Abstract
Purpose
We previously identified interleukin-27 (IL-27) as a sepsis diagnostic biomarker in critically ill children. The current study tested the performance of IL-27 alone and in combination with PCT for diagnosing sepsis in critically ill adults.
Methods
Serum samples were made available from a prior prospective study of sepsis biomarkers in critically ill adults. The primary analysis used receiver operating characteristic (ROC) curves to evaluate the performance of IL-27 and PCT. Secondary analysis explored IL-27 performance in subgroups of patients with sepsis secondary to lung and non-lung sources of infection. The net reclassification improvement (NRI) was used to estimate the incremental predictive ability of IL-27 compared to PCT alone. Classification and Regression Tree (CART) analysis was used to generate an IL-27- and PCT-based decision tree.
Results
There were 145 patients with sepsis and 125 without sepsis. The ROC curve for IL-27 was inferior (area under the curve [AUC]: 0.68; 95% CI: 0.62 – 0.75) to that of PCT (AUC: 0.84; 95% CI: 0.79 – 0.89). Similar findings were observed when comparing patients with a lung source of infection and those without sepsis. For sepsis patients with a non-lung source of infection, adding IL-27 to PCT improved discrimination (NRI = 0.685; p < 0.001). The AUC for the CART-derived decision tree was 0.92 (95% CI: 0.88 – 0.96) and was significantly greater than that of PCT alone.
Conclusions
When used in combination with PCT, IL-27 may improve classification of critically ill adults with sepsis secondary to a non-lung source of infection.
Keywords: sepsis, diagnosis, biomarkers, decision tree, interleukin-27, procalcitonin
INTRODUCTION
There is an unmet need for diagnostic biomarkers of sepsis in critically ill patients [1–3]. Procalcitonin (PCT) is currently used as a sepsis diagnostic biomarker, but its performance in critically ill patients has been questioned [4]. Consequently, investigators continue to search for additional sepsis biomarkers that can enhance or complement the diagnostic test characteristics of PCT [5–8].
Using genome-wide expression analysis, we identified interleukin-27 (IL-27) as a candidate diagnostic gene for sepsis [9, 10]. IL-27 is a heterodimeric cytokine belonging to the IL-6 and IL-12 family of cytokines, and is produced by antigen presenting cells upon exposure to microbial products and inflammatory stimuli [11]. IL-27 is a T-cell regulator, having both pro- and anti-inflammatory effects [12, 13], and is rapidly induced in a murine model of septic peritonitis [14]. Furthermore, genetic ablation of an IL-27 subunit or neutralization of IL-27 via a soluble IL-27 receptor fusion protein is protective in a murine model of septic peritonitis [14]. Thus, it is biologically plausible that IL-27 can serve as a sepsis diagnostic biomarker.
Also plausible in the search for diagnostic biomarkers of sepsis is that different biomarkers are differentially produced depending of the source of infection [15]. This would naturally reflect the heterogeneity inherent to the complex syndrome of sepsis. Thus, it is important to consider biomarker performance in different subgroups of patients being evaluated for sepsis secondary to different potential sources of infection.
We have demonstrated that serum IL-27 protein concentrations can differentiate between critically ill children with sterile inflammation and those with laboratory-confirmed bacterial infections [10]. In addition, we demonstrated that combining both IL-27 and PCT more accurately identified critically ill children with and without bacterial infection, compared to either biomarker alone. In the current study, we test the ability of IL-27 alone and in combination with PCT to differentiate critically ill adults with and without sepsis. Secondarily, we explored whether the diagnostic accuracy of IL-27 was dependent on the source of infection.
METHODS
Study subjects
This retrospective diagnostic study used data from a biorepository generated during a prospective study investigating sepsis biomarkers in critically ill adults [5]. The enrollment procedures for the study have been previously described in detail [5]. Briefly, 300 consecutive patients admitted to the intensive care unit of the University Hospital of Nancy, France, were prospectively enrolled without any exclusion criteria. Adjudication of sepsis or no sepsis classifications was performed by duplicate review of medical records by investigators blinded to biomarker data; consensus was achieved in all cases. All serum samples used in the current study were drawn within 12 hours of admission. The original consent included provisions for secondary analyses of biological samples and clinical data, as approved by the Institutional Review Board of the University Hospital of Nancy, France.
