spikes (pre-post-pre-post-pre-post) as used to induce STD-LTP in striatal neurons (Shen et al. 2008) reversed the direction of plasticity from STD-LTPGABA to STD-LTDGABA (Fig. 5) suggesting that STD-LTDGABA dominates over STD-LTPGABA for complex pre- and postsynaptic spike pairings. This complex pattern may actually represent positive (pre-post) and negative (post-pre) timing, given than each pre-post spiking pair is only separated by 15 ms. This brief time interval is still within the critical window for STDP induction.
Neither pre- nor postsynaptic spiking per se induced STDP at GABAergic synapses onto DA neurons indicating an associative component of the inhibitory STDP in the VTA. Similarly, STDP of GABAergic synapses onto layer II excitatory stellate neurons exhibits an asymmetrical window, where pairing of pre- and postsynaptic spikes (positive timing) leads to LTP and pairing of post- and presynaptic spikes (negative timing) induces LTD (Haas et al. 2006). The induction of STDP at immature GABAergic synapses between mossy fibres and CA3 neurons in the hippocampus also obeyed the same classical rules of STDP induction, although the action of GABA in neonatal hippocampal slices is depolarizing and the locus of STDP expression is presynaptic (i.e. a change in the probability of GABA release rather than insertion or removal of postsynaptic GABAARs). It is also noteworthy to mention that STDP protocols did not affect the measurements of chloride reversal potentials in DA neurons here, excluding the possibility of an ‘ionic shift plasticity’ where the induction of GABAergic STDP is affected by a change in the function of neuronal Cl− transporter KCC2 and the resulting shift in the Cl− gradient (Woodin et al. 2003).
The signalling pathways involved in STDP induction may share many features with conventional LTP and LTD such as Ca2+ rise, and NMDARs/phospholipase C as spike-timing detectors (Caporale & Dan, 2008). We found that NMDARs are critical for the induction of GABAergic STDP. We still expect that conventional GABAergic LTP/LTD and GABAergic STDP in the VTA involve some overlapping mechanisms. For example, we previously showed that LTDGABA induced in response to a typical LFS-pairing protocol is postsynaptic, D2 dopamine receptor (D2R)-mediated, but NMDAR independent. Moreover, it requires an InsP3 receptor-mediated increase in postsynaptic Ca2+ and involves the A-kinase anchoring protein (AKAP)–protein kinase A–calcineurin signalling cascade (Dacher et al. 2013). Although the source of calcium entry for induction of STD-LTDGABA and LTDGABA differs, both are expressed postsynaptically so it is likely that STD-LTDGABA and LTDGABA share similar expression rules of plasticity involving the AKAP150 signalling pathway. On the other hand, postsynaptic NMDAR-dependent STD-LTPGABA may differ mechanistically from HFS-induced LTPGABA as the latter is expressed presynaptically. The induction of LTPGABA is postsynaptic, NMDAR- and Ca2+-dependent but its expression is presynaptic and requires the nitric oxide–protein kinase G signalling cascade. Our future studies will identify the detailed molecular underpinning of both forms of plasticity. Given that the neuromodulators such as DA are found to strongly influence STDP (Pawlak & Kerr, 2008; Shen et al. 2008) and the fact that DA release inside the VTA plays an important role in mediating the addictive properties of drugs of abuse, it will also be of great interest to further investigate how DA signalling would affect glutamatergic and GABAergic STDP in the VTA.
In summary, we demonstrated that the learning rules governing spike timing GABAergic plasticity of VTA DA neurons obey the typical Hebbian rules of STDP. Since glutamatergic and GABAergic inputs onto DA neurons are critically involved in fine tuning the firing patterns of DA cells (Lobb et al. 2010), such plasticity could be physiologically recruited where glutamatergic transmission is closely correlated with postsynaptic DA firing. A preceding firing of glutamatergic afferents could provide an inhibitory braking mechanism by the induction of STD-LTPGABA, thereby generating pauses in the firing of DA neurons. On the other hand, a succeeding firing of glutamatergic afferents could promote STD-LTDGABA, thereby facilitating bursts in the firing of DA neurons. Therefore, such inhibitory STDP may have important functional implications for the regulation of DA cell firing in natural reward as well as the reinforcing actions of addictive drugs.
Translational perspective
Boosting the brain's reward response by drugs of abuse occurs through activation of a subset of neurons located in the ventral tegmental area (VTA). It is proposed that the brain may, in fact, be learning to crave drugs. The interaction of drugs with the mechanisms associated with learning (i.e. synaptic plasticity) in addiction-related areas of the brain such as the VTA reinforces addictive behaviours. Therefore, an understanding of how neurons in these brain regions form such cellular memories will point to new directions in the pharmacotherapy of drug addiction. Dopamine release from VTA dopamine neurons controls reward-motivated learning and also mediates the addictive properties of drugs. Here we tested whether the temporal sequence of neuronal firings, known as spike timing-dependent plasticity (STDP), is a critical element in synaptic plasticity of inhibitory synapses in the VTA dopamine neurons. The regulation of dopamine neural excitability by GABAergic STDP in the VTA has the potential to alter the patterns of circuit activation in the VTA projection areas, providing a natural mechanism for reward circuits to perform reward-related learning. The findings of this study have important implications for changes in reward circuitry related to drug seeking and relapse. Moreover, our study will help elucidate how STDP that contributes to reward information processing can be hijacked by drugs of abuse. Understanding the functional interaction between addictive drugs and GABAergic STDP in the VTA could ultimately identify critical targets in the neurocircuitry of drug addiction for novel pharmacological, surgical and genetic therapeutic interventions.
