Abstract
High choline kinase-alpha(Chk-α) expression is frequently observed in cancer cells, making it a novel target for pharmacological and molecular inhibition. Since inhibiting agents are delivered systemically it is important to determine Chk-α expression levels in endothelial cells that line both normal and tumor vasculature, and the effect of Chk-α downregulationon these cells. Here we characterized Chk-α expression and the effect of its downregulation in human umbilical vein endothelial cells (HUVEC) compared to MDA-MB-231 human breast cancer cells. We used siRNA to downregulate Chk-α expression. Basal mRNA levels of Chk-α were approximately three fold lower in HUVEC compared to MDA-MB-231 breast cancer cells. Consistent with the differences in Chk-α protein levels, phosphocholine levels were approximately 10 fold lower in HUVEC than in MDA-MB-231 cells. Transient transfection with siRNA-Chk resulted in comparable levels of mRNA and protein in MDA-MB-231 breast cancer cells and HUVEC. However there was a significant reduction of proliferation in MDA-MB-231 cells but not in HUVEC. No significant difference in CD31 immunostaining was observed in tumor sections obtained from mice injected with control luciferase-short hairpin (sh)RNA or Chk-shRNA lentivirus. These data suggest that systemically delivered agents that downregulate Chk-α in tumors will not affect endothelial cell proliferation during delivery, and further support the development of Chk-α downregulation as a cancer-specific treatment.
Keywords: breast cancer, endothelial cells, choline kinase, MR spectroscopy, phosphocholine, proliferation, angiogenesis
Introduction
Phosphatidylcholine (PtdCho), the major phospholipid component of mammalian cells, plays critical roles in membrane structure and provides the substrate to produce lipid second messengers such as phosphatidic acid and diacylglycerol. In PtdCho biosynthesis (Kennedy pathway), choline kinase (Chk) is the initial enzyme that catalyses the phosphorylation of choline (Cho) to yield phosphocholine (PC) in the presence of ATP and magnesium(1, 2).The increase of cellular PC and total choline-containing compounds is one of the most widely established characteristics of cancers including breast, ovaries, prostate, lung, and bladder cancers (1, 3–8). This elevation is closely related to malignant transformation, invasion, and metastasis and is due, in large part, to increased expression of Chk (5, 9).Chk-α is overexpressed at the mRNA level in ovarian, breast and bladder cancer cell lines and is closely associated with tumor progression and invasiveness(7, 8).Chk is required for the activation of phosphatidylinositol 3-kinase (PI3K)/AKT and mitogen-activated protein kinase (MAPK) signaling pathways since PC serves as a vital metabolic reservoir for the production of PtdCho(11).
Because of these findings Chk-α is being considered as a novel target in cancer treatment. Targeting Chk-α can provide treatment strategies for selective therapy to use either singly or in combination with conventional chemotherapeutic agents, since Chk-α is usually up-regulated in cancer cells but not in normal cells(12, 13). Chk inhibition using the pharmacologic inhibitor MN58b was found to reduce proliferation of cancer cells in vitro and xenografts in vivo(14–18). We have used small interfering RNA (siRNA) and short hairpin RNA (shRNA)to target Chk-α (siRNA-Chk and Chk-shRNA) and showed that both transient transfection and stable expression of siRNA-Chk and Chk-shRNA, significantly reduced proliferation in breast cancer cells (12) and tumors (19). Treatment with a combination of siRNA-Chk and the commonly used anticancer drug 5-fluorouracil (5–FU) resulted in a larger reduction of cell viability/proliferation than treatment with 5-FU alone in breast cancer cell lines(20). Downregulation of Chk-α in nonmalignant immortalized MCF-12A human epithelial cells resulted in an almost negligible effect on PC and proliferation (20).
Here, for the first time, we have investigated the expression of Chk-α in human umbilical vein endothelial cells (HUVEC) to further validate the specificity of Chk-α increase in cancer cells. Endothelial cells are a key component of vasculature and are exposed to agents that are delivered systemically. We have investigated the effects of siRNA-Chk on endothelial cell proliferation to determine if Chk-α downregulation may damage systemic vasculature. We have examined changes in proliferation and PC levels of human umbilical vein endothelial cells (HUVEC) following transient siRNA-Chk transfection, and compared the results with siRNA-Chk transfected human breast cancer cells (MDA-MB-231). Downregulating Chk-α mRNA to comparable levels in both cell linesdid not affect endothelial cell proliferation,in contrast to the significant decrease of proliferation observed in breast cancer cells. Since tumor vasculature plays an important role in tumor growth and metastasis (21–23), it was also important to determine the contribution of endothelial cell effects on reduced tumor growth observed following transduction of Chk-shRNA following tail vein injection of lentiviral vectors(19). We therefore performed immunostaining of the endothelial cell marker CD31 in tumor sections obtained from mice from injected with either control luciferase (Luc)-shRNA or Chk-shRNA virus. There was no difference in CD31 density between the control and Chk-α targeted tumors. These data confirm the specificity of Chk-α expression in cancer cells, and within the confines of the current study, the absence of effects on endothelial cell proliferation or endothelial cell density following Chk-α downregulation.
Experimental details
Cell culture and siRNA transfection
MDA-MB-231 human breast cancer cells were purchased from American Type Culture Collection (ATCC, Manassas, VA) and were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS).HUVEC were obtained from VEC technologies, Inc. (Rensselaer, NY) and maintained in MCDB-131 complete medium (VEC technologies, Inc.). Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 in air. HUVEC used in all the experiments were passaged for less than 30 days.