Measurement of IL-27 serum protein concentrations
Serum IL-27 protein concentrations were measured for the current study using a magnetic bead multi-plex platform (EMD Millipore Corporation, Billerica, MA) and a Luminex® 100/200 System (Luminex Corporation, Austin, TX), according the manufacturers’ specifications. PCT concentrations were measured in the original study using an immunoassay with a sandwich technique and a chemiluminescent detection system (LumiTest; Brahms Diagnositica, Berlin, Germany).
Statistical Analysis
Initially, biomarker data are described using medians and interquartile ranges. Biomarker comparisons between groups used the Mann-Whitney U-test (SigmaStat Software, Systat Software, Inc., San Jose, CA). Receiver operating characteristic (ROC) curves and the respective area under the curve (AUC) were constructed and compared using SigmaStat Software. Classification and regression tree (CART) analysis was conducted using the Salford Predictive Modeler v6.6 (Salford Systems, San Diego, CA) [9, 16, 17]. Biomarker test characteristics are reported using diagnostic test statistics with 95% confidence intervals computed using the score method as implemented by VassarStats Website for Statistical Computation [18].
The net reclassification improvement (NRI) was used to estimate the incremental predictive ability of IL-27 compared to using PCT alone [19]. The NRI ranges between −2 and +2. A score of -2 indicates that all true positives are reclassified as false negatives and all true negatives are reclassified as false positives, and no false classifications are reclassified as true classifications. Conversely, when the score is 2, adding the information correctly reclassifies every case. The NRI was computed using the R-package Hmisc.
RESULTS
Primary Analysis
The clinical characteristics of the study subjects were previously published [5]. In the original cohort of 300 subjects, there were 154 with sepsis and 146 without sepsis. Remaining serum samples were available for the current study from 145 critically ill adults with sepsis and 125 without sepsis. Among the patients with sepsis, 87 (60%) had a lung source of infection. The next three most common sources of infection were the abdomen (n = 19 [13%]), the urinary tract (n = 11 [8%]), and the central nervous system (n = 8 [6%]). Forty-one sepsis patients (28%) had a documented infection secondary to a gram negative organism and 42 sepsis patients (29%) had a documented infection secondary to a gram positive organism. Fifty-eight sepsis patients (40%) had no organism identified, but met clinical criteria for sepsis. The remaining 4 sepsis patients had documented infection secondary to either a virus or an intracellular pathogen. Table 1 provides the median (IQR) IL-27 and PCT serum concentrations. IL-27 and PCT serum concentrations were greater in the subjects with sepsis, compared to the subjects without sepsis.
Table 1.
IL-27 and PCT concentrations.
| No Sepsis N = 125 |
All Subjects With Sepsis N = 145 |
Sepsis, Lung Source of Infection N = 87 |
Sepsis, Non-Lung Source of Infection N = 58 |
|
|---|---|---|---|---|
| Median ng/ml IL-27 (IQR) | 1.5 (1.0 – 2.3) | 2.5 (1.4 – 5.2)* | 2.0 (1.1 – 3.9)* | 3.7 (2.0 – 6.0)* |
| Median ng/ml PCT (IQR) | 0.3 (0.1 – 1.0) | 6.0 (1.2 – 25.2)* | 3.8 (1.0 – 19.6)* | 11.5 (2.0 – 41.4)* |
P < 0.001 vs. No Sepsis.
The AUC [95% CI] for the PCT ROC curve (0.840 [0.792 – 0.888]) was significantly greater than that of IL-27 (0.683 [0.620 –0.746], p <0.001). Tables 2A and 2B provide the diagnostic test characteristics for IL-27 and PCT at different cut points. PCT performed better than IL-27 as a sepsis diagnostic biomarker at all cut points.
