Abstract
X-ray crystallographic analysis of a bovine antibody (BLV1H12) revealed a unique scaffold in its ultralong heavy chain complementarity determining region 3 (CDR3H) region that folds into a solvent exposed, antiparallel β-stranded “stalk” fused with a disulfide cross-linked “knob” domain. This unusual variable region motif provides a novel approach for generating chimeric antibodies with novel activities. Toward this end, human erythropoietin (hEPO) was substituted for the “knob” domain in this antibody to afford an antibody-hEPO (Ab-hEPO) fusion protein that efficiently expresses in mammalian cells. Ab-hEPO proliferated TF-1 cells with a potency comparable to that of hEPO (EC50 ~ 0.03 nM) and exhibits a significantly extended plasma half-life (>6 days) in mice relative to hEPO (~4 hours). Mice treated with the Ab-hEPO fusion protein show sustained elevated hematocrit for more than two weeks. This work demonstrates the utility of BLV1H12 CDR3 fusions as a novel approach for generating potent polypeptides with enhanced pharmacological properties.
Introduction
The ability to incorporate biologically active proteins and peptides directly into the hypervariable loops of antibodies may provide a general approach for modifying or enhancing the pharmacological properties of various cytokines, growth factors, peptide hormones and ion channel blockers. For example, the resulting fusion proteins are likely to have increased serum half-lives due to their size and interaction with the neonatal Fc receptors (FcRn). They may also express at higher levels in mammalian cells, be more easily purified, or have enhanced solubility and proteolytic stability. Moreover, antibody chimeras will have increased avidity due to the bivalent nature of the antibody molecule; additional binding interactions between the antibody CDR loops and the target receptor may also lead to increased potency or specificity. Finally, it may be possible to graft two or more distinct proteins or peptides into the CDRs to afford fusion proteins with dual activities.
Recently, we identified a bovine antibody (BLV1H12) with an ultralong heavy chain CDR3 (CDR3H) region that facilitates engineering of such CDR fusions. The Xray crystal structure revealed an unusual CDR3H region that folds as a disulfide-bonded “knob” domain fused to a solvent accessible, antiparallel β-stranded “stalk” that protrudes from the antibody surface (Figure 1).(1) Unlike conventional antibodies with CDR loops of 10–15 residues in length, the novel architecture of this ultralong CDR3H provides an attractive platform for the creation of antibody chimeras with novel pharmacological activities.(1–5) Indeed, we recently showed that bovine granulocyte colony-stimulating factor (bGCSF), when substituted into this ultralong CDR3H region, exhibits enhanced serum half-life in mice.(6)