RNA interference experiments
siRNA targeting Chk-α (siRNA-Chk) was designed with the sequence 5’-CAUGCUGUUCCAGUGCUCC-3’ and purchased as a duplex from Thermo Fisher scientific Inc. (Lafayette, CO). DharmaFECT (D-FECT; Thermo Fisher scientific Inc. Lafayette, CO) was utilized for transient transfection as per the manufacturer’s instructions. Briefly, cells were cultured for 24 h in antibiotic free culture media and transiently transfected with 100 nM siRNA using D-FECT for 48 h.
mRNA quantification
Total RNA was isolated from cells at 48 hours post transfection using a Qiagen kit (Qiagen Inc. Valencia, CA). Quantitative real-time PCR (q-RT-PCR) was performed using iQ SYBR Green Supermix and gene-specific primers in the iCycler real-time PCR detection system (Bio-Rad, Hercules, CA), with cDNA which was synthesized using qScript (Quanta Bioscience, Gaithersburg, MD). All primers were designed using Beacon designer software7.6. The expression of RNA for Chk-α relative to the house keeping gene, hypoxanthine phosphoribosyltransferase-1 (HPRT-1), was calculated based on the threshold cycle (Ct) as R=2−Δ(ΔCt, where ΔCt= Ct of Chk-α- Ct of HPRT-1 and Δ(ΔCt)=ΔCt of Chk-α silenced sample - ΔCt control sample. Fold difference in Chk-α expression between MDA-MB-231 and HUVEC was obtained by comparing their respective ΔCtvalues under control conditions. Values expressed are from three or four independent experiments.
Immunoblot analysis
Total protein from cells was extracted at 48 h post-transfection using RIPA buffer [50 mM Tris, pH 7.4,150 mM NaCl, 1 mM EDTA, 1% Triton-100, 1% sodium deoxycholate, 0.1% SDS,1 mM phenylmethylsulfonyl fluoride (PMSF), sodium orthovanadate (1mM), sodium fluoride (1mM), dithiothreitol (1mM)], and a protease inhibitor cocktail at 1:200 dilution (Sigma-Aldrich, St. Louis, MO). Cell lysates were kept on ice for 30 minutes, and then centrifuged at 13,000 rpm for 30 minutes at 4 °C. Protein concentrations were estimated using the Bio-Rad DC assay (Bio-Rad, Hercules, CA.). SDS-PAGE was run on a 7.5% reducing gel loaded with 50 µg of total protein from samples and the proteins were transferred to a nitrocellulose membrane (Bio-Rad) overnight at 4 °C. After blocking in 5% milk-TBST (TBS Tween), the membrane was separately probed with a custom-made polyclonal antibody against Chk-α (Proteintech Group, Inc., Chicago, IL) as previously described (12). Mouse monoclonal antibody against GAPDH (Sigma-Aldrich, St. Louis, MO) was used as a loading control. Secondary antibodies used were horseradish peroxidase conjugated anti-mouse and anti-rabbit IgG (GE Healthcare UK Limited, Little Chalfont, Buckinghamshire). Reactions were recorded on Blue Bio film (Denville Scientific, Metuchen, NJ) following use of Super Signal West Pico Substrate (Pierce Biotech. Rockford, IL). The films were scanned and densitometry was done using the Gel-analysis-method in ImageJ (NIH, Bethesda, MD). Chk-α was detected at approximately 48 kD.
Cell extraction and magnetic resonance spectroscopy (MRS)
Cells were cultured for 24 h in antibiotic-free culture medium and transfected with or without 100 nM siRNA-Chk for 48 h using D-FECT. Adherent cells were collected by trypsinization and counted using a hemacytometer after staining with trypan blue. More than 1x107 cells were used for cell extraction. Water-soluble as well as lipid extracts were obtained from untreated control, D-FECT only, and siRNA-Chk-treated cells using the dual-phase extraction method(12). Briefly, the pelleted cells were mixed with 4mL of ice-cold methanol and vigorously vortexed. After keeping samples on ice for 15 minutes, 4 mL of chloroform were added, vortexed vigorously and kept on ice for 10 minutes. Finally, 4 mL of water were added and shaken well. All operations were performed on ice and samples were stored at 4 °C overnight for phase separation and later centrifuged at about 20,000×g at 4 °C for 30 minutes. The water/methanol phase containing water-soluble cellular metabolites such as choline (Cho), PC and glycerophosphocholine (GPC) was treated with ~100 mg of chelex beads (Sigma-Aldrich, St. Louis, MO) to remove any divalent cations. After removing the beads, methanol was evaporated using a rotary evaporator. The remaining water phase was lyophilized. Cell extracts were resuspended in 0.6 mL of deuterated water (D2O) containing 2.4×10−7 mol of 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid (TSP; Sigma-Aldrich, St. Louis, MO) as an internal standard for MR spectral analysis. Fully relaxed 1H MR spectra of water-soluble extracts were acquired on a Bruker Avance 11.7T spectrometer (Bruker BioSpin Corp., Billerica, MA) with flip angle = 30°, sweep width = 10,000 Hz, repetition time = 11.2 seconds, block size = 32,766, and scans = 128.
Signal integrals of N-(CH33 of PC at ~3.226 ppm were determined and normalized to cell numbers and compared to the standard. To determine concentrations of cell samples, peak integration (IPC) from 1H spectra for PC were compared to that of the internal standard TSP according to the equation:
| equation (1) |
In this equation, [PC] represents the intracellular concentration of PC expressed as mmol/L (mM). ATSP is the number of moles of TSP in the sample, N is the cell number, and V is cell volume (MDA-MB-231: 2,050 µm3and HUVEC: 4,530 µm3). Because the number of protons contributing to the signal of PC at ~3.226ppm and the TSP peak at 0 ppm is the same, correction for differences in the number of protons was not required. To determine the cell volume, cell size was determined by trypsinizing the cells and measuring the diameter (d) of 100 randomly selected cells using an optical microscope and calculated as [(4π/3)x(d/2)3]. The resulting PC concentrations were averaged for three or four independent experiments.
