Abstract
The ionotropic glutamate receptors are primary mediators of fast excitatory neurotransmission, and their properties are determined both by their subunit composition and their association with auxiliary subunits. The neuropilin and tolloid-like 1 and 2 proteins (Neto1 and Neto2) have been recently identified as auxiliary subunits for kainate-type glutamate receptors. Heteromeric kainate receptors (KARs) can be assembled from varying combinations of low-affinity (GluK1–GluK3) and high-affinity (GluK4–GluK5) subunits. To better understand the functional impact of auxiliary subunits on KARs, we examined the effect of Neto1 on the responses of recombinant homomeric and heteromeric KARs to varying concentrations of glutamate. We found that co-expression of Neto1 with homomeric GluK2 receptors had a small effect on sensitivity of the receptors to glutamate, but decreased the onset of desensitization while speeding recovery from desensitization. In the absence of Neto1, addition of GluK5 subunits to form GluK2/GluK5 heteromeric receptors slowed the onset of desensitization at low glutamate concentrations, compared with GluK2 homomers. Co-expression of Neto1 with GluK2/GluK5 receptors further enhanced these effects, essentially eliminating desensitization at μm glutamate concentrations without altering the EC50 for activation by glutamate. In addition, a prominent rebound current was observed upon removal of the agonist. The rate of recovery from desensitization was increased to the same degree by Neto1 for both homomeric GluK2 and heteromeric GluK2/GluK5 receptors. Expression of Neto1 with GluK1/GluK5, GluK3/GluK5 or GluK2/GluK4 receptors produced qualitatively similar effects on whole-cell currents, suggesting that the impact of Neto1 on the desensitization properties of heteromeric receptors was not subunit dependent. These results provide greater insight into the functional effects of the auxiliary subunit Neto1 on both homomeric and heteromeric KARs. Alteration of the characteristics of desensitization at both sub-maximal and saturating glutamate concentrations could influence the responsiveness of these receptors to repeated stimuli. As a result, assembly of KARs with the Neto auxiliary subunits could change the kinetic properties of the neuronal response to glutamatergic input.
Key points
Kainate receptors (KARs) are a subtype of ionotropic glutamate receptors, which mediate excitatory neurotransmission. KARs can be regulated through assembly with the auxiliary subunits neuropilin and tolloid-like 1 and 2 proteins (Neto1 and Neto2).
We characterized the effect of Neto1 on the glutamate sensitivity and desensitization properties of recombinant receptors containing different combinations of the five different KAR subunits (GluK1–GluK5).
We found that Neto1 reduces the onset of desensitization and speeds recovery from desensitization of both homomeric (K2) and heteromeric (with K4 or K5) receptors.
The largest impact of Neto1 was seen at sub-maximal glutamate concentrations, suggesting that one functional role is to reduce desensitization in partially bound receptors.
Neto1 co-assembly with neuronal KARs may alter the kinetics of the postsynaptic response, regulating the efficacy of glutamate neurotransmission.
Introduction
Fast synaptic transmission mediated by the excitatory neurotransmitter glutamate is produced through activation of ionotropic receptors belonging to three distinct subclasses: NMDA; AMPA; and kainate receptors (KARs; Traynelis et al. 2010). The KARs are tetramers composed of combinations of GluK1–GluK3 (formerly GluR5–GluR7) and GluK4–GluK5 (formerly KA1 and KA2) subunits. The GluK1–GluK3 subunits are also referred to as ‘low-affinity’ subunits, with KDs for kainate near 50 nm, while the GluK4–GluK5 subunits have ‘higher affinity’, with KDs in the range of 5–15 nm (Werner et al. 1991; Sommer et al. 1992; Herb et al. 1992). While GluK1–GluK3 subunits can form homomeric receptors, the GluK4 or GluK5 subunits only function in heteromeric assemblies with GluK1–GluK3 subunits. These heteromeric KARs predominate in the CNS (Petralia et al. 1994). The KARs perform diverse functions throughout the brain, acting in both pre- and postsynaptic locations to regulate neurotransmitter release and neuronal excitability (Pinheiro & Mulle, 2006; Jane et al. 2009; Contractor et al. 2011).
In addition to the pore-forming subunits (GluK1–GluK5), recent studies have identified neuropilin and tolloid-like 1 and 2 proteins (Neto1 and Neto2) as auxiliary subunits of KARs (Zhang et al. 2009; Straub et al. 2011a; Copits & Swanson, 2012). Although Neto1 has also been shown to interact with NMDA receptors (Ng et al. 2009; Tang et al. 2011; but see Straub et al. 2011a), neither Neto1 nor Neto2 appear to associate with AMPA receptors. The Neto proteins associate with KARs both in vitro and in vivo to modulate their properties. In heterologous expression systems, Neto1 slowed deactivation and desensitization of GluK2/GluK5 heteromeric receptors (Straub et al. 2011a). In contrast, it was found to enhance the onset of desensitization of GluK1 receptors (Copits et al. 2011). Neto1 increased the rate of recovery from desensitization for both the homomeric (GluK1) and heteromeric (GluK2/GluK5) receptors (Copits et al. 2011; Straub et al. 2011a).
Neuronal KAR-mediated currents can be observed in a variety of brain regions, but have been best studied at mossy fibre synapses onto CA3 pyramidal cells where they control spike transmission and network activity (Contractor et al. 2011). At this synapse, Neto1 is highly expressed (Straub et al. 2011a), and genetic deletion of Neto1 (but not Neto2) has effects on KAR-mediated EPSCs (Straub et al. 2011a; Tang et al. 2011). In the absence of Neto1, postsynaptic responses showed faster decay and reduced amplitudes, although the expression of KAR subunits was unaffected (Straub et al. 2011a) or only slightly reduced at the postsynaptic density (Tang et al. 2011). Thus, Neto1 appears to play a crucial role in regulating the kinetic properties of these KAR-mediated currents. The postsynaptic response is most likely mediated by heteromeric KARs composed of GluK2/GluK4 and GluK2/GluK5 subunit combinations (Contractor et al. 2003; Darstein et al. 2003; Fernandes et al. 2009).
Previous studies have examined the effect of Neto1 on only two KAR isoforms (GluK1 and GluK2/GluK5). KARs of different subunit composition have distinct biophysical properties and roles in synaptic transmission (Jane et al. 2009; Contractor et al. 2011). Therefore, we compared the effect of Neto1 on the behaviour of homomeric or heteromeric KARs composed of a variety of different subunit combinations, focusing on GluK5-containing receptors. In addition, the previous studies used only maximally effective concentrations of agonist. We examined the effects of Neto1 on the sensitivity of the receptors to glutamate, as well as the kinetic properties of responses to non-saturating agonist concentrations. For GluK5-containing heteromeric receptors, we found that Neto1 eliminated desensitization at sub-maximal glutamate concentrations without altering the concentration dependence of activation. At saturating agonist concentrations, Neto1 slowed the onset of desensitization of GluK2 homomers, but not GluK2/GluK5 heteromers. For both receptors the rate of recovery from desensitization was enhanced. Our results demonstrate that the functional impact of Neto1 on KAR desensitization can be dependent upon the level of receptor activation, and suggest that this auxiliary subunit stabilizes a high-affinity, non-desensitized state in partially bound receptors.