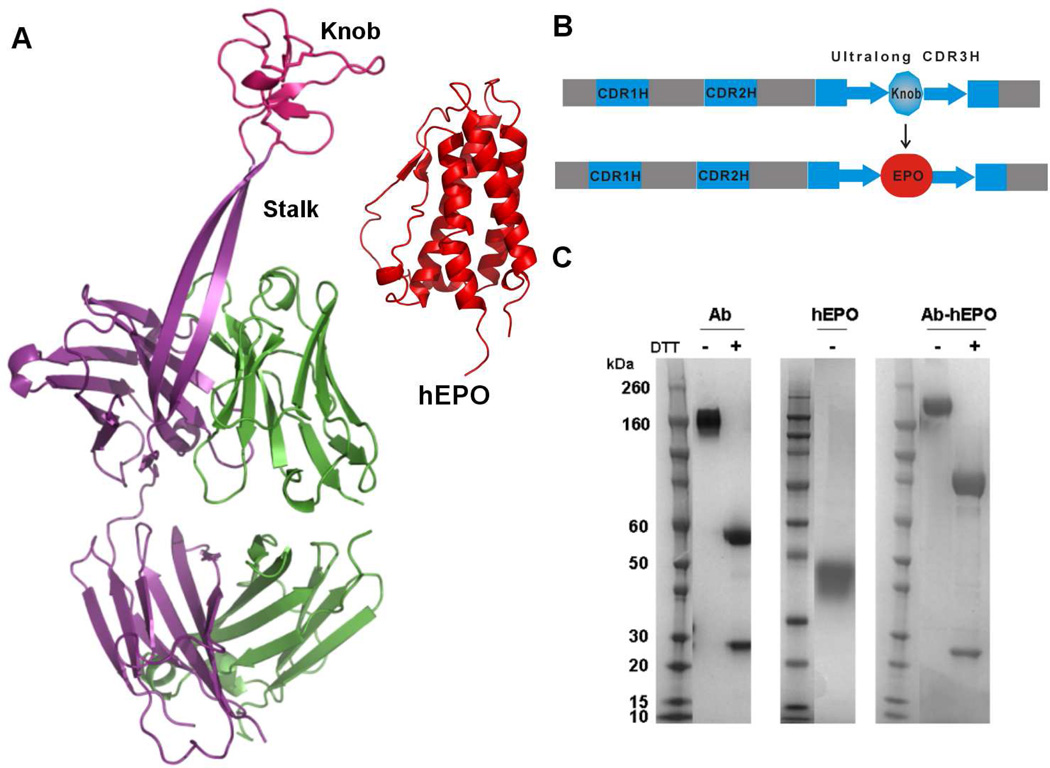
Figure 1.
Grafting of human erythropoietin (hEPO) onto the “stalk” region of bovine antibody BLV1H12. (A) X-ray crystal structures of bovine antibody BLV1H12 Fab fragment (PDB ID: 4K3D) and hEPO (PDB ID: 1EER). (B) Scheme for generation of antibody-hEPO fusion protein. (C) SDS-PAGE gel of purified BLV1H12 full-length IgG (Ab), hEPO, and Ab-hEPO.
Erythropoietin (EPO), a cytokine mainly produced by kidney in adult, is a 34-kDa glycoprotein which stimulates erythroid progenitor cell differentiation and maturation and thus increases the erythrocyte population.(7, 8) Recombinant human EPO (hEPO) and its mimetics have been used clinically to treat anemia associated with chronic kidney disease and cancer chemotherapy.(8–12) However, their short circulating half-lives, which necessitate frequent subcutaneous (s.c.) administrations, have led to the development of second generation modified EPOs (e.g., darbepoetin alfa, methoxy polyethylene glycol-epoetin, etc.) with improved serum half-lives.(9) To further explore the generality of the BLV1H12 antibody scaffold as a platform for generating biologically active fusion proteins, we asked whether grafting hEPO into the ultralong CDR3H region would afford an antibody-hEPO chimera with high potency and long serum half-life. Here we show that direct grafting of hEPO into the ultralong CDR3H region of this bovine antibody results in an efficiently expressed fusion protein that stimulates TF-1 cell proliferation in a dose-dependent manner. Remarkably, this antibody-hEPO fusion protein (Ab-hEPO) potently stimulates erythropoiesis in mice and sustains high levels of hematocrit for more than two weeks.
Results and Discussion
The folded, disulfide-bonded “knob” domain of the bovine antibody BLV1H12 is separated from the immunoglobulin domain by a 20 Å solvent exposed, antiparallel β-stranded “stalk” (Figure 1A). Thus, it is likely that fusion of the N- and C-termini of hEPO with the corresponding β-stranded “stalk” will not interfere with folding of either the antibody or hEPO. Moreover, because the receptor binding surface of EPO is on the opposite face of the molecule to the chain termini, the fusion protein should still retain its erythropoietic activity. To generate the Ab-hEPO fusion protein, a synthetic hEPO gene was ligated into the ultralong CDR3H region of a chimeric BLV1H12 full-length IgG (Ab) with a human IgG1 Fc fragment through overlap PCR. The “knob” domain (Cys108-Tyr146) of the Ab was replaced by the hEPO fragment with its N- and Ctermini fused to the ascending and descending β-strands of the “stalk”, respectively, with GGGGS linkers (Figure 1B). We reasoned that the flexible linkers may facilitate protein folding and promote favorable interactions of the fused hEPO with its receptor.