Proliferation assay
Approximately 4000 MDA-MB-231 cells and 5000 HUVEC were seeded in each well of a 96 well plate and cultured overnight. Twenty-four hours later, 100nM siRNA was transfected transiently using D-FECT for 48 h, changed to fresh culture medium and cultured for another 3 days. MTS assays (Promega Corp. Madison, WI) were performed as per manufacturer’s protocol. Untreated control, D-FECT treatment without siRNA and non-targeting siRNA (Thermo Fisher scientific Inc. Lafayette, CO) were used as negative controls and values were compared to untreated values.
Immunohistochemistry
Establishment of MDA-MB-231 human breast cancer xenografts, lentivirus shRNA vector, production of lentivirus particles and in vivo transduction of lentivirus has been previously reported (19). All surgical procedures and animal handling were performed in accordance with protocols approved by the Johns Hopkins University Institutional Animal Care and Use Committee, and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes for Health. Formalin-fixed, 5µm thick tumor sections obtained from five animals in the Luc-shRNA group and four animals in the Chk-shRNA group, respectively, were stained for CD31using rat monoclonal CD31 antibody (PECAM), which specifically recognizes mouse CD31 (DIA 310, clone SZ31, Rat IgG2A, Dianova, Hamburg, Germany) at a dilution of 1:30 overnight at 4°C. Following this treatment, sections were incubated with rat specific secondary antibody conjugated with horseradish peroxidase (Vector Laboratories, Burlingame, CA, USA) for 30 minutes before developing color with 3,3’-diaminobenzidine. After each incubation, sections were washed three times with phosphate-buffered saline with 0.2% tween (PBST) for 10 s each. All sections were counterstained with hematoxylin (Vector Laboratories, Burlingame, CA, USA) for one minute followed by a water wash and mounting with DAKO faramount aqueous mounting solution (DAKO, Carpinteria, CA, USA). Photomicrographs were taken on an Olympus microscope (PA, USA) equipped with a CCD camera.
Digital imaging and quantitative image analysis
Images of tumor sections stained for CD31 from the Luc-shRNA virus injected mice (n=5) and Chk-shRNA virus injected mice (n=4) were digitally captured using the Aperio ScanScope XT slide scanner (Aperio Technologies, Vista, CA, USA). Images were further analyzed and membrane staining quantified using manufacturer supplied software and immunohistochemisty algorithms (Aperio Technologies). These algorithms are based on the spectral differentiation between brown (positive) and blue (counter) staining. The algorithms also calculate total percentage positivity for membrane staining for the individual cells in the selected regions, and quantify the intensity and completeness of the membrane staining. Cells were individually classified as 0, 1+, 2+ and 3+ based on their membrane staining intensity. Percentage positive 3+ staining was calculated relative to the total number of nuclei, and values were expressed as mean ± standard deviation.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). The significance of differences in PC levels, MTS assay, and CD31 immunostaining, between the control and treated groups were evaluated using a one-tailed Student’s t-test. P values of < 0.05 were considered significant.
Results
Chk-α mRNA and protein levels following siRNA-Chk transfection
Quantitative real-time PCR (q-RT-PCR) experiments were performed to determine the efficacy of transient siRNA-Chk transfection in MDA-MB-231 cells and HUVEC. The ΔCt value (differences between the cycle threshold of Chk and housekeeping gene HPRT-1) was high in HUVEC, corresponding to low Chk mRNA levels (Figure 1). The differences in the ΔCt values of HUVEC and MDA-MB-231 (2.40±0.11 vs 0.93±0.60) clearly indicated the low basal expression of Chk mRNA in HUVEC. Upon transfection with siRNA-Chk, the ΔCt values for HUVEC were 2.40±0.11 vs 2.76±0.41, which was not significant. However, the ΔCt value for MDA-MB-231 showed a statistically significant increase (P = 0.004) from 0.93±0.60 in control to 2.65±0.17 in siRNA-Chk transfected cells indicating a decrease in Chk mRNA levels. The ΔCt values of siRNA-Chk transfected HUVEC and MDA-MB-231 cells were comparable indicating similar levels of Chk mRNA expression. A 68.3 ± 10.8% reduction of Chk-α mRNA levels was observed in MDA-MB-231 cells treated with 100 nM siRNA-Chk compared to untreated cells. In HUVEC, a 21.5 ± 23.7% reduction was observed after treatment with siRNA-Chk.
Figure 1.
The difference of cycle number required by
q-RT-PCR∆Ct) for Chk-α mRNA in MDA-MB-231 breast
cancer cells ( , n=4) and HUVEC (
, n=4) and HUVEC ( ,
n=3) compared to the mRNA levels of the housekeeping gene hypoxanthine
phosphoribosyltransferase-1 (HPRT-1). Samples of mRNA were prepared at 48 h
after treatment. Columns are mean, and bars are SD.
,
n=3) compared to the mRNA levels of the housekeeping gene hypoxanthine
phosphoribosyltransferase-1 (HPRT-1). Samples of mRNA were prepared at 48 h
after treatment. Columns are mean, and bars are SD.