Methods
Culture and transfection of HEK-293T cells
HEK-293T cells (GenHunter, Nashville, TN, USA) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 IU ml−1 penicillin and 100 μg ml−1 streptomycin. Cells were passaged with a 0.025% trypsin/0.01% EDTA solution in phosphate-buffered saline (10 mm Na2HPO4, 150 mm NaCl, pH 7.3). Full-length cDNAs for the GluK or Neto1 subunits in mammalian expression vectors were transfected into cells using calcium phosphate precipitation as previously described (Mott et al. 2010). Rat GluK1, GluK2(Q), GluK4 and GluK5 plasmids were provided by S. Heinemann (Salk Institute, San Diego, CA, USA), and human GluK3 was provided by A. Srivastava (Greenwood Genetics Center, Greenwood, SC, USA). Human Neto 1 was provided by S. Tomita (Yale University, New Haven, CN, USA). Plasmids were transfected at ratios of 1:3:4 (GluK1–GluK3:GluK4–GluK5:Neto1), previously shown to optimize formation of heteromeric receptors (Barberis et al. 2008) and Neto1 assembly with KARs (Fisher & Mott, 2012). To identify transfected cells, we co-transfected 1 μg of a cDNA encoding a single-chain antibody recognizing the hapten 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (phOx; Chesnut et al. 1996). Positively transfected cells were isolated using phOx-coated beads 18–28 h after transfection and plated onto glass coverslips treated with poly-l-lysine and collagen.
Electrophysiological recordings
Whole-cell recordings were performed on isolated HEK-293T cells 40–52 h after transfection under voltage-clamp conditions. Patch pipettes were pulled from borosilicate glass with an internal filament (World Precision Instruments, Sarasota, FL, USA) on a two-stage puller (Narishige, Japan) to a resistance of 5–10 MΩ and filled with a solution containing (in mm): CsGluconate, 130; CsCl, 5; Hepes, 10; CsBapta, 5; MgCl2, 2; MgATP, 2; NaGTP, 0.3; with pH 7.4 and osmolarity 290–300 mosmol l−1. Cells were continually perfused with an external solution containing (in mm): NaCl, 150; KCl, 3; Hepes, 10; CaCl2, 1; MgCl2, 0.4; at pH 7.4 and osmolarity 295–305 mosmol l−1. For whole-cell recordings glutamate was applied to cells using a stepper solution exchanger (SF-77B, Warner Instruments, Hamden, CT, USA). The time course of drug application was <50 ms in the whole-cell recording configuration as determined using a diluted external solution applied to the electrode. For outside-out patch recordings the 3-barrel square glass of the drug applicator was pulled to a final size near 200 μm. Rise times (10–90%) of the junction potential at the open tip were consistently faster than 400 μs and were tested using a diluted external solution. Current recordings were amplified (Axopatch 200B; Molecular Devices, Foster City, CA, USA), filtered (1 kHz) and digitized at 10 kHz using a Digidata 1320 analog to digital board (Molecular Devices) and stored on a computer hard drive for off-line analysis.
Analysis of whole-cell and outside-out patch currents
Whole-cell currents were analysed using the programs Clampfit (pClamp9.2 suite, Molecular Devices, Foster City, CA, USA) and Prism (Graphpad, San Diego, CA, USA). Concentration–response data were fit with a four-parameter logistic equation: Current =[Minimum current + (Maximum current – Minimum Current)]/1 + (10∧(log EC50– log [Glutamate]) ×n), where n represents the Hill number. All fits were made to normalized data with current expressed as a percentage of the maximum response to glutamate for each cell. Peak currents and log EC50 values were compared using unpaired Student's t tests with a significance level of P= 0.05. Currents from outside-out patch recordings were analysed with the pClamp9.2 suite of programs (Molecular Devices). The desensitization rate was determined by fitting the decay current with the Levenberg–Marquardt least squares method with increasing numbers of exponential functions until additional components did not significantly improve the fit (F test of the sum of squared residuals).
Results
Neto1 shifts the concentration dependence of desensitization
Co-expression of the Neto1 auxiliary subunit with recombinant KARs has been shown to alter both agonist binding and desensitization kinetics in response to saturating agonist concentrations (Copits et al. 2011; Straub et al. 2011a). To examine the effect of the KAR subunit composition on modulation by Neto1 and to better understand the mechanisms underlying this modulation, we first compared the effect of glutamate concentration on the properties of GluK2 and GluK2/GluK5 receptors co-expressed with Neto1 in transiently transfected HEK-293T cells.
GluK2 homomeric receptors
Homomeric GluK2 receptors are characterized by a relatively low sensitivity to glutamate (EC50 > 100 μm), and by rapid and complete desensitization in response to even sub-EC50 glutamate concentrations (Heckmann et al. 1996; Pasternain et al. 1998; Barberis et al. 2008; Fig. 1). Co-transfection of Neto1 with GluK2 caused a modest increase in glutamate sensitivity (Table 1; Fig. 1A and B). This is consistent with the previously reported increase in kainate binding affinity when GluK2 subunits were co-expressed with Neto1 in oocytes (Straub et al. 2011a). We found that addition of Neto1 did not significantly change the peak amplitude of the response to maximally effective glutamate concentrations (Table 1). Neto1 slowed the onset and reduced the extent of desensitization of the whole-cell current (Fig. 1A). At low μm concentrations, GluK2 receptors with Neto1 showed little desensitization. A lack of desensitization observed at the macroscopic level does not necessarily indicate that microscopic transitions to desensitized states do not occur, only that they are not apparent under the conditions of these recordings. Although desensitization increased with increasing glutamate concentrations, the 5 s application never produced complete desensitization, and a steady-state current nearly 10% of the peak current remained even in response to 10 mm glutamate (Figs 1A and 2C).
Figure 1. Effect of Neto1 on GluK2 homomeric receptors.
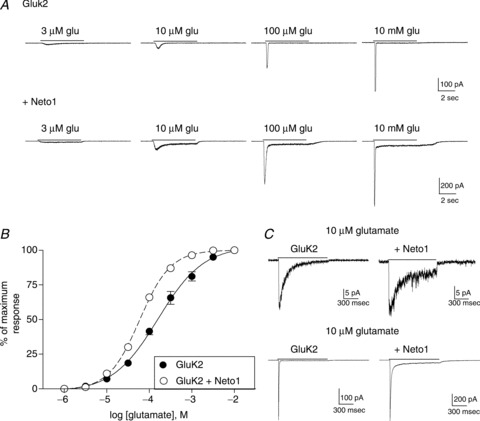
A, whole-cell current responses from HEK-293T cells transiently transfected with GluK2 alone or GluK2 + Neto1 (1:4 cDNA ratio). Glutamate was applied for 5 s (continuous line) at the concentration indicated to cells voltage-clamped at −70 mV. B, the peak current response at each glutamate concentration was measured and normalized to the maximum response for each cell. Symbols indicate the mean ± SEM (n= 5 cells) of the response of GluK2 homomers either with (open circles) or without (filled circles) Neto1. Averaged data were fit with a four-parameter logistic equation (solid or dashed line). C, 10 μm or 10 mm glutamate was rapidly applied for 1 s (continuous line) to outside-out patches from cells transfected with either GluK2 alone or GluK2 + Neto1. Patches were voltage-clamped at −70 mV.