The Ab, hEPO (with C-term HisTag) and Ab-hEPO fusion protein were subsequently expressed in freestyle HEK293 cells by transient transfection. Proteins were secreted into culture medium, followed by purification using protein G chromatography for Ab and Ab-hEPO, and Ni-NTA chromatography for hEPO. The purified proteins were analyzed by SDS-PAGE gel (Figure 1C). Under non-reducing conditions, Ab migrates as a single band of ~160 kDa due to N-glycosylation in the Fc region; glycosylated hEPO migrates at 34 kDa; and Ab-hEPO migrates at ~200 kDa. In the presence of 50 mM dithiothreitol (DTT), the light chains of Ab and Ab-hEPO migrate at 23 kDa; and the heavy chains of Ab and Ab-hEPO migrate at 55 kDa and 80 kDa, respectively. Mass spectral analyses of the heavy chains of Ab, Ab-hEPO and hEPO indicate that hEPO fused into the CDR3H region is heterogeneously glycosylated, as is the case with hEPO (Figure S1–S3). After treatment with peptide-N-glycosidase and DTT, mass spectral analysis of the heavy chains of Ab and Ab-hEPO afford a mass difference of 15748 Da (Figure S4–S6), corresponding to two fused hEPO molecules per IgG molecule. The final yields of the Ab and Ab-hEPO fusion protein are similar, both around 17 mg/L, and both proteins can be concentrated to over 10 mg/mL in PBS (pH 7.4) without aggregation. Gel filtration analyses indicate that Ab and Ab-hEPO in PBS (pH 7.4) have apparent molecular weights of 160 kDa and 200 kDa, respectively, as expected, and that aggregation is less than 3% of the total amount of protein in both samples on the basis of integrated UV absorbance peaks at 280 nm (Figure S7). The comparable yields and solubilities of Ab and Ab-hEPO suggest that the fusion protein is folded correctly.