Table 2.
| A: Diagnostic test characteristics of IL-27 at different cut points in all sepsis patients. | ||||||
|---|---|---|---|---|---|---|
| Cut point (≥) | Sensitivity | Specificity | PPV | NPV | +LR | −LR |
| 1 ng/ml | 88% (81 – 92) | 23% (16 – 32) | 57% (50 – 63) | 62% (46 – 75) | 1.1 (1.0 – 1.3) | 0.5 (0.3 – 0.9) |
| 2 ng/ml | 63% (54 – 71) | 62% (52 – 70) | 65% (57 – 73) | 59% (50 – 67) | 1.6 (1.3 – 2.1) | 0.6 (0.5 – 0.8) |
| 3 ng/ml | 43% (35 – 52) | 86% (79 – 92) | 79% (68 – 87) | 57% (49 – 64) | 3.2 (2.0 – 5.2) | 0.7 (0.6 – 0.8) |
| 4 ng/ml | 33% (26 – 41) | 92% (85 – 96) | 83% (70 – 91) | 54% (47 – 61) | 4.1 (2.2 – 7.8) | 0.7 (0.6 – 0.8) |
| 5 ng/ml | 26% (19 – 34) | 96% (90 – 99) | 88% (74 – 96) | 53% (46 – 59) | 6.6 (2.7 – 16.1) | 0.8 (0.7 – 0.8) |
| B: Diagnostic test characteristics of PCT at different cut points in all sepsis patients. | ||||||
|---|---|---|---|---|---|---|
| Cut point (≥) | Sensitivity | Specificity | PPV | NPV | +LR | −LR |
| 0.5 ng/ml | 88% (82 – 93) | 64% (55 – 72) | 74% (67 – 80) | 82% (73 – 89) | 2.5 (1.9 – 3.1) | 0.2 (0.1 – 0.3) |
| 1 ng/ml | 80% (72 – 86) | 74% (66 – 82) | 78% (71 – 85) | 76% (68 – 83) | 3.1 (2.3 – 4.3) | 0.3 (0.2 – 0.4) |
| 2 ng/ml | 67% (59 – 74) | 86% (79 – 92) | 85% (77 – 91) | 69% (61 – 76) | 4.9 (3.1 – 7.8) | 0.4 (0.3 – 0.5) |
| 3 ng/ml | 60% (52 – 68) | 90% (83 – 94) | 87% (78 – 93) | 66% (58 – 73) | 5.8 (3.4 – 9.8) | 0.4 (0.4 – 0.5) |
| 4 ng/ml | 57% (49 – 65) | 92% (85 – 96) | 89% (81 – 94) | 65% (57 – 72) | 7.2 (3.9 – 13.2) | 0.5 (0.4 – 0.6) |
Secondary Analysis
Because the lung was the most common source of infection, we conducted a secondary analysis comparing patients with a lung source of infection (n = 87) and patients with a non-lung source of infection (n = 58). Table 1 provides the median IL-27 and PCT serum concentrations in these two subgroups. IL-27 and PCT concentrations were higher in both sepsis subgroups, compared to the subjects without sepsis.
For differentiating between subjects with a lung source of infection and those without sepsis, the AUC for PCT was significantly greater than that for IL-27 (0.806 [0.743 – 0.868] vs. 0.617 [0.538 – 0.696], p < 0.001). For differentiating between those with a non-lung source of infection and those without sepsis, the AUC for PCT was also significantly greater than that for IL-27 (0.890 [0.836 – 0.944] vs. 0.783 [0.708 – 0.859], p = 0.02). However, the AUC for IL-27 in the sepsis subgroup with a non-lung source of infection was improved relative to that for other sepsis patients. Tables 3A and 3B provide the test characteristics for IL-27 and PCT at different cut points in the sepsis subgroup with a non-lung source of infection. Collectively, these secondary analyses suggest that IL-27 expression in sepsis may be dependent on the source of infection and may thus have diagnostic value in sepsis patients with a non-lung source of infection, even if not in patients with a lung source of infection.
Table 3.