** represents P< 0.01 against untreated or D-FECT control.
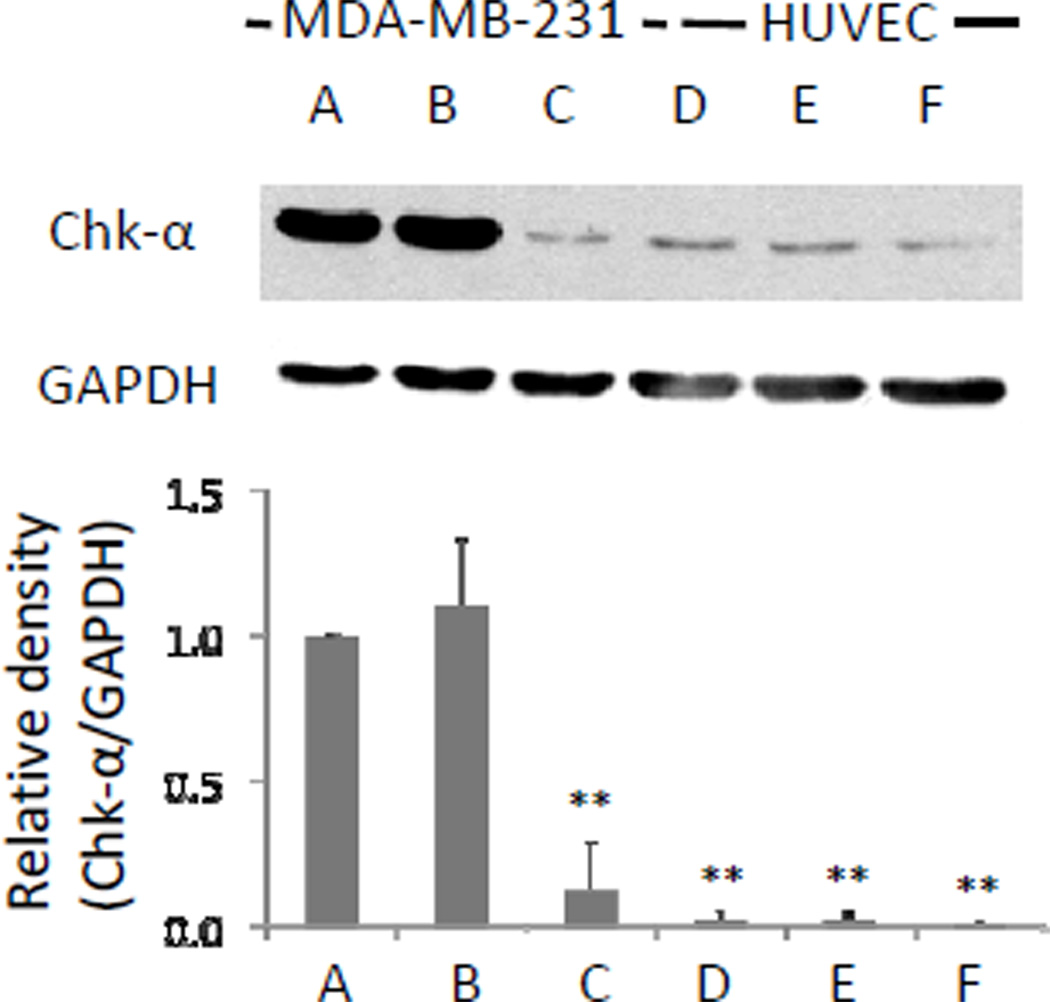
Basal Chk-α protein levels in HUVEC were significantly lower compared to MDA-MB-231 cells as identified in immunoblot assays with the Chk-α antibody (Figure 2). A significant reduction of Chk-α protein expression levels in MDA-MB-231 cells was observed after transient siRNA-Chk transfection compared to untreated cells and D-FECT treated cells (P<0.01). HUVEC showed downregulation to a lesser extent following transfection with siRNA-Chk that was not significant compared to untreated cells and D-FECT treated cells. The levels of Chk-α protein in MDA-MB-231 cells and HUVEC were not significantly different after transfection with siRNA-Chk (Figure 2).
Figure 2.
Chk-α protein expression levels in (A) untreated control, (B) D-FECT treated, and (C) 100nM siRNA-ChktransfectedMDA-MB-231 breast cancer cells;(D) untreated control, (E) D-FECT treated, and (F) 100nM siRNA-Chk transfected HUVEC. Cell lysates were prepared at 48 h after treatment. 50 µg of protein was loaded on 7.5% reducing gel. GAPDH levels were determined as loading controls. Relative density changes (Chk-α/GAPDH) in Chk-α were normalized to the Chk-α immunoreactive band in untreated MDA-MB-231 breast cancer cells (A), which was set to 1.Columns are mean, and bars are SD. ** represents P < 0.01 against untreated control and D-FECT control in MDA-MB-231, n=3 in each group.