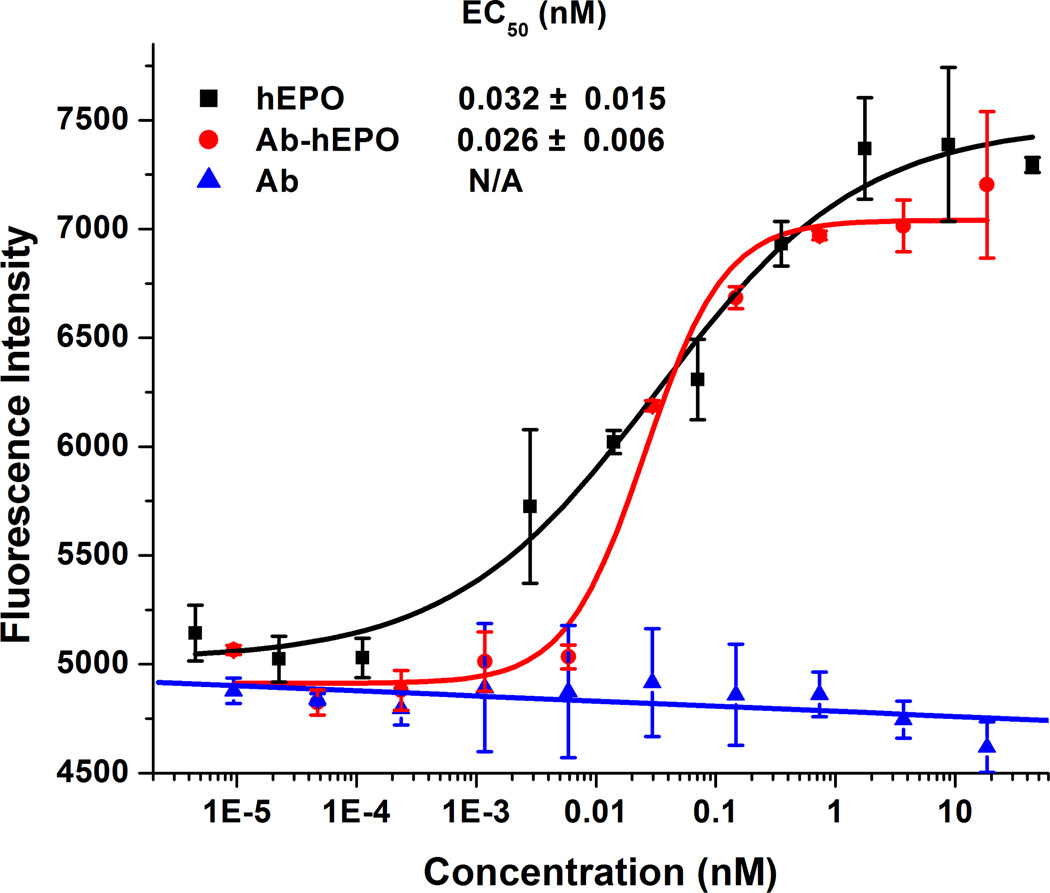
We next examined the activity of Ab-hEPO using TF-1 cells that are growth-dependent on hEPO.(13) Prior to treatment, cells were washed three times to remove residual GM-CSF from the growth media, followed by treatment with various concentrations of Ab, hEPO and Ab-hEPO for 72 hours. Cell viability in each well was quantified using an Alamar Blue assay. Both hEPO and Ab-hEPO stimulate TF-1 cell proliferation in a dose-dependent manner (Figure 2), whereas Ab by itself has no proliferative activity, indicating that the observed activities of the Ab-hEPO fusion protein result from the fused hEPO. The EC50 is 0.032 ± 0.015 nM for hEPO and 0.026 ± 0.006 nM for Ab-hEPO, indicating that the fusion protein has similar activity to hEPO in stimulating TF-1 cells proliferation. Notably, Ab-hEPO is as potent as other modified EPOs in stimulating EPO-dependent cell growth on the basis of a comparison with published results (EC50: 0.07 nM for hEPO-Fc fusion protein, 0.26 nM for darbepoetin alfa, and 0.07 nM for PEG-hEPO).(14–16)
Figure 2.
Ab-hEPO fusion protein stimulates proliferation of TF-1 cells in a dose-dependent manner. Cells cultured in RPMI-1640 medium with 10% FBS were treated with various concentrations of hEPO, BLV1H12 full-length IgG (Ab), and Ab-hEPO. Cell viability was quantified using an Alamar Blue (Invitrogen) assay.
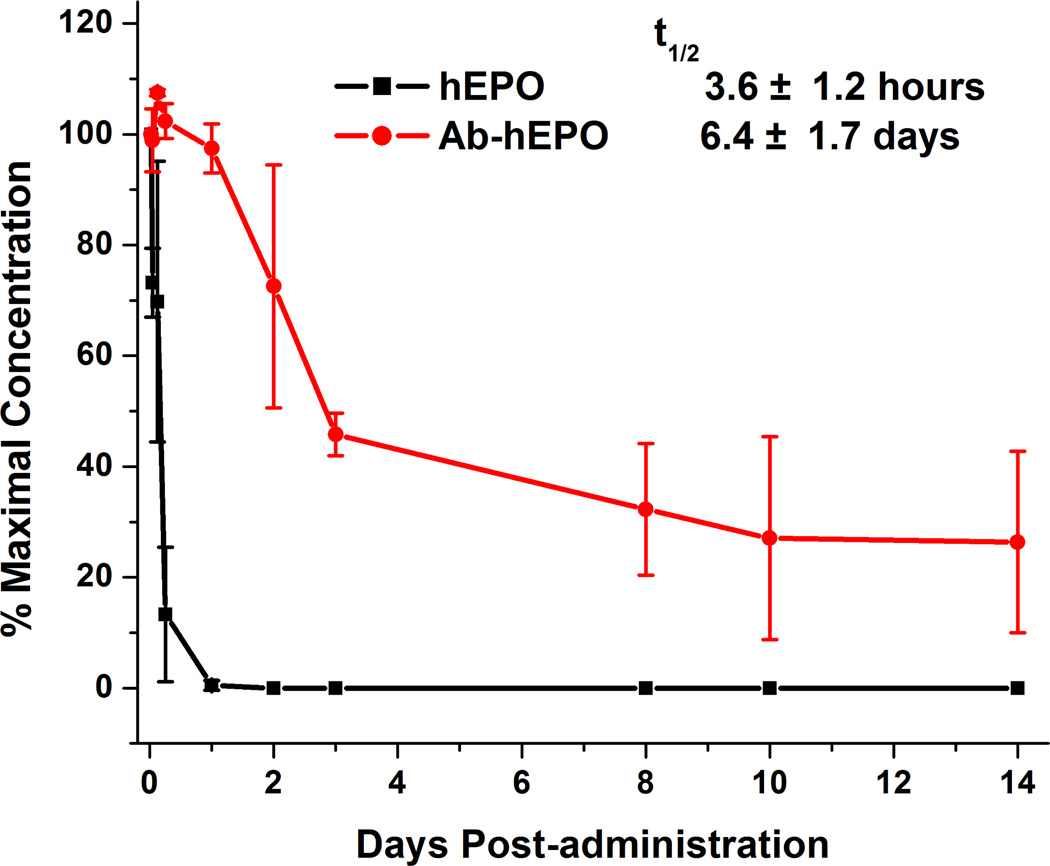