| A: Diagnostic test characteristics of IL-27 at different cut points in sepsis patients with a non-lung source of infection. | ||||||
|---|---|---|---|---|---|---|
| Cut point (≥) | Sensitivity | Specificity | PPV | NPV | +LR | −LR |
| 1 ng/ml | 91% (80 – 97) | 23% (16 – 32) | 36% (28 – 44) | 85% (68 – 94) | 1.2 (1.1 – 1.3) | 0.4 (0.2 – 0.9) |
| 2 ng/ml | 79% (66 – 88) | 62% (52 – 70) | 49% (39 – 59) | 87% (77 – 92) | 2.1 (1.6 – 2.7) | 0.3 (0.2 – 0.6) |
| 3 ng/ml | 55% (42 – 68) | 86% (79 – 92) | 65% (50 – 78) | 81% (73 – 87) | 4.1 (2.5 – 6.7) | 0.5 (0.4 – 0.7) |
| 4 ng/ml | 47% (34 – 60) | 92% (85 – 96) | 73% (56 – 86) | 79% (71 – 85) | 5.8 (3.0 – 11.1) | 0.6 (0.5 – 0.7) |
| 5 ng/ml | 36% (24 – 50) | 96% (90 – 99) | 81% (60 – 93) | 76% (69 – 83) | 9.1 (3.6 – 22.8) | 0.7 (0.5 – 0.8) |
| B: Diagnostic test characteristics of PCT at different cut points in sepsis patients with a non-lung source of infection. | ||||||
|---|---|---|---|---|---|---|
| Cut point (≥) | Sensitivity | Specificity | PPV | NPV | +LR | −LR |
| 0.5 ng/ml | 93% (82 – 98) | 64% (55 – 72) | 55% (44 – 64) | 95% (88 – 98) | 2.6 (2.0 – 3.3) | 0.1 (0.0 – 0.3) |
| 1 ng/ml | 86% (74 – 93) | 74% (66 – 82) | 61% (50 – 71) | 92% (85 – 96) | 3.4 (2.5 – 4.6) | 0.2 (0.1 – 0.4) |
| 2 ng/ml | 76% (63 – 86) | 86% (79 – 92) | 72% (59 – 82) | 88% (81 – 93) | 5.6 (3.5 – 8.9) | 0.3 (0.2 – 0.4) |
| 3 ng/ml | 72% (59 – 83) | 90% (83 – 94) | 76% (63 – 86) | 76% (63 – 86) | 7.0 (4.1 – 11.9) | 0.3 (0.2 – 0.5) |
| 4 ng/ml | 69% (55 – 80) | 92% (85 – 96) | 80% (66 – 89) | 86% (79 – 92) | 8.6 (4.6 – 16.0) | 0.3 (0.2 – 0.5) |
Combining IL-27 and PCT
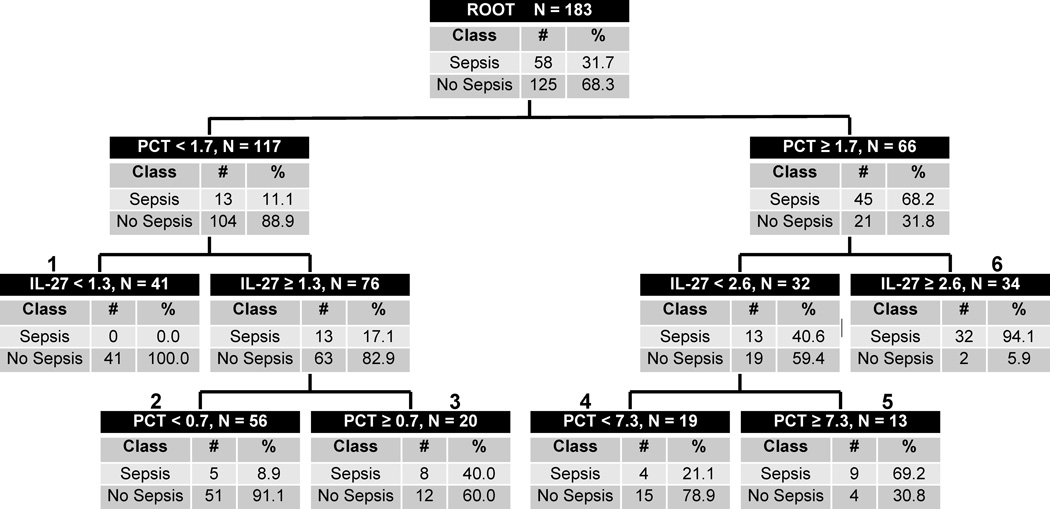
To assess further IL-27 as a sepsis diagnostic biomarker in critically ill patients with a non-lung source of infection, we derived a decision tree incorporating both IL-27 and PCT. Figure 1 shows the derived decision tree, consisting of a very low sepsis probability terminal node (terminal node 1), two high sepsis probability terminal nodes (terminal nodes 5 and 6), and three intermediate sepsis probability nodes (nodes 2 to 4). Of the 41 cases in the very low sepsis probability node, none (0%) had sepsis. Of the 47 cases in the high sepsis probability nodes, 41 (87%) had sepsis. The proportion with sepsis in the remaining terminal nodes varied from about 9% to about 40%. The diagnostic test characteristics of the decision tree are as follows (95% C.I.): sensitivity of 85% (72 – 92); specificity of 86% (78 – 91); positive predictive value of 73% (61 – 83); negative predictive value of 92% (85 – 96), positive likelihood ratio of 5.9 (3.8 – 9.1); and negative likelihood ratio of 0.2 (0.1 – 0.3).