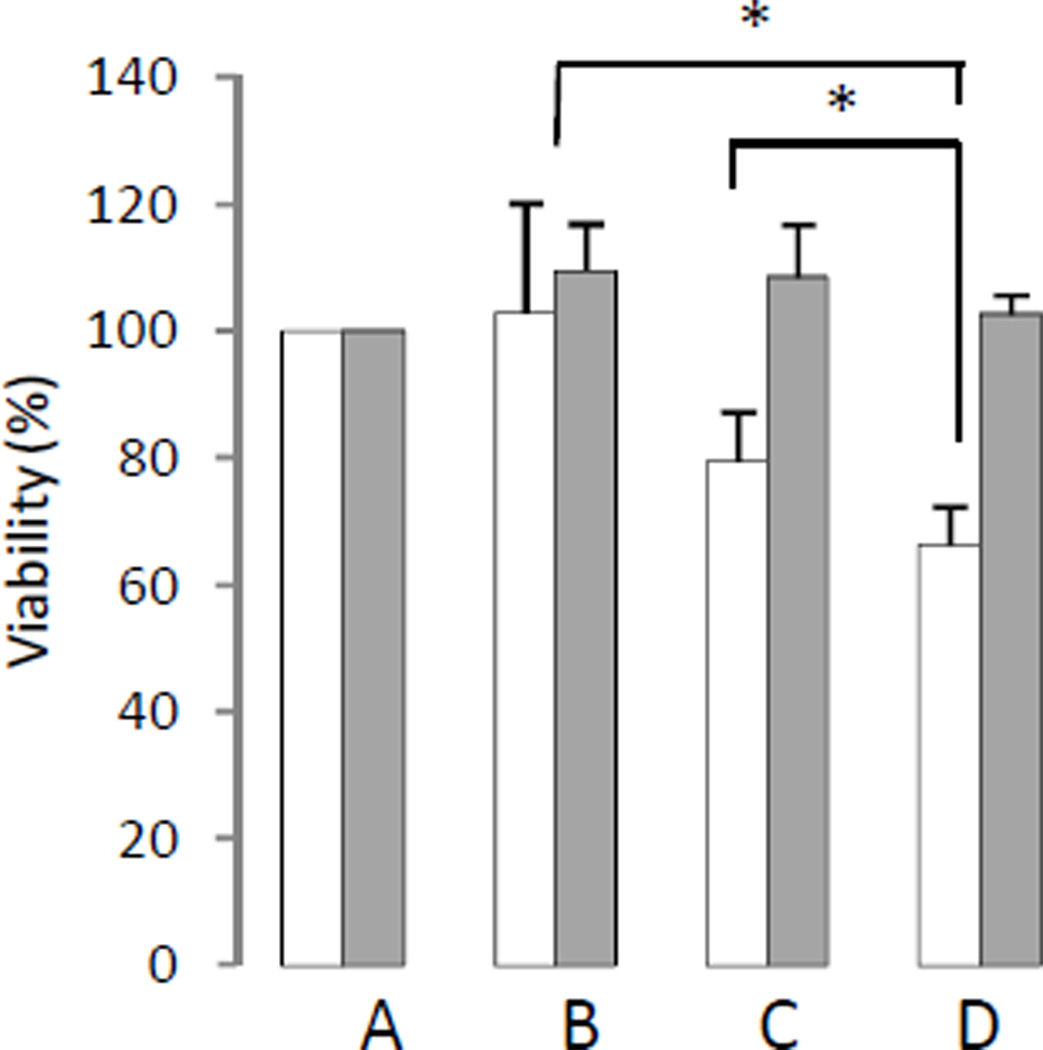
Cell viability/proliferation assay safter transient siRNA-Chk transfection of MDA-MB-231 and HUVEC
MDA-MB-231 cells showed a significant reduction of viability/proliferation following transient transfection using siRNA-Chk compared to D-FECT (P = 0.027) and non-targeting siRNA treated cells(P = 0.042) (Figure 3). In contrast, there was no reduction of viability/proliferation in HUVEC after siRNA-Chk transfection compared to control cells (Figure 3).
Figure 3.
Cell viability/proliferation (%) as determined by MTS assay in
MDA-MB-231 breast cancer cells ( )
and HUVEC (
)
and HUVEC ( ). (A) Untreated control cells set
to100%, (B)D-FECT treated cells, (C)100nM non-targeting siRNA
transfected cells, and (D)100nM siRNA-Chk transfected cells. MTS assays were
performed 3 days after 48 h treatment. Columns are mean, and bars are SD.
). (A) Untreated control cells set
to100%, (B)D-FECT treated cells, (C)100nM non-targeting siRNA
transfected cells, and (D)100nM siRNA-Chk transfected cells. MTS assays were
performed 3 days after 48 h treatment. Columns are mean, and bars are SD.
* represents P < 0.05 against D-FECT or non-targeting siRNA, n=3 per group.
MRS of MDA-MB-231 and HUVEC after transient siRNA-Chk transfection
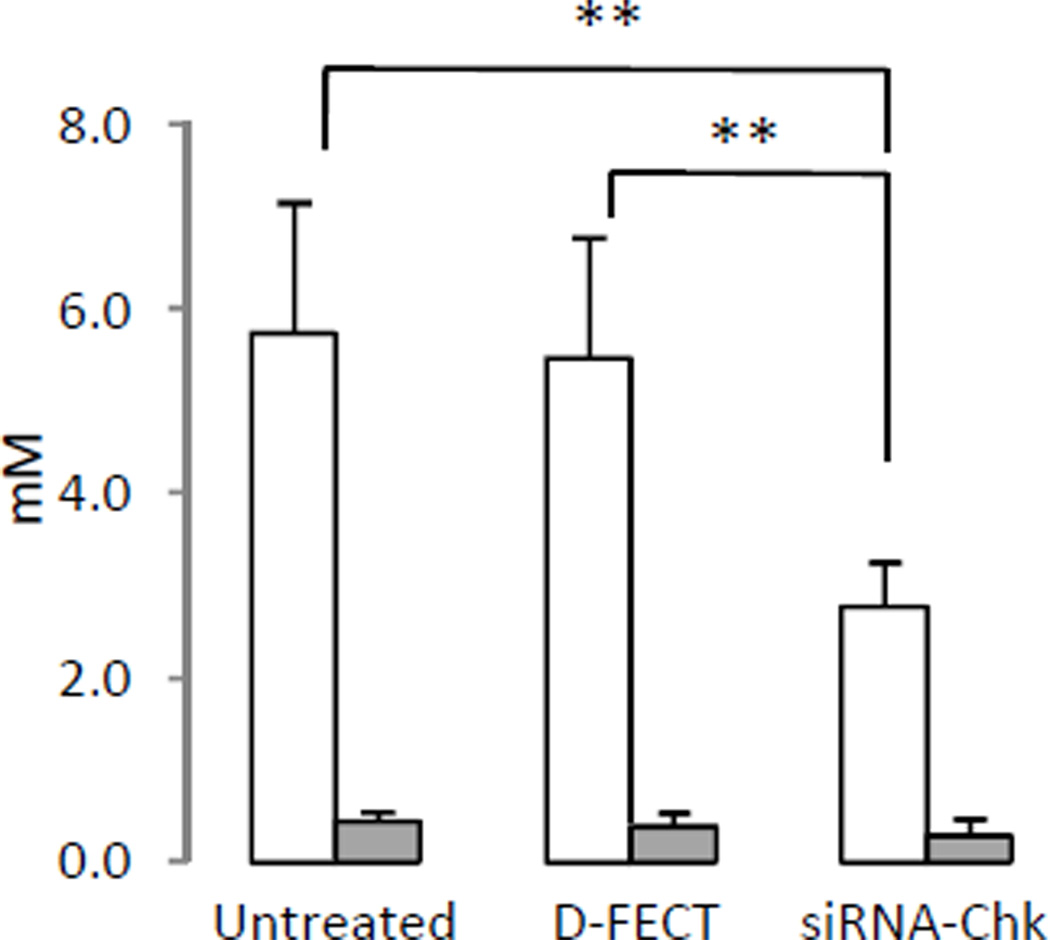
Representative 1H MR spectra from untreated and siRNA-Chk treated MDA-MB-231 cells and HUVEC are shown in Figure 4 (A-B). As evident in the spectra, PC levels were more than 10 fold lower in HUVEC than in MDA-MB-231 breast cancer cells. PC levels from multiple experiments are summarized in Figure 5. Following siRNA-Chk transfection, PC levels significantly decreased by more than half (5.73±1.43 mM to 2.76±0.49 mM) in MDA-MB-231 cells (P = 0.009). In HUVEC the decrease of PC was about 37% (0.44±0.10 mM to 0.28±0.19 mM). Although there was some reduction of PC with siRNA-Chk treatment in HUVEC compared to untreated cells this was not statistically significant.
Figure 4.
Representative 1H MR spectra of the 3.20 ppm to 3.25 ppm region of water-soluble extracts from (A) MDA-MB-231 breast cancer cells and (B) HUVEC. Spectra were acquired with 30° flip angle, 10,000 Hz sweep width, 11.2 s repetition time, 32K block size and 128 scans.
Figure 5.
Levels of PC quantitated from 1H MR spectra of MDA-MB-231
breast cancer cells ( , n=4) and HUVEC (
, n=4) and HUVEC ( ,
n=3).Columns are mean, and bars are SD.
,
n=3).Columns are mean, and bars are SD.
** represents P ≤ 0.01 against untreated or D-FECT control.
Effect of Chk downregulation on endothelial cells in vivo