Table 1.
Summary of characteristics from whole-cell recordings
| Peak amplitude (pA) | Glutamate EC50 (μm) | Steady-state current 10 μm glutamate, (% of peak) | ||||
|---|---|---|---|---|---|---|
| Subunit composition | −Neto1 | +Neto1 | −Neto1 | +Neto1 | −Neto1 | +Neto1 |
| GluK2 | 654 ± 135 | 547 ± 74 | 186.0 ± 34.2 | 60.0 ± 2.6*** | 0.88 ± 0.33 | 7.12 ± 0.87*** |
| (n= 5) | (n= 5) | |||||
| GluK2/GluK5 | 391 ± 26 | 467 ± 179 | 6.0 ± 1.2 | 5.0 ± 0.3 | 10.1 ± 1.9 | 98.6 ± 1.4*** |
| (n= 5) | (n= 5) | |||||
| GluK2/GluK4 | 447 ± 92 | 561 ± 118 | 43.2 ± 7.2 | 9.1 ± 1.2*** | 2.6 ± 1.3 | 96.8 ± 2.0*** |
| (n= 5) | (n= 5) | |||||
| GluK1/GluK5 | 200 ± 40 | 335 ± 180 | 7.8 ± 1.3 | 5.2 ± 0.2 | 3.7 ± 1.4 | 87.8 ± 2.8*** |
| (n= 5) | (n= 5) | |||||
| GluK3/GluK5 | 55 ± 6 | 194 ± 35** | 6.0 ± 0.7 | 4.8 ± 0.5 | 6.4 ± 0.8 | 91.2 ± 2.1*** |
| (n= 5) | (n= 5) | |||||
**P≤ 0.01 or ***P≤ 0.001 indicate a significant difference from the same subunit combination without Neto1 (unpaired Student's t test).
Figure 2. Effect of Neto1 on GluK2/GluK5 heteromeric receptors.
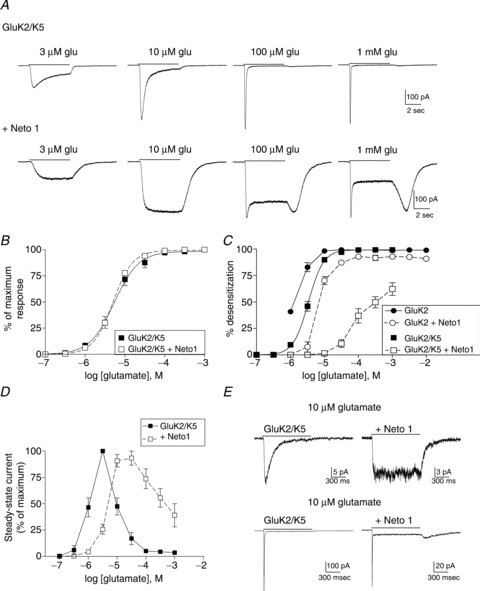
A, cells were transiently transfected with GluK2/GluK5 alone or plus Neto1. Whole-cell currents are shown in response to glutamate applied for 5 s (continuous line) at the concentration indicated to cells voltage-clamped at −70 mV. B, the peak current response was measured and normalized to the maximum response for each cell. Symbols indicate the mean ± SEM (n= 5 cells) of the response of GluK2/GluK5 receptors with (open squares) or without (filled squares) Neto1. Averaged data were fit with a four-parameter logistic equation (solid or dashed line). C, the extent of desensitization was measured by subtracting the steady-state /peak current ratio from 1 and multiplying by 100 to give percentage desensitization. Steady-state current was measured at the end of the 5 s application. Symbols show mean ± SEM (n= 5 cells). D, the amplitude of the steady-state current was measured at the end of the 5 s application and normalized to the maximum steady-state current for each cell. Symbols show mean ± SEM (n= 5 cells). E, 10 μm or 10 mm glutamate was rapidly applied for 1 s (continuous line) to outside-out patches from cells transfected with either GluK2/GluK5 alone or GluK2/GluK5 + Neto1. Patches were voltage-clamped at −70 mV.
The whole-cell recording configuration does not permit accurate measurement of the fast phases of desensitization. To quantify the effects of Neto1 on the onset of desensitization, we used rapid application of 10 μm or 10 mm glutamate to excised outside-out patches (Fig. 1C). Addition of Neto1 reduced desensitization of GluK2 receptors for both glutamate concentrations. The average time constant of desensitization in response to a 1 s application of 10 μm glutamate was significantly slowed, from 142.1 ± 12.3 ms (n= 4, GluK2) to 386.2 ± 64.2 ms (n= 5, +Neto1, P≤ 0.05). The characteristics of desensitization in response to 10 mm glutamate, a maximally effective concentration, were also altered by Neto1. For GluK2 alone, the onset of desensitization was best fit with a single exponential component with an average time constant of 6.2 ± 0.3 ms (n= 3). In the presence of Neto1, the decay was fit with the sum of two exponentials with average time constants of 20.4 ± 2.8 ms (90.5 ± 5.5% of the total area) and 147.2 ± 20.5 ms, producing a weighted mean average of 32.2 ± 3.8 ms (n= 3, P≤ 0.05 compared with GluK2 alone). In addition, unlike for GluK2 alone, desensitization was not complete, and a steady-state current averaging 5.9 ± 1.6% of the peak current remained at the end of the 1 s application. These results are consistent with our findings from whole-cell recordings.
GluK2/GluK5 heteromeric receptors
Addition of the GluK5 subunit to form K2/K5 heteromeric receptors increases glutamate sensitivity, slows deactivation, and slows the rate of onset and the extent of desensitization at low (<100 μm) agonist concentrations (Barberis et al. 2008; Fisher & Mott, 2011; Yan et al. 2013). Earlier studies have suggested that the heteromeric receptor is activated by glutamate binding to the higher affinity GluK5 subunit, and that binding to the lower affinity GluK2 subunit is required for desensitization (Mott et al. 2010; Fisher & Mott, 2011). Straub et al. (2011a) reported that co-expression of Neto1 with GluK2/GluK5 heteromers in mammalian cells slowed both desensitization and deactivation in response to 10 mm glutamate and caused faster recovery from desensitization. We extended these studies by characterizing the response of these receptors to sub-maximal glutamate levels.