Figure 1.
The CART-derived decision tree for sepsis diagnosis in patients with a non-lung source of infection, based on IL-27 and PCT. Each node provides the total number of subjects in the node, the IL-27 or PCT serum concentration-based decision rule, and the number of patients with and without sepsis, with the respective rates. Terminal nodes 1, 2, and 4 are considered low sepsis probability nodes, whereas terminal nodes 3, 5, and 6 are considered high sepsis probability nodes. To calculate the diagnostic test characteristics, all subjects in the low probability terminal nodes (n = 116) were classified as predicted no sepsis, whereas all subjects in the high probability terminal nodes (n = 67) were classified as predicted sepsis.
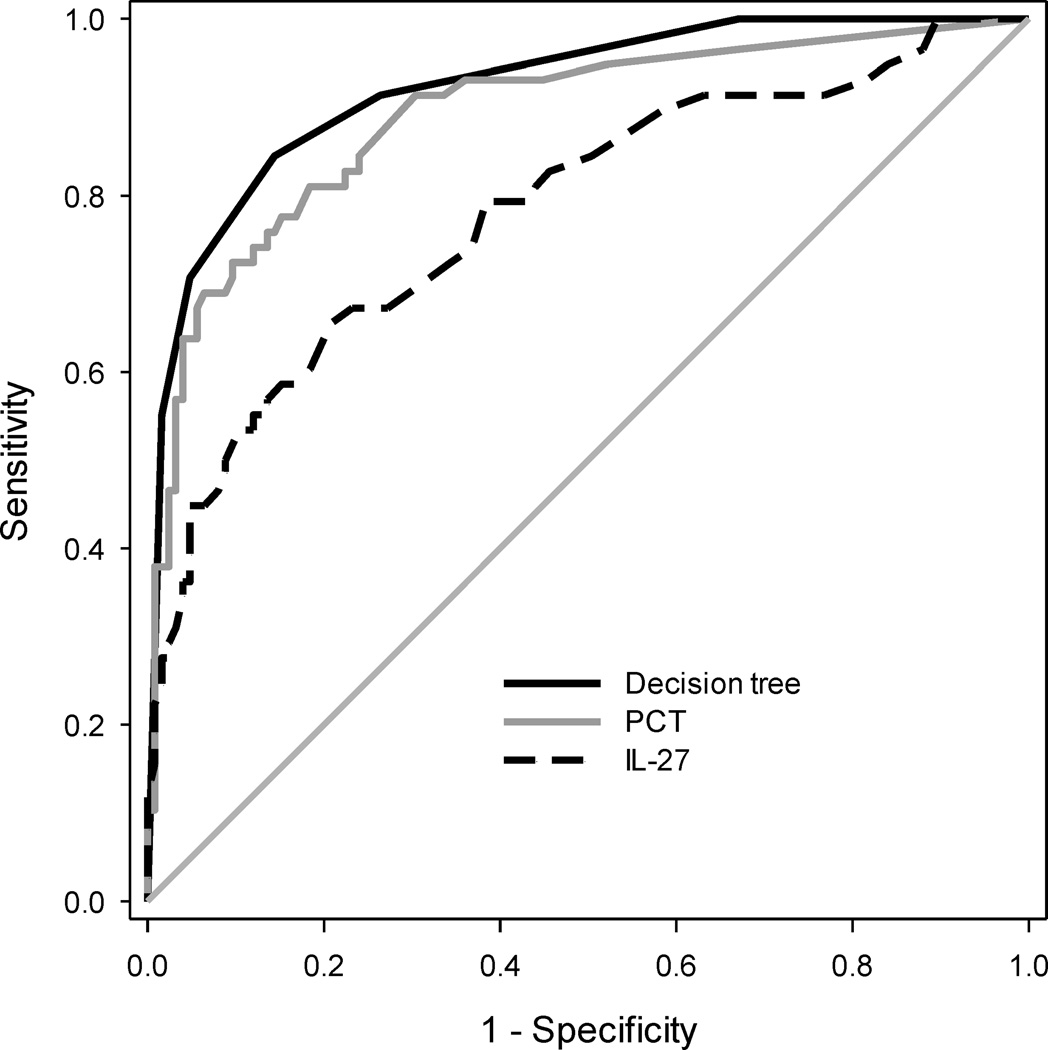
Figure 2 shows the ROC curves for the decision tree, PCT alone, and IL27 alone in the sepsis patients with a non-lung source of infection. The AUC of the decision tree (0.92 [0.88 – 0.96]) was significantly greater than that of PCT (p = 0.02) and IL-27 alone (p < 0.001). Further, when adding the IL-27 data to the PCT data, the NRI was 0.69 (0.37 – 1.00; p < 0.001). This suggests that in critically ill patients with sepsis secondary to a non-lung source of infection, IL-27 may add diagnostic information beyond that provided by PCT alone.