We investigated CD31 immunostaining density in tumor sections of mice injected with Luc-shRNA virus and Chk-shRNA virus. As shown in Figure 6A, there was no difference in the distribution of CD31 positive cells in viable tumor regions. Quantification of cells with high CD31 staining intensity showed no statistically significant difference in the number of endothelial cells between the two groups (Figure 6B).
Figure 6.
(A) High resolution scanned images of representative 5µm thick
formalin fixed, paraffin-embedded tumor sections stained with CD31 obtained from
representative Luc-shRNA injected (a, b, c) and Chk-shRNA injected (d, e,
f)mice. (B) Quantification of the percent CD31 positive cells in viable regions
of Luc-shRNA treated tumors ( ,
n=5) and Chk-shRNA treated tumors (
,
n=5) and Chk-shRNA treated tumors ( ,
n=4) show no statistically significant difference between the groups. Columns
are mean, and bars are SD.
,
n=4) show no statistically significant difference between the groups. Columns
are mean, and bars are SD.
Discussion
Several studies have identified the link between Chk and cancer, and Chk is being actively investigated as a novel target in cancer treatment (5, 14). Recently, Wu et al found that Chk-α is crucial for the early development of mouse embryos by using Chk-α -deficient mice (24). Mice that lack Chk-β survive to adulthood, but develop hindlimb muscular dystrophy and forelimb bone deformity (25, 26).In cancer, Chk-α but not Chk- β overexpression is predominantly observed (7, 8, 13).
While several studies have characterized Chk-α levels in nonmalignant cells (12, 13, 27, 28), expression of this enzyme in human endothelial cells has not been previously characterized. Previous studies have shown low Chk-α expression levels in primary cultures of human mammary epithelial cells (HMEC), nonmalignant HMEC, MCF-10A and MCF-12A cells (12, 13, 27, 28). Here we have confirmed that human endothelial cells also express low Chk-α compared to human breast cancer cells. However, we also previously observed that HUVEC exposed to conditioned medium from MDA-MB-231 cells showed an approximately 20% increase of PC(29). It is therefore possible that endothelial cells as well as other stromal cells that are found within tumors may show increased Chk-α expression due to paracrine signaling from cytokines released by cancer cells, which should be further investigated. Future investigations should also focus on the effect of Chk-α downregulation on cells of the immune system, which also represent important components of tumor microenvironment and host response.