In contrast to the GluK2 homomeric receptors, we found that co-transfection of Neto1 with GluK2 and GluK5 did not change the glutamate sensitivity of whole-cell current activation (Fig. 2A and B; Table 1). However, Neto1 had a dramatic effect on the desensitization characteristics of these heteromeric receptors (Fig. 2). In the absence of Neto1, GluK2/GluK5 receptors typically first began to desensitize at concentrations near 1–3 μm glutamate. As the agonist concentration increased, desensitization became faster and more complete, and at mm concentrations was very similar to that of the GluK2 homomer. A small rebound current could be observed following removal of agonist at high glutamate concentrations, which has been attributed to the large difference in glutamate affinity between the GluK2 and GluK5 subunits (Mott et al. 2010; Fisher & Mott, 2011). With the addition of Neto1, the concentration dependence of desensitization for GluK2/GluK5 heteromers was substantially altered (Fig. 2). Glutamate at levels up to 10 μm produced non-desensitizing whole-cell responses, and at higher concentrations desensitization was less complete than in the absence of Neto1 (Fig. 2A and C). At the end of the 5 s application a large rebound current was observed (Fig. 2A). These characteristics are strikingly similar to those seen in heteromeric receptors when the glutamate sensitivity of the GluK2 subunit was reduced by a mutation within the agonist binding site (Fisher & Mott, 2011). However, we found that Neto1 actually increased the glutamate sensitivity of GluK2 homomeric receptors (Fig. 1), making it unlikely that decreased activation of the GluK2 subunit in the heteromer underlies this change in receptor behaviour. A more reasonable explanation is that the rebound current arises from alteration of the desensitization kinetics. The averaged data in Fig. 2C were fit with logistic equations to determine the glutamate EC50 for the onset of desensitization. Because substantial desensitization was observed with GluK2 receptors even at the lowest concentrations tested, the minimum response was fixed at zero for this data set. In the absence of Neto1, the values of EC50 for onset of desensitization were 1.2 μm (GluK2) and 6.7 μm (GluK2/GluK5). These levels are far lower than the glutamate concentrations required to fully activate GluK2 homomeric receptors (see Fig. 1; Table 1). In the presence of Neto1, higher concentrations are required to produce desensitization, and the values of EC50 are shifted to 6.7 μm (GluK2 + Neto1) and 84.9 μm (GluK2/GluK5 + Neto1). We also measured the steady-state current amplitude and found a similar pattern (Fig. 2D). Addition of Neto1 causes a rightward shift in the concentration–response relationship of the steady-state current, as the maximum amplitude of these currents is now reached at a higher glutamate concentration.
Using recordings from excised outside-out patches, we measured the onset of desensitization in response to 1 s applications of 10 μm or 10 mm glutamate (Fig. 2E). As we observed in whole-cell recordings, GluK2/GluK5 receptors with Neto1 showed no desensitization in response to 10 μm glutamate (n= 3 patches), while in the absence of Neto1 the time constant of decay averaged 160.2 ± 9.1 ms (n= 6). Rapid desensitization in both cases was produced with 10 mm glutamate, averaging 5.9 ± 0.6 ms (n= 4, GluK2/GluK5 alone) and 6.2 ± 0.6 ms (n= 3, +Neto1). However, in the presence of Neto1, desensitization was not complete, with a steady-state current averaging 8.2 ± 0.2% of the peak current. As with the whole-cell recordings, a rebound current could be observed after the end of the glutamate application. These results with 10 mm glutamate differ from those of Straub et al. (2011a) using the same subunit combinations. Compared with our findings, they observed a greater effect of Neto1 on the rate of desensitization but less impact on the steady-state current, and did not report a rebound current following agonist removal.
Recovery from desensitization – GluK2 and GluK2/GluK5
One consistent effect of the Neto proteins on KARs is an increase in the rate of recovery from desensitization (Zhang et al. 2009; Straub et al. 2011a,b; Copits et al. 2011). We compared the effect of Neto1 on GluK2 and GluK2/GluK5 receptors using paired, 100 ms applications of 10 mm glutamate (Fig. 3). Recovery time constants for homomeric and heteromeric receptors without Neto1 averaged 1.67 ± 0.24 s (n= 5, GluK2) and 1.74 ± 0.19 s (n= 4, GluK2/GluK5), similar to previous reports (Heckmann et al. 1996; Mott et al. 2010; Straub et al. 2011a). We found that recovery was significantly faster in the presence of Neto1 for both receptor isoforms (P≤ 0.01), with average time constants of 0.24 ± 0.12 s (n= 4) for GluK2 + Neto1 and 0.25 ± 0.05 s (n= 3) for GluK2/GluK5 + Neto1. In both the presence and absence of Neto1, recovery was not affected by inclusion of the GluK5 subunit, consistent with the view that the desensitization properties of heteromeric receptors are determined primarily by the GluK2 subunits.
Figure 3. Effect of Neto1 on recovery from desensitization.
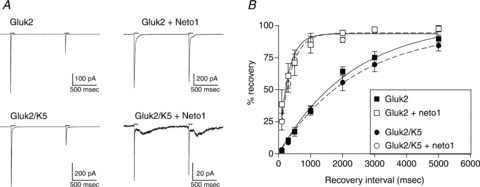
A, outside-out patches were excised from cells transfected with the subunits indicated. Pairs of 100 ms applications of 10 mm glutamate (continuous lines) were applied 1 s apart to patches voltage-clamped at −70 mV. B, paired pulses of 10 mm glutamate (100 ms) were applied to outside-out patches at intervals ranging from 100 ms to 5 s. The peak amplitude of the 2nd response was divided by that of the 1st response and multiplied by 100 to give percentage recovery. Data were fitted with a single exponential shown by the continuous line (GluK2) or dashed line (GluK2/GluK5).
The effect of Neto1 on heteromeric receptors does not depend upon the GluK1–GluK3 subunit subtype
The tetrameric KARs can be assembled from a combination of five different subunits. Earlier studies suggested that the functional effect of Neto1 on homomeric receptors was subunit dependent, slowing desensitization of GluK2 homomers, but speeding desensitization of GluK1 homomers (Copits et al. 2011). To determine if the effect of Neto1 on the properties of heteromeric receptors was also influenced by the subunit composition, we compared the characteristics of GluK1/GluK5 and GluK3/GluK5 in the absence and presence of Neto1 (Fig. 4). Just as we observed for GluK2/GluK5 receptors, co-expression with Neto1 did not change the glutamate sensitivity of activation (Fig. 4B; Table 1). The only subunit combination for which Neto1 significantly altered maximum current amplitude was GluK3/GluK5, which increased about 350% when Neto1 was co-transfected (Table 1).
Figure 4. Neto 1 effects on GluK1/GluK5 and GluK3/GluK5 heteromeric receptors.