Figure 2.
ROC curves in sepsis patients with a non-lung source of infection for the decision tree, PCT, and IL-27. The respective AUCs with 95% C.I. were: 0.923 (0.883 – 0.963), 0.890 (0.836 – 0.944), and 0.783 (0.708 – 0.859). P = 0.02, decision tree versus PCT alone.
DISCUSSION
This study represents the first test of IL-27 as a sepsis diagnostic biomarker in critically ill adults. In both the overall sepsis cohort and in the sepsis subgroup with a lung source of infection, the AUC for IL-27 was below 0.7 and the diagnostic test characteristics of IL-27 were inferior to that of PCT. When differentiating between a non-lung source of infection and those without sepsis, however, the AUC for IL-27 approached 0.8. Although the diagnostic test characteristics of IL-27 were also inferior to that of PCT in this subgroup, a decision tree incorporating both IL-27 and PCT suggested an improvement of the overall diagnostic accuracy relative to PCT alone. Compared to PCT alone, when a low IL-27 was measured in conjunction with a low PCT, the negative predictive value for sepsis was correctly increased, and when a high IL-27 was added to a high PCT, the positive predictive value for sepsis was correctly increased. Further support that adding IL-27 to PCT improved discrimination is provided by the NRI. In particular, when differentiating between sepsis patients with a non-lung source of infection and patients without sepsis, a low IL-27 helped to identify more reliably the patients without sepsis when compared to PCT alone. We do note that the NRI has been criticized as having the potential to inflate the incremental prognostic impact of a new biomarker when used in isolation [20]. The NRI is this study, however, was consistent with changes in traditional diagnostic test statistics, including the AUC.
The decision tree based on IL-27 and PCT has potential to provide a clinically relevant sepsis probability range, which is otherwise not captured by a single biomarker with a single cut point yielding a dichotomous risk estimate for sepsis. For example, patients in terminal node 1 have extremely low probability for sepsis (0.0%), whereas patients in terminal node 6 have extremely high probability for sepsis (94.1%), thus potentially allowing for biomarker data to directly inform clinical decision making. Alternatively, patients in the remaining terminal nodes have variable, intermediate probabilities for sepsis, thus requiring interpretation and integration of biomarker data with the clinical context for decision-making. These assertions require prospective validation.
Our results contrast with our prior study involving critically ill children that demonstrated IL-27 was not only additive, but also outperformed PCT with a specificity and positive predictive value for sepsis of >90% [9]. Several factors may account for the differences between the pediatric and adult studies. Differences in sample storage conditions could affect the stability of IL-27, and therefore the measurement of IL-27 between the two studies. It is also possible that the IL-27 response of children is different than that of adults, as there are clinical and experimental data demonstrating significantly different responses to inflammatory challenges between developing, pediatric hosts and mature, adult hosts [21–24]. We are not aware of any existing data demonstrating a developmental influence on IL-27 expression during infection and so the potential relationship between developmental age and IL-27 expression is worthy of further investigation. Ultimately, IL-27 may prove to be a more effective sepsis diagnostic biomarker in children than in adults.