We previously observed that transient transfection and stable expression of siRNA and short hairpin RNA (shRNA) against Chk-α (Chk-shRNA) significantly reduced proliferation in breast cancer cells (12) and tumors (19). To downregulate Chk-α in human breast tumor xenografts in vivowe systemically administered lentivirus expressing Chk-shRNA through the tail vein and observed a significant decrease of tumor growth and proliferation, together with a decrease of Chk-α and PC in these tumors(19). We have examined these tumor sections to identify changes in the expression of CD31, which is an endothelial cell marker, and found no changes in the distribution of endothelial cells in the viable areas of the tumor upon Chk-α targeting. Since systemic administration exposes endothelial cells in the vasculature of malignant and nonmalignant tissues to the treatment, here we have determined the effects of Chk-α downregulation on human endothelial cells. Consistent with our previous studies(12), transfection with siRNA-Chk resulted in a significant decrease of Chk-α mRNA, Chk-α protein, PC levels and cell proliferation in MDA-MB-231. The 2−ΔΔCt is the most commonly used method to determine relative changes in gene expression from real-time quantitative PCR experiments(30). Assuming a 100% efficiency between cycles, there is a doubling of amplicons (PCR product) between two cycles (31).On comparing the Chk-α mRNA ΔCts of HUVEC and MDA-MB-231, ΔCt of HUVEC (2.4 ±0.11) was approximately 1.5 Ct higher than MDA-MB-231 cells (0.93±0.60). Applying the 2−ΔΔCt calculation, we can conclude that Chk-α mRNA expression is approximately three fold less in HUVEC than in MDA-MB-231 cells. In HUVEC the basal levels of Chk-α mRNA, Chk-α protein and PC were very low to start with, and downregulation occurred to a much lesser extent after transfection with siRNA-Chk. Interestingly, following siRNA-Chk transfection, Chk-α mRNA levels of MDA-MB-231 breast cancer cells and HUVEC were comparable. The MTS assay results showed no significant reduction of proliferation in HUVEC after siRNA-Chk transfection, in contrast to MDA-MB-231 cells that showed a significant reduction of proliferation. These data suggest that Chk-α downregulation by siRNA transfection targeting cancer cells will most likely not affect endothelial cell proliferation during systemic administration.
It is possible that the lack of a significant effect on the proliferation of HUVEC was due to the fact that the already low level of Chk-α was not significantly downregulated in the endothelial cells. However, we observed no differences in CD31 density in tumors targeted in vivo with Chk-shRNA lentivirus compared to control tumors. The attenuated tumor growth observed following Chk-α targeting by us (19) and others (15–17) was therefore most likely due to the effects of Chk-α downregulationon cancer cells. Since normal tissue damage is a major cause of toxicity in cancer treatment, identifying targets that are cancer specific is critical for improving treatment outcome and quality of life. Our data suggest that Chk-α may represent one such cancer-specific target.
Acknowledgments
This work was supported by NIH R01 CA73850, R01 CA82337, R01 CA138515, R01 CA136576, and P50 CA103175. We thank Dr. V.P. Chacko and Dr. Ioannis Stasinopoulos for expert technical support and Ms. Yelena Mironchik for valuable technical assistance.
Sponsors: This work was supported by NIH R01 CA73850, R01 CA82337, R01 CA138515, R01 CA136576, and P50 CA103175.
Abbreviations
- Cho
choline
- Chk
choline kinase
- D-FECT
DharmaFECT
- HUVEC
human umbilical vein endothelial cells
- Luc
luciferase
- PC
phosphocholine
- PtdCho
Phosphatidylcholine
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
References
- 1.Negendank W. Studies of human tumors by MRS: a review. NMR Biomed. 1992;5:303–324. doi: 10.1002/nbm.1940050518. [DOI] [PubMed] [Google Scholar]
- 2.Aoyama C, Liao H, Ishidate K. Structure and function of choline kinase isoforms in mammalian cells. Prog Lipid Res. 2004;43:266–281. doi: 10.1016/j.plipres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999;59:80–84. [PubMed] [Google Scholar]
- 4.Ackerstaff E, Pflug BR, Nelson JB, Bhujwalla ZM. Detection of increased choline compounds with proton nuclear magnetic resonance spectroscopy subsequent to malignant transformation of human prostatic epithelial cells. Cancer Res. 2001;61:3599–3603. [PubMed] [Google Scholar]
- 5.Ramirez de Molina A, Gutierrez R, Ramos MA, Silva JM, Silva J, Bonilla F, Sanchez JJ, Lacal JC. Increased choline kinase activity in human breast carcinomas: clinical evidence for a potential novel antitumor strategy. Oncogene. 2002;21:4317–4322. doi: 10.1038/sj.onc.1205556. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez de Molina A, Sarmentero-Estrada J, Belda-Iniesta C, Taron M, Ramirez de Molina V, Cejas P, Skrzypski M, Gallego-Ortega D, de Castro J, Casado E, Garcia-Cabezas MA, Sanchez JJ, Nistal M, Rosell R, Gonzalez-Baron M, Lacal JC. Expression of choline kinase alpha to predict outcome in patients with early-stage non-small-cell lung cancer: a retrospective study. Lancet Oncol. 2007;8:889–897. doi: 10.1016/S1470-2045(07)70279-6. [DOI] [PubMed] [Google Scholar]