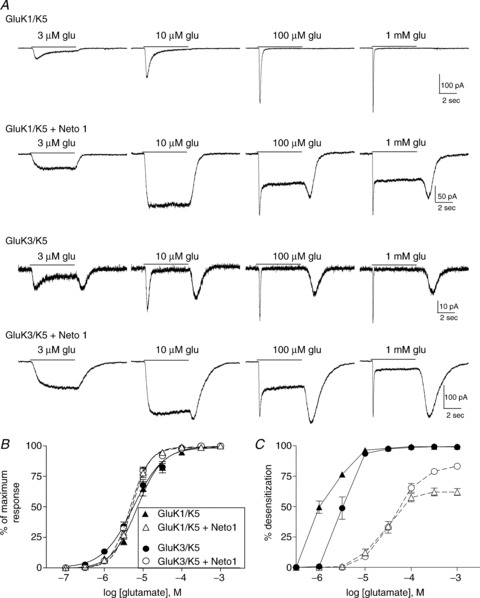
A, whole-cell currents from cells transfected with the indicated subunits. Glutamate was applied for 5 s (continuous line) at the concentration shown to cells voltage-clamped at −70 mV. B, the peak current response at each glutamate concentration was normalized to the maximum response for each cell. Symbols indicate the mean ± SEM of the response of heteromeric receptors either with (open symbols, dashed line) or without Neto1 (filled symbols, continuous line). Averaged data were fit with a four-parameter logistic equation. C, the extent of desensitization was measured by subtracting the steady-state/peak current ratio from 1 and multiplying by 100 to give percentage desensitization. Steady-state current was measured at the end of the 5 s application. Symbols (mean ± SEM) represent subunit combinations with or without Neto1, as indicated in B.
The impact of Neto1 on desensitization of the whole-cell current was similar for heteromeric receptors containing GluK1, GluK2 or GluK3 in combination with GluK5 (Fig. 4). In the absence of Neto1, GluK1/GluK5 receptors showed substantial desensitization in response to low μm levels of glutamate, and the rate and extent of desensitization increased in a concentration-dependent manner, consistent with the behaviour of GluK1 homomers (Sommer et al. 1992). GluK3 subunits are characterized by a very low sensitivity to glutamate (EC50 > 10 mm for homomeric receptors) as well as rapid onset of desensitization (Schiffer et al. 1997; Perrais et al. 2009). We found that the GluK3/GluK5 heteromers have high glutamate sensitivity, but rapidly desensitize in response to concentrations above 1 μm and exhibit a large rebound current following removal of agonist (Fig. 4A). This is consistent with a relatively rapid recovery from the desensitized state, and could be produced by a large separation between the agonist affinities for the two different subunits (Mott et al. 2010; Fisher & Mott, 2011). As with the GluK2/GluK5 receptors (Fig. 2), addition of Neto1 to GluK1/GluK5 or GluK3/GluK5 shifted the concentration dependence of desensitization to the right, and reduced the extent of desensitization at all glutamate concentrations (Fig. 4A and C). These results suggest that the functional impact of Neto1 is similar at heteromeric receptors containing the GluK5 subunit in combination with any of the GluK1, GluK2 or GluK3 subunits.
We also examined GluK2/GluK4 heteromeric receptors, to determine if the effects of Neto1 were dependent on the identity of the ‘high-affinity’ subunit. Like GluK5, co-assembly with GluK4 increased glutamate sensitivity compared with the GluK2 homomeric receptor, although the presence of GluK4 had less impact on the receptor's kinetic properties (Fig. 5A and B). The addition of Neto1 to GluK2/GluK4 receptors reduced the extent of desensitization in whole-cell recordings, and produced a prominent rebound current (Fig. 5A and C). As with GluK2/GluK5, Neto1 changed the glutamate EC50 for the extent of desensitization from 1.3 μm (GluK2/GluK4) to 63 μm (GluK2/GluK4 + Neto1). The functional impact was therefore qualitatively similar to that seen with GluK2/GluK5 receptors. Unlike GluK2/GluK5, Neto1 also increased glutamate sensitivity of GluK2/GluK4 receptors (Fig. 5B; Table 1), demonstrating a distinct functional impact at the GluK4 subunit that is not observed with GluK5-containing receptors.
Figure 5. Effect of Neto 1 on the properties of GluK2/GluK4 heteromeric receptors.
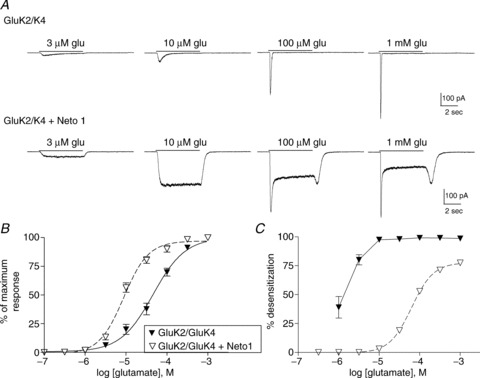
A, representative whole-cell currents from cells transfected with GluK2 and GluK4 with or without Neto1. Glutamate was applied for 5 s (continuous line) at the concentration indicated to cells voltage-clamped at −70 mV. B, the peak current response at each glutamate concentration was normalized to the maximum response for each cell. Symbols indicate the mean ± SEM of the response of receptors either with (open symbols, dashed line) or without Neto1 (filled symbols, continuous line). Averaged data were fit with a four-parameter logistic equation. C, the extent of desensitization was measured by subtracting the steady-state/peak current ratio from 1 and multiplying by 100 to give percentage desensitization. Steady-state current was measured at the end of the 5 s application. Symbols (mean ± SEM) represent GluK2/GluK4 with (open) or without (filled) Neto1, as in B.
Discussion
We examined the impact of the auxiliary subunit Neto1 on the properties of recombinant homomeric and heteromeric KARs with varying subunit composition. For all isoforms studied, co-expression of Neto1 shifted the concentration dependence for the onset of desensitization towards higher levels of glutamate. Little or no macroscopic desensitization was observed at low glutamate concentrations (<30 μm), particularly for heteromeric GluK4- or GluK5-containing receptors. Neto1 also increased the rate of recovery from desensitization for both homomeric (GluK2) and heteromeric (GluK2/GluK5) receptors and increased the steady-state current, even at saturating agonist concentrations. We found that the effect of Neto1 on the glutamate sensitivity of channel activation was subunit dependent and relatively modest. The glutamate EC50 was unchanged for all GluK5-containing isoforms, and was significantly reduced only for GluK2 homomers and GluK2/GluK4 heteromers. This is consistent with the slight enhancement of [3H]kainate binding affinity for GluK2 homomers previously observed (Straub et al. 2011a). Neto1 also had little effect on the peak current amplitude of GluK1- and GluK2-containing receptors, but increased the maximum response of GluK3/GluK5 receptors. This selective effect at GluK3-containing receptors might arise through changes in kinetic properties. GluK3-containing receptors have been found to strongly desensitize without channel opening and, as a result, reducing desensitization would be expected to increase current amplitude (Perrais et al. 2009). Therefore, our results are in line with previous reports that Neto1 has little impact on surface expression of recombinant receptors (Copits et al. 2011; Straub et al. 2011a; Copits & Swanson, 2012).