To examine if fusion of hEPO into the novel CDR3H domain within the bovine antibody increases its serum half-life, we carried out a pharmacokinetic (PK) study of hEPO and Ab-hEPO in mice. Single doses of hEPO (0.18 mg/kg) and Ab-hEPO (1.5 mg/kg) in PBS (pH 7.4) were intravenously injected into CD1 mice (three per group). Plasma from each injection group was collected at day 0 to day 14 and analyzed by ELISA using anti-human IgG Fc and anti-hEPO antibodies. Compared with hEPO which was quickly cleared within 24 hours, Ab-hEPO remained above 20% of the maximal concentration even after 8 days (Figure 3). The estimated half-lives are 3.6 hours for hEPO and 6.4 days for Ab-hEPO by assuming a one-compartment model with first-order elimination. These PK results show that fusion of EPO into the ultralong CDR3H region of BLV1H12 can significantly extend its plasma half-life, which is much longer than those of other modified EPOs in rodents (half-lives: 29 hours for hEPO-Fc fusion protein, 14 hours for darbepoetin alfa, and 23 hours for PEG-hEPO) (15, 17), which is likely a result of the increased molecular size, FcRn-assisted recycling, and resistance to proteolytic cleavage in the context of antibody framework. Thus, this unique CDR3H motif has been used to extend the half-lives of both EPO and the 4-helix-bundle protein GCSF, suggesting this may be a general strategy for generating long-lived protein and peptide therapeutics.
Figure 3.
Pharmacokinetics in mice. hEPO (0.18 mg/kg) and Ab-hEPO (1.5 mg/kg) in PBS (pH 7.4) were administrated by intravenous (i.v.) injection into CD1 mice (three per group). Blood was collected from day 0 to day 14 and analyzed by ELISA using antihuman IgG Fc and anti-hEPO antibodies. Data were normalized by taking maximal concentration at the first time point (30 minutes).