- 7.Hernando E, Sarmentero-Estrada J, Koppie T, Belda-Iniesta C, Ramirez de Molina V, Cejas P, Ozu C, Le C, Sanchez JJ, Gonzalez-Baron M, Koutcher J, Cordon-Cardo C, Bochner BH, Lacal JC, Ramirez de Molina A. A critical role for choline kinase-alpha in the aggressiveness of bladder carcinomas. Oncogene. 2009;28:2425–2435. doi: 10.1038/onc.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iorio E, Ricci A, Bagnoli M, Pisanu ME, Castellano G, Di Vito M, Venturini E, Glunde K, Bhujwalla ZM, Mezzanzanica D, Canevari S, Podo F. Activation of phosphatidylcholine cycle enzymes in human epithelial ovarian cancer cells. Cancer Res. 2010;70:2126–2135. doi: 10.1158/0008-5472.CAN-09-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glunde K, Jie C, Bhujwalla ZM. Molecular causes of the aberrant choline phospholipid metabolism in breast cancer. Cancer Res. 2004;64:4270–4276. doi: 10.1158/0008-5472.CAN-03-3829. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama C. Function of choline kinase. Seikagaku. 2010;82:305–309. [PubMed] [Google Scholar]
- 11.Yalcin A, Clem B, Makoni S, Clem A, Nelson K, Thornburg J, Siow D, Lane AN, Brock SE, Goswami U, Eaton JW, Telang S, Chesney J. Selective inhibition of choline kinase simultaneously attenuates MAPK and PI3K/AKT signaling. Oncogene. 2010;29:139–149. doi: 10.1038/onc.2009.317. [DOI] [PubMed] [Google Scholar]
- 12.Glunde K, Raman V, Mori N, Bhujwalla ZM. RNA interference-mediated choline kinase suppression in breast cancer cells induces differentiation and reduces proliferation. Cancer Res. 2005;65:11034–11043. doi: 10.1158/0008-5472.CAN-05-1807. [DOI] [PubMed] [Google Scholar]
- 13.Eliyahu G, Kreizman T, Degani H. Phosphocholine as a biomarker of breast cancer: molecular and biochemical studies. Int J Cancer. 2007;120:1721–1730. doi: 10.1002/ijc.22293. [DOI] [PubMed] [Google Scholar]
- 14.Lacal JC. Choline kinase: a novel target for antitumor drugs. IDrugs. 2001;4:419–426. [PubMed] [Google Scholar]
- 15.Hernandez-Alcoceba R, Fernandez F, Lacal JC. In vivo antitumor activity of choline kinase inhibitors: a novel target for anticancer drug discovery. Cancer Res. 1999;59:3112–3118. [PubMed] [Google Scholar]
- 16.Ramirez de Molina A, Banez-Coronel M, Gutierrez R, Rodriguez-Gonzalez A, Olmeda D, Megias D, Lacal JC. Choline kinase activation is a critical requirement for the proliferation of primary human mammary epithelial cells and breast tumor progression. Cancer Res. 2004;64:6732–6739. doi: 10.1158/0008-5472.CAN-04-0489. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Gonzalez A, Ramirez de Molina A, Fernandez F, Ramos MA, del Carmen Nunez M, Campos J, Lacal JC. Inhibition of choline kinase as a specific cytotoxic strategy in oncogene-transformed cells. Oncogene. 2003;22:8803–8812. doi: 10.1038/sj.onc.1207062. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Gonzalez A, Ramirez de Molina A, Fernandez F, Lacal JC. Choline kinase inhibition induces the increase in ceramides resulting in a highly specific and selective cytotoxic antitumoral strategy as a potential mechanism of action. Oncogene. 2004;23:8247–8259. doi: 10.1038/sj.onc.1208045. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamachary B, Glunde K, Wildes F, Mori N, Takagi T, Raman V, Bhujwalla ZM. Noninvasive detection of lentiviral-mediated choline kinase targeting in a human breast cancer xenograft. Cancer Res. 2009;69:3464–3471. doi: 10.1158/0008-5472.CAN-08-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori N, Glunde K, Takagi T, Raman V, Bhujwalla ZM. Choline kinase down-regulation increases the effect of 5-fluorouracil in breast cancer cells. Cancer Res. 2007;67:11284–11290. doi: 10.1158/0008-5472.CAN-07-2728. [DOI] [PubMed] [Google Scholar]
- 21.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 22.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 23.Ribatti D. Judah Folkman, a pioneer in the study of angiogenesis. Angiogenesis. 2008;11:3–10. doi: 10.1007/s10456-008-9092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu G, Aoyama C, Young SG, Vance DE. Early embryonic lethality caused by disruption of the gene for choline kinase alpha, the first enzyme in phosphatidylcholine biosynthesis. J Biol Chem. 2008;283:1456–1462. doi: 10.1074/jbc.M708766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu G, Sher RB, Cox GA, Vance DE. Understanding the muscular dystrophy caused by deletion of choline kinase beta in mice. Biochim Biophys Acta. 2009;1791:347–356. doi: 10.1016/j.bbalip.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Sher RB, Aoyama C, Huebsch KA, Ji S, Kerner J, Yang Y, Frankel WN, Hoppel CL, Wood PA, Vance DE, Cox GA. A rostrocaudal muscular dystrophy caused by a defect in choline kinase beta, the first enzyme in phosphatidylcholine biosynthesis. J Biol Chem. 2006;281:4938–4948. doi: 10.1074/jbc.M512578200. [DOI] [PubMed] [Google Scholar]
- 27.Glunde K, Serkova NJ. Therapeutic targets and biomarkers identified in cancer choline phospholipid metabolism. Pharmacogenomics. 2006;7:1109–1123. doi: 10.2217/14622416.7.7.1109. [DOI] [PubMed] [Google Scholar]
- 28.Gallego-Ortega D, Ramirez de Molina A, Ramos MA, Valdes-Mora F, Barderas MG, Sarmentero-Estrada J, Lacal JC. Differential role of human choline kinase alpha and beta enzymes in lipid metabolism: implications in cancer onset and treatment. PLoS One. 2009;4:e7819. doi: 10.1371/journal.pone.0007819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori N, Natarajan K, Chacko VP, Artemov D, Bhujwalla ZM. Choline phospholipid metabolites of human vascular endothelial cells altered by cyclooxygenase inhibition, growth factor depletion, and paracrine factors secreted by cancer cells. Mol Imaging. 2003;2:124–130. doi: 10.1162/15353500200303127. [DOI] [PubMed] [Google Scholar]
- 30.Kenneth JL, Thomas D. Schmittgen. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCt Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Dean Fraga TM. Current Protocols Essential Laboratory Techniques. John Wiley and Sons, Inc.; 2008. Steven Fenster. UNIT 10.3 Real-Time PCR; pp. 10.3.1–10.3.33. [Google Scholar]