Our studies also demonstrate the distinct properties associated with each of the five KAR subunits. Homomeric receptors composed of GluK1–GluK3 subunits differ in agonist affinity and desensitization kinetics (Perrais et al. 2010), and these characteristics also contribute to some aspects of the behaviour of heteromeric receptors. In homomeric KARs, the onset of desensitization has only a small dependence upon glutamate concentration (Heckmann et al. 1996), and even low μm levels of glutamate cause rapid and complete desensitization. This pattern changes in heteromers containing GluK4 or GluK5 subunits, suggesting that the process of glutamate-induced desensitization differs in these receptors. In the absence of Neto1, the concentration-dependent kinetics of heteromeric KARs can be ascribed to the unique properties of the individual subunits (Swanson et al. 2002; Mott et al. 2010; Fisher & Mott, 2011). Thus, at heteromeric GluK2/GluK5 receptors, glutamate binding to the high-affinity GluK5 subunit opens the channel and rapid desensitization occurs only when the lower-affinity GluK2 subunit is activated by agonist. The non-desensitizing properties associated with the high-affinity GluK4 or GluK5 subunits contribute to the slow kinetics of heteromeric KARs at non-saturating glutamate concentrations. This theory is further supported by the behaviour we observed for GluK3/GluK5 receptors in this study. GluK3 homomeric receptors are characterized by a low affinity for activation, with a glutamate EC50 in the mm range, and are likely to desensitize without opening, especially at low agonist concentrations (Schiffer et al. 1997; Perrais et al. 2009). In contrast, co-assembly of GluK5 with GluK3 in a heteromer enabled receptor activation at low μm glutamate concentrations, similar to the glutamate sensitivity of other GluK5-containing heteromers. We found that the onset of desensitization of the GluK3/GluK5 heteromeric receptors begins in the μm range, consistent with levels that desensitize (but do not activate) GluK3 homomeric receptors. This suggests that glutamate binding to GluK5 subunits in these receptors was responsible for receptor activation, whereas agonist binding to GluK3 subunits produced desensitization. The relatively low affinity of these GluK3 subunits would allow rapid recovery and produce the rebound current we observed upon agonist washout.
It is clear from the properties of homomeric receptors that the Neto proteins can modulate the function of GluK1 or GluK2 subunits. It is not known if they can also alter the properties of GluK4 or GluK5 subunits, which must co-assemble with one of the GluK1–GluK3 subunits to form heteromeric receptors. Interaction of Neto1 with GluK2/GluK4 or GluK2/GluK5 receptors slowed the onset of desensitization in response to sub-maximal glutamate concentrations and caused a prominent rebound current following removal of higher glutamate concentrations. This ‘tail’ current is consistent with rapid recovery from desensitization, allowing re-activation of the receptors through the glutamate bound to the high-affinity GluK4/GluK5 subunits. It is interesting that many of the effects of Neto1 are comparable to those of adding the GluK5 subunit to homomeric receptors. Both Neto1 and GluK5 increase glutamate sensitivity, slow the onset of desensitization at sub-maximal glutamate concentrations, and reduce voltage-dependent block by intracellular polyamines (Barberis et al. 2008; Fisher & Mott, 2011, 2012). Because binding of glutamate to the GluK5 subunit does not normally appear to contribute to the onset of desensitization, it may be difficult to discern whether Neto1 has any functional impact on this subunit. Further studies will be necessary to clarify whether there are interactions between these subunits. We found similar effects of Neto1 on heteromeric GluK5-containing receptors regardless of the GluK1–GluK3 partner. Interestingly, Copits et al. (2011) previously reported that Neto1 accelerated desensitization of GluK1 homomers, in contrast to its effects on GluK2 homomers. This suggests that the presence of GluK5 may regulate the interaction of Neto1 with GluK1. With GluK2 homomers, we found that Neto1 had a comparable effect slowing desensitization at both low and high agonist concentrations, but was effective only at low agonist concentrations for GluK2/GluK5. This subunit dependence might arise from preferential assembly of Neto1 with either GluK2 or GluK5, or may reflect differences in the structural changes that underlie desensitization states in homomeric and heteromeric receptors.
Neto1 did not alter the glutamate sensitivity of activation of GluK5-containing receptors, but caused a fivefold increase in the glutamate sensitivity of GluK2/GluK4 receptors. This suggests that Neto1 interacts with GluK4- and GluK5-containing receptors differently. The GluK4 subunit appears to make a greater contribution to desensitization of heteromeric receptors than does the GluK5 subunit (Mott et al. 2010; Copits & Swanson, 2012), which may account for its modulation by Neto1. However, the observed shift in glutamate sensitivity might also have arisen from a change in receptor assembly. GluK4 subunits do not always readily incorporate into functional receptors in expression systems (see Perrais et al. 2010) and, as a result, the receptor population is likely to be heterogeneous, with some homomeric GluK2 receptors contributing to the response. If co-expression of Neto1 preferentially enhanced the formation of higher-affinity heteromeric receptors, an apparent increase in glutamate sensitivity would result.
The effects of Neto1 on the properties of GluK2/GluK5 receptors that we observed differ substantially from those previously reported by Straub et al. (2011a). They found that Neto1 significantly slowed both desensitization and deactivation of the channels in response to 10 mm glutamate or 1 mm kainate, and that no rebound current occurred at the end of agonist application. They also reported that Neto1 co-expression had only a small effect on the steady-state current when glutamate was the agonist. These characteristics are similar to our results using GluK2 homomeric receptors, and it is possible that in their studies the co-transfection of GluK2 and GluK5 produced a heterogeneous population of receptors with a prominent homomeric component. The kinetic properties they reported for receptors in the absence of Neto1 are also more consistent with homomeric receptors, with fast deactivation rates in response to 1 ms applications of 10 mm glutamate (weighted mean time constants of ∼1.5 ms). These values would be expected from GluK2 receptors but not GluK2/GluK5 heteromers, which deactivate with time constants closer to 50 ms (Barberis et al. 2008).