Acknowledgments
The opinions and assertions contained herein are the private opinions of the authors and are not to be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense or the Government of the United States. We are grateful to Dr Brian Cox for his constructive comments on the earlier version of the manuscript.
Glossary
- bAP
back-propagating action potential
- CV
coefficient of variation
- DA
dopamine
- GABAAR
GABAA receptor
- HFS
high frequency stimulation
- LFS
low frequency stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- NMDAR
NMDA receptor
- PPR
paired-pulse ratio
- STDP
spike timing-dependent plasticity
- VTA
ventral tegmental area
Additional information
Competing interests
The authors declare no conflict of interest.
Author contributions
J.N.K., M.D. and F.S.N. designed the research; J.N.K., M.D., M.E.A. and F.S.N. performed the experiments; J.N.K., M.D. and F.S.N. analysed the data; J.N.K., M.D., M.E.A. and F.S.N. wrote the paper and all authors approved the final submitted version.
Funding
This work was supported by a DOD-intramural grant from the Uniformed Services University (USUHS), and a Whitehall Foundation Research Grant to F.S.N. The funding agencies did not contribute to writing this article or deciding to submit it.
References
- Brown MT, Bellone C, Mameli M, Labouebe G, Bocklisch C, Balland B, Dahan L, Lujan R, Deisseroth K, Luscher C. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS One. 2010;5:e15870. doi: 10.1371/journal.pone.0015870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Chergui K, Charlety PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci. 1993;5:137–144. doi: 10.1111/j.1460-9568.1993.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Creed MC, Luscher C. Drug-evoked synaptic plasticity: beyond metaplasticity. Curr Opin Neurobiol. 2013;23:553–558. doi: 10.1016/j.conb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Dacher M, Gouty S, Dash S, Cox BM, Nugent FS. A-kinase anchoring protein–calcineurin signaling in long-term depression of GABAergic synapses. J Neurosci. 2013;33:2650–2660. doi: 10.1523/JNEUROSCI.2037-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacher M, Nugent FS. Morphine-induced modulation of LTD at GABAergic synapses in the ventral tegmental area. Neuropharmacology. 2011;61:1166–1171. doi: 10.1016/j.neuropharm.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Dan Y. Spike-timing-dependent synaptic modification induced by natural spike trains. Nature. 2002;416:433–438. doi: 10.1038/416433a. [DOI] [PubMed] [Google Scholar]
- Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77:942–954. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JS, Nowotny T, Abarbanel HD. Spike-timing-dependent plasticity of inhibitory synapses in the entorhinal cortex. J Neurophysiol. 2006;96:3305–3313. doi: 10.1152/jn.00551.2006. [DOI] [PubMed] [Google Scholar]
- Holmgren CD, Zilberter Y. Coincident spiking activity induces long-term changes in inhibition of neocortical pyramidal cells. J Neurosci. 2001;21:8270–8277. doi: 10.1523/JNEUROSCI.21-20-08270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V, North RA. Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science. 1992;258:665–667. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- Lamsa KP, Kullmann DM, Woodin MA. Spike-timing dependent plasticity in inhibitory circuits. Front Synaptic Neurosci. 2010;2:8. doi: 10.3389/fnsyn.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien CC, Mu Y, Vargas-Caballero M, Poo MM. Visual stimuli-induced LTD of GABAergic synapses mediated by presynaptic NMDA receptors. Nat Neurosci. 2006;9:372–380. doi: 10.1038/nn1649. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb CJ, Wilson CJ, Paladini CA. A dynamic role for GABA receptors on the firing pattern of midbrain dopaminergic neurons. J Neurophysiol. 2010;104:403–413. doi: 10.1152/jn.00204.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Malenka RC. Spike timing-dependent long-term potentiation in ventral tegmental area dopamine cells requires PKC. J Neurophysiol. 2008;100:533–538. doi: 10.1152/jn.01384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008a;28:14018–14030. doi: 10.1523/JNEUROSCI.4035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008b;28:1385–1397. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Wanat MJ, Soden ME, Ahmad K, Zweifel LS, Bamford NS, Palmiter RD. Attenuating GABAA receptor signaling in dopamine neurons selectively enhances reward learning and alters risk preference in mice. J Neurosci. 2011;31:17103–17112. doi: 10.1523/JNEUROSCI.1715-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval HZ, Bear MF, Cooper LN. A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc Natl Acad Sci U S A. 2002;99:10831–10836. doi: 10.1073/pnas.152343099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumaran S, Mohajerani MH, Cherubini E. At immature mossy-fiber–CA3 synapses, correlated presynaptic and postsynaptic activity persistently enhances GABA release and network excitability via BDNF and cAMP-dependent PKA. J Neurosci. 2009;29:2637–2647. doi: 10.1523/JNEUROSCI.5019-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolu S, Eddine R, Marti F, David V, Graupner M, Pons S, Baudonnat M, Husson M, Besson M, Reperant C, Zemdegs J, Pages C, Hay YA, Lambolez B, Caboche J, Gutkin B, Gardier AM, Changeux JP, Faure P, Maskos U. Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol Psychiatry. 2013;18:382–393. doi: 10.1038/mp.2012.83. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse. 1996;22:195–208. doi: 10.1002/(SICI)1098-2396(199603)22:3<195::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Gerkin RC, Nauen DW, Bi GQ. Coactivation and timing-dependent integration of synaptic potentiation and depression. Nat Neurosci. 2005;8:187–193. doi: 10.1038/nn1387. [DOI] [PubMed] [Google Scholar]
- Woodin MA, Ganguly K, Poo MM. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl− transporter activity. Neuron. 2003;39:807–820. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]