It is possible that differences in enrollment criteria for the pediatric and adult cohorts may account for the observed differences in the performance of IL-27 between these two groups. Pediatric patients were required to meet criteria for systemic inflammatory response syndrome (SIRS) and were classified as having sepsis based on laboratory confirmation of a positive culture for known bacterial pathogens, and the majority of these positive cultures were from the blood compartment [9, 25, 26]. In contrast, the adult cohort did not require meeting criteria for SIRS [5]. Patients in the adult cohort were enrolled consecutively, upon admission to the intensive care unit, irrespective of SIRS criteria, and were subsequently classified as having sepsis based on laboratory and clinical criteria. In addition, a majority of the patients in the adult cohort had a primary lung source of infection. This is an important limitation of our study because it may not be representative of all critically ill populations. For example, it is possible that surgical patients or patients suffering from major trauma may have a lower prevalence of lung infections. Thus, while the pediatric and adult cohorts are both clinically representative, they also reflect different clinical contexts that could influence biomarker performance. This further supports our contention that different biomarkers may have more or less utility in different populations with this highly heterogeneous condition.
In post hoc analyses, we noted that the AUC for IL-27 was 0.768 in subjects with sepsis secondary to a gram negative organism, whereas the AUC was 0.639 in subjects with sepsis secondary to a gram positive organism. Thus, future studies of IL-27 as a sepsis diagnostic biomarker should consider the bacterial etiology of sepsis. In addition, future studies may also consider the ability of IL-27 to discriminate between different levels of sepsis severity.
In conclusion, as a general sepsis diagnostic biomarker, IL-27 may not be as effective in critically ill adults as in critically ill children. However, in critically ill adults with sepsis secondary to a non-lung source of infection, IL-27 may add to the sepsis diagnostic accuracy of PCT. Further study of IL-27 as a candidate sepsis biomarker is warranted.
ACKNOWLEDGEMENTS
Supported by National Institutes of Health Grants RC1HL100474, RO1GM064619, and R01GM099773. Supported in part by an Institutional Clinical and Translational Science Award, NIH/NCRR 5UL1RR026314.
The authors thank the investigators that took part in the original prospective study that generated the database used in the current study.
Hector R. Wong: Dr. Wong and the Cincinnati Children’s Hospital Research Foundation have submitted a provisional patent application for the use of IL-27 as a sepsis diagnostic biomarker.
ABBREVIATIONS
- PCT
procalcitonin
- IL-27
interleukin-27
- ROC
receiver operating characteristic
- AUC
area under the curve
- CART
classification and regression tree
- NRI
net reclassification improvement
- IQR
interquartile range
- PPV
positive predictive value
- NPV
negative predictive value
- +LR
positive likelihood ratio
- −LR
negative likelihood ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
HRW conceived and developed the study, obtained funding for the study, directly took part in the analyses, and wrote the manuscript. CJL collaborated in the initial design of the study and in obtaining funding, oversaw the statistical analyses, and edited the manuscript. PL conducted all biomarker measurements, managed all biological specimens, and edited the manuscript. KWH assisted with statistical analysis and edited the manuscript. SG was the lead investigator for the original study that generated the prospective database used in the current study, assisted with data analysis, and edited the manuscript. All authors read and approved the final manuscript.
AUTHOR COMPETING INTERESTS
The remaining authors have no competing interests to report.