At GluK2/GluK5 heteromeric receptors, the effect of Neto1 on the onset of desensitization was concentration dependent, producing a significant change at sub-maximal agonist concentrations, but not saturating concentrations. This suggests that, at least in heteromeric receptors, Neto1 primarily regulates the kinetic properties of partially bound receptors. Neto1 seems to stabilize the open state(s) and/or destabilize the desensitized state(s) of the channel. Similarly, Neto2 increases the open probability and burst length of individual GluK2 receptors (Zhang et al. 2009). Significant progress has been made towards understanding the structural mechanisms that regulate desensitization of ionotropic glutamate receptors (Sun et al. 2002; Horning & Mayer, 2004; Armstrong et al. 2006; Weston et al. 2006; Schauder et al. 2013). KARs, like AMPA receptors, have been proposed to function largely as a ‘dimer-of-dimers’ in which the onset of desensitization is regulated by residues that contribute to the dimer interface for each pair of subunits (Zhang et al. 2006; Chaudhry et al. 2009; Das et al. 2010; Gonzalez et al., 2010; Nayeem et al. 2011). According to this proposal, desensitization occurs when agonist binding causes a conformational change within the ligand-binding domain, leading to disruption of the dimer interface and transition to an energetically favourable desensitized state (see reviews by Traynelis et al. 2010; Mayer, 2011). Recent work has expanded this mechanism by demonstrating that fully occupied GluK2 receptors assume a stable desensitized state by undergoing a major conformational rearrangement in which the ligand-binding domains no longer form dimer assemblies, but emerge instead as four separate protomers (Schauder et al. 2013). Schauder et al. (2013) suggested that conformational changes of individual dimers within the receptor may represent an intermediate desensitized state. Accordingly, in a partially bound receptor, one fully occupied dimer pair could enter the desensitized state, closing the channel. The other dimer pair may be in a different conformation, with one or neither of the subunits bound to the agonist. The conformation of the desensitized receptor may thus depend upon agonist concentration, consistent with an earlier proposal suggesting the existence of multiple desensitized states (Patneau & Mayer, 1991). The heteromeric KAR is likely composed of two dimers, each of which contains one GluK2 and one GluK5 subunit (Kumar et al. 2011; Reiner et al. 2012). The higher affinity of the GluK5 subunits increases the likelihood that they will be bound before the GluK2, leading to a condition in which one binding site is occupied by glutamate in each dimer. Our previous studies suggest that this produces channel activation but not desensitization (Fisher & Mott, 2011). With increasing levels of glutamate, the agonist binds to the GluK2 subunits, and occupancy of both subunits within a dimer produces desensitization. Neto1 could regulate this process in a manner dependent upon the conformation of the desensitized state of the receptor. In heteromeric receptors, Neto1 had its greatest effect at sub-maximal concentrations, suggesting that it modulates the interaction between subunits forming a dimer in partially bound receptors. In these partially bound receptors Neto1 may stabilize the dimer interface, allowing the channel to remain open, despite agonist binding to both subunits in the dimer. In contrast, Neto1 had a reduced effect on desensitization at maximal glutamate concentrations, when all four subunits are bound to the agonist. Our data suggest that Neto1 only minimally modulates the conformational changes associated with transitions into this desensitized state in the heteromeric receptor. However, the increase in recovery from desensitization suggests that it may reduce the stability of this closed but fully bound state. The effect of Neto1 on the glutamate EC50 for onset of desensitization is also consistent with this type of model. In the presence of Neto1, the EC50 for desensitization of both GluK2 homomers and GluK2/GluK5 heteromers shifts to the right, although the EC50 for activation is either slightly decreased or unchanged. This change is consistent with the idea that in the absence of Neto1, partially bound receptors can produce complete desensitization while, in the presence of Neto1, fully bound receptors may be required for desensitization.
While the effects of agonist concentration on the modulation produced by Neto1 could arise from differences in the ability of the auxiliary subunit to influence various desensitized states of the receptor, the concentration dependence could also result from a rapidly regulated interaction between Neto1 and the KAR subunits. Either glutamate itself or the conformational changes associated with activation could disrupt the interaction between the pore-forming and auxiliary subunits, removing the positive modulation produced by Neto1 and causing a rapid ‘depotentiation’ that would appear to be desensitization. Recovery of Neto1 to its site as glutamate unbinds could produce the observed rebound current. A similar mechanism has been demonstrated for TARP modulation of AMPA receptors, as high concentrations of glutamate induce rapid dissociation of stargazin (γ-2) from neuronal or recombinant AMPA receptors (Morimoto-Tomita et al. 2009). Future studies uncovering the structural basis of the KAR–Neto interaction may shed light on the potential for this process to occur.
Our studies focused on Neto1 modulation of KARs because it appears to have an important role in the CA3 region of the hippocampus (Straub et al. 2011a; Tang et al. 2011). While Neto1 and Neto2 have high structural homology to one another, they show different expression patterns in the brain and have distinct effects on channel function. Unlike Neto1, Neto2 appears to have an important functional role in regulation of surface expression and synaptic targeting of KARs in neurons (Copits & Swanson, 2012). Their effects on channel kinetics are generally comparable, except at GluK1 homomeric receptors where they have opposing effects (Copits et al. 2011). In addition, at the receptors that have been studied so far, Neto2 has a larger impact than Neto1 on the onset of desensitization, and its effect appears to be less dependent upon subunit composition (Zhang et al. 2009; Copits et al. 2011; Straub et al. 2011a,b; Copits & Swanson, 2012). Further work may clarify the functional differences between these two auxiliary subunits.
Synaptic KAR currents differ from those mediated by AMPA receptors in their slow kinetics, decaying in tens or hundreds of milliseconds. These slow kinetics provide an important synaptic mechanism through which KARs encode temporal information (Castillo et al. 1997; Vignes & Collingridge, 1997; Mulle et al. 1998). Studies using Neto1 knockout animals show that the presence of Neto1 impacts the properties of KAR EPSCs at mossy fibre synapses in the hippocampus and suggest that this regulation is crucial for conferring the slow decay kinetics associated with KAR-mediated postsynaptic currents (Straub et al. 2011a). These KARs are likely heteromeric receptors containing GluK4 or GluK5 subunits, which may be associated with Neto1. Rapid recovery from desensitization conferred by Neto1 may give these neurons the ability to respond to fast stimulation frequencies. Our results also suggest that tonic KAR-mediated currents could be produced at low (<100 μm) glutamate concentrations. If expression of the Neto proteins is regulated by activity, changes in the proportion of Neto-associated KARs may provide a mechanism for altering the properties of KAR-mediated neurotransmission.
Acknowledgments
We thank Dr Morris Benveniste (Morehouse School of Medicine) for helpful comments and suggestions regarding this study, and for a critical reading of the manuscript.
Glossary
- KAR
kainate receptor
- Neto
neuropilin and tolloid-like
- phOx
4-ethoxymethylene-2-phenyl-2-oxazolin-5-one
Additional information
Competing interests
None.
Author contributions
J.L.F. conducted the experiments. J.L.F. and D.D.M. designed the experiments, analysed data and wrote the paper.
Funding
This work was supported by a grant from NIH-NINDS (NS065869 to D.D.M. and J.L.F.). The contents are solely the responsibility of the authors, and do not necessarily represent the official views of NINDS or NIH.