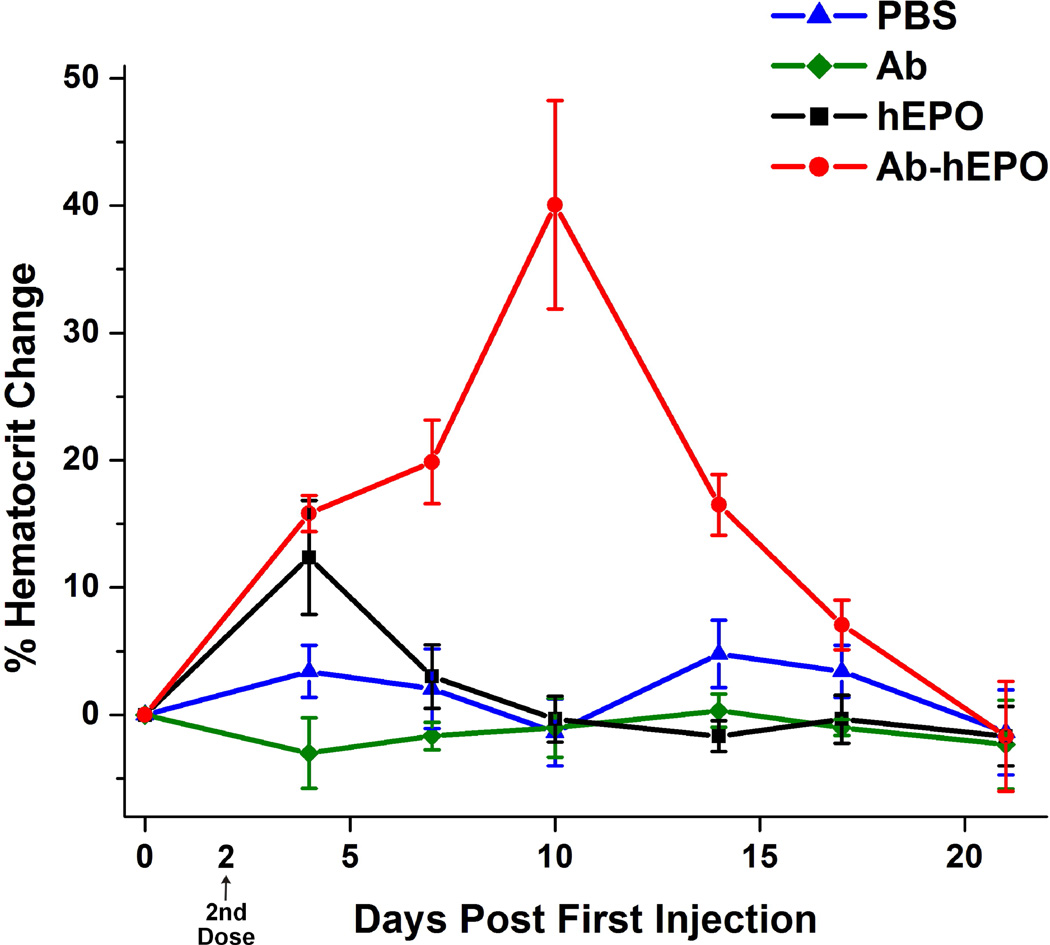
We next examined if the increased plasma half-life of Ab-hEPO translates to extended erythropoiesis stimulating activity in mice by measuring hematocrit levels at different time points post subcutaneous dosing. Remarkably, mice treated with Ab-hEPO exhibit sustained high levels of hematocrit for more than two weeks in comparison to hEPO (Figure 4). For mice treated with two doses of Ab-hEPO (810 µg/kg) at day 0 and day 2, hematocrit levels increased >15% at day 4 post-administration; at day 10 there was a >40% increase in hematocrit levels. In comparison, for mice treated with hEPO protein, the hematocrit levels increased by only 12% at day 4 and then decreased to normal levels within 6 days, which is consistent with the short circulating half-life of hEPO. As controls, mice treated with PBS and Ab showed no hematocrit increases.
Figure 4.
Pharmacodynamics in mice. Vehicle (PBS, pH 7.4), Ab (810 µg/kg), hEPO (90 µg/kg) and Ab-hEPO (810 µg/kg) were subcutaneously administrated into CD1 mice (three per group) on day 0 and day 2, followed by the measurements of hematocrit.
In conclusion