REFERENCES
- 1.Marshall JC, Reinhart K. Biomarkers of sepsis. Crit Care Med. 2009;37(7):2290–2298. doi: 10.1097/CCM.0b013e3181a02afc. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan JM, Wong HR. Biomarker discovery and development in pediatric critical care medicine. Pediatr Crit Care Med. 2011;12(2):165–173. doi: 10.1097/PCC.0b013e3181e28876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Standage SW, Wong HR. Biomarkers for pediatric sepsis and septic shock. Expert Rev Anti Infect Ther. 2011;9(1):71–79. doi: 10.1586/eri.10.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. The Lancet Infect Dis. 2007;7(3):210–217. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 5.Gibot S, Bene MC, Noel R, Massin F, Guy J, Cravoisy A, Barraud D, De Carvalho Bittencourt M, Quenot JP, Bollaert PE, et al. Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med. 2012;186(1):65–71. doi: 10.1164/rccm.201201-0037OC. [DOI] [PubMed] [Google Scholar]
- 6.Wang HJ, Zhang PJ, Chen WJ, Jie D, Dan F, Jia YH, Xie LX. Characterization and Identification of novel serum microRNAs in sepsis patients with different outcomes. Shock. 2013;39(6):480–487. doi: 10.1097/SHK.0b013e3182940cb8. [DOI] [PubMed] [Google Scholar]
- 7.Rivers EP, Jaehne AK, Nguyen HB, Papamatheakis DG, Singer D, Yang JJ, Brown S, Klausner H. Early biomarker activity in severe sepsis and septic shock and a contemporary review of immunotherapy trials: not a time to give up, but to give it earlier. Shock. 2013;39(2):127–137. doi: 10.1097/SHK.0b013e31827dafa7. [DOI] [PubMed] [Google Scholar]
- 8.Monaghan SF, Thakkar RK, Tran ML, Huang X, Cioffi WG, Ayala A, Heffernan DS. Programmed death 1 expression as a marker for immune and physiological dysfunction in the critically ill surgical patient. Shock. 2012;38(2):117–122. doi: 10.1097/SHK.0b013e31825de6a3. [DOI] [PubMed] [Google Scholar]
- 9.Wong HR, Cvijanovich NZ, Hall M, Allen GL, Thomas NJ, Freishtat RJ, Anas N, Meyer K, Checchia PA, Lin R, et al. Interleukin-27 is a novel candidate diagnostic biomarker for bacterial infection in critically ill children. Crit Care. 2012;16(5):R213. doi: 10.1186/cc11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scicluna BP, van der Poll T. Interleukin-27: a potential new sepsis biomarker exposed through genome-wide transcriptional profiling. Crit Care. 2012;16(6):188. doi: 10.1186/cc11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojno ED, Hunter CA. New directions in the basic and translational biology of interleukin-27. Trends Immunol. 2012;33(2):91–97. doi: 10.1016/j.it.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16(6):779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 13.Villarino AV, Larkin J, 3rd, Saris CJ, Caton AJ, Lucas S, Wong T, de Sauvage FJ, Hunter CA. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174(12):7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- 14.Wirtz S, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203(8):1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med. 2011;9:107. doi: 10.1186/1741-7015-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller R, Mockel M. Logistic regression and CART in the analysis of multimarker studies. Clin Chim Acta. 2008;394(1–2):1–6. doi: 10.1016/j.cca.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Wong HR, Salibury S, Xiao Q, Cvijanovich NZ, Hall M, Allen GL, Thomas NJ, Freishtat RJ, Anas N, Meyer K, et al. The pediatric sepsis biomarker risk model. Crit Care. 2012;16(5):R174. doi: 10.1186/cc11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Computation VWfS: http://faculty.vassar.edu/lowry/VassarStats.html [Google Scholar]
- 19.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 20.Hilden J, Gerds TA. A note on the evaluation of novel biomarkers: do not rely on integrated discrimination improvement and net reclassification index. Stat Med. 2013 doi: 10.1002/sim.5804. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125(5):1031–1041. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol. 2010;37(2):439–479. doi: 10.1016/j.clp.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wynn JL, Cvijanovich NZ, Allen GL, Thomas NJ, Freishtat RJ, Anas N, Meyer K, Checchia PA, Lin R, Shanley TP, et al. The influence of developmental age on the early transcriptomic response of children with septic shock. Mol Med. 2011;17(11–12):1146–1156. doi: 10.2119/molmed.2011.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, Moldawer LL. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112(5):1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong HR, Shanley TP, Sakthivel B, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, Kalyanaraman M, Tofil NM, et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics. 2007;30(2):146–155. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong HR, Cvijanovich N, Allen GL, Lin R, Anas N, Meyer K, Freishtat RJ, Monaco M, Odoms K, Sakthivel B, et al. Genomic expression profiling across the pediatric systemic inflammatory response syndrome, sepsis, and septic shock spectrum. Crit Care Med. 2009;37(5):1558–1566. doi: 10.1097/CCM.0b013e31819fcc08. [DOI] [PMC free article] [PubMed] [Google Scholar]