References
- Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127:85–97. doi: 10.1016/j.cell.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Barberis A, Sachidhanandam S, Mulle C. GluR6/KA2 kainate receptors mediate slow-deactivating currents. J Neurosci. 2008;28:6402–6406. doi: 10.1523/JNEUROSCI.1204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Chaudhry C, Weston MC, Schuck P, Rosenmund C, Mayer ML. Stability of ligand-binding domain dimer assembly controls kainate receptor desensitization. EMBO J. 2009;28:1518–1530. doi: 10.1038/emboj.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut JD, Baytan AR, Russell M, Chang MP, Bernard A, Maxwell IH, Hoeffler JP. Selective isolation of transiently transfected cells from a mammalian cell population with vectors expressing a membrane anchored single-chain antibody. J Immunol Meth. 1996;193:17–27. doi: 10.1016/0022-1759(96)00032-4. [DOI] [PubMed] [Google Scholar]
- Contractor A, Mulle C, Swanson GT. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 2011;34:154–163. doi: 10.1016/j.tins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fibre synapses in KA2 −/− mice. J Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copits BA, Robbins JS, Frausto S, Swanson GT. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J Neurosci. 2011;31:7334–7340. doi: 10.1523/JNEUROSCI.0100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copits BA, Swanson GT. Dancing partners at the synapse: auxiliary subunits that shape kainate receptor function. Nat Rev Neurosci. 2012;13:675–686. doi: 10.1038/nrn3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. Distribution of kainate receptor subunits at hippocampal mossy fibre synapses. J Neurosci. 2003;23:8013–8019. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U, Kumar J, Mayer ML, Plested AJ. Domain organization and function in GluK2 subtype kainate receptors. Proc Natl Acad Sci U S A. 2010;107:8463–8468. doi: 10.1073/pnas.1000838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes HB, Catches JS, Petralia RS, Copits BA, Xu J, Russell TA, Swanson GT, Contractor A. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signalling. Neuron. 2009;63:818–829. doi: 10.1016/j.neuron.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD. Distinct functional roles of subunits within the heteromeric kainate receptor. J Neurosci. 2011;31:17113–17122. doi: 10.1523/JNEUROSCI.3685-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD. The auxiliary subunits Neto1 and Neto2 reduce voltage-dependent inhibition of recombinant kainate receptors. J Neurosci. 2012;32:12928–12933. doi: 10.1523/JNEUROSCI.2211-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J, Du M, Parameshwaran K, Suppiramaniam V, Jayaraman V. Role of dimer interface in activation and desensitization in AMPA receptors. Proc Natl Acad Sci U S A. 2010;107:9891–9896. doi: 10.1073/pnas.0911854107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann M, Bufler J, Franke C, Dudel J. Kinetics of homomeric GluR6 glutamate receptor channels. Biophys J. 1996;71:1743–1750. doi: 10.1016/S0006-3495(96)79375-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Horning MS, Mayer ML. Regulation of AMPA receptor gating by ligand binding core dimers. Neuron. 2004;41:379–388. doi: 10.1016/s0896-6273(04)00018-2. [DOI] [PubMed] [Google Scholar]
- Jane DE, Lodge D, Collingridge GL. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology. 2009;56:90–113. doi: 10.1016/j.neuropharm.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Kumar J, Schuck P, Mayer ML. Structure and assembly mechanism for heteromeric kainate receptors. Neuron. 2011;71:319–331. doi: 10.1016/j.neuron.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML. Emerging models of glutamate receptor ion channel structure and function. Structure. 2011;19:1370–1380. doi: 10.1016/j.str.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto-Tomita M, Zhang W, Straub C, Cho C-H, Kim KS, Howe JR, Tomita S. Autoinactivation of neuronal AMPA receptors via glutamate-regulated TARP interaction. Neuron. 2009;61:101–112. doi: 10.1016/j.neuron.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott DD, Rojas A, Fisher JL, Dingledine RJ, Benveniste M. Subunit-specific desensitization of heteromeric kainate receptors. J Physiol. 2010;588:683–700. doi: 10.1113/jphysiol.2009.185207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Nayeem N, Mayans O, Green T. Conformational flexibility of the ligand-binding domain dimer in kainate receptor gating and desensitization. J Neurosci. 2011;31:2916–2924. doi: 10.1523/JNEUROSCI.4771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D, Pitcher GM, Szilard RK, Sertié A, Kanisek M, Clapcote SJ, Lipina T, Kalia LV, Joo D, McKerlie C, Cortez M, Roder JC, Salter MW, McInnes RR. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLOS Biol. 2009;7:e41. doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternain AV, Rodriguez-Moreno A, Villarroel A, Lerma J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology. 1998;37:1249–1259. doi: 10.1016/s0028-3908(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991;6:785–798. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- Perrais D, Coussen F, Mulle C. Atypical functional properties of GluK3-containing kainate receptors. J Neurosci. 2009;29:15499–15510. doi: 10.1523/JNEUROSCI.2724-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D, Veran J, Mulle C. Gating and permeation of kainate receptors: differences revealed. Trends Pharmacol Sci. 2010;31:516–522. doi: 10.1016/j.tips.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol. 1994;349:85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res. 2006;326:457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- Reiner A, Arant RJ, Isacoff EY. Assembly stoichiometry of the GluK2/GluK5 kainate receptor complex. Cell Reports. 2012;1:234–240. doi: 10.1016/j.celrep.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder DM, Kuybeda O, Zhang J, Klymko K, Bartesaghi A, Borgnia MJ, Mayer ML, Subramaniam S. Glutamate receptor desensitization is mediated by changes in quaternary structure of the ligand binding domain. Proc Natl Acad Sci U S A. 2013;110:5921–5926. doi: 10.1073/pnas.1217549110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer HH, Swanson GT, Heinemann SF. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 1997;19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Sommer B, Burnashev N, Verdoorn TA, Keinanen K, Sakmann B, Seeburg PH. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992;11:1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Hunt DL, Yamasaki M, Kim KS, Watanabe M, Castillo PE, Tomita S. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto 1. Nat Neurosci. 2011a;14:866–873. doi: 10.1038/nn.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Zhang W, Howe JR. Neto2 modulation of kainate receptors with different subunit compositions. J Neurosci. 2011b;31:8078–8082. doi: 10.1523/JNEUROSCI.0024-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Green T, Sakai R, Contractor A, Che W, Kamiya H, Heinemann SF. Differential activation of individual subunits in heteromeric kainate receptors. Neuron. 2002;34:589–598. doi: 10.1016/s0896-6273(02)00676-1. [DOI] [PubMed] [Google Scholar]
- Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW, McInnes RR. Neto1 is an auxiliary subunit of native synaptic kainate receptors. J Neurosci. 2011;31:10009–10018. doi: 10.1523/JNEUROSCI.6617-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Werner P, Voigt M, Keinanen K, Wisden W, Seeburg PH. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature. 1991;351:742–744. doi: 10.1038/351742a0. [DOI] [PubMed] [Google Scholar]
- Weston MC, Schuck P, Ghosal A, Rosenmund C, Mayer ML. Conformational restriction blocks glutamate receptor desensitization. Nat Struct Molec Biol. 2006;13:1120–1127. doi: 10.1038/nsmb1178. [DOI] [PubMed] [Google Scholar]
- Yan D, Yamasaki M, Straub C, Watanabe M, Tomita S. Homeostatic control of synaptic transmission by distinct glutamate receptors. Neuron. 2013;78:687–699. doi: 10.1016/j.neuron.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Nayeem N, Nanao MH, Green T. Interface interactions modulating desensitization of the kainate-selective ionotropic glutamate receptor subunit GluR6. J Neurosci. 2006;26:10033–10042. doi: 10.1523/JNEUROSCI.2750-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumicka A, Morimoto-Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, Tomita S. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–396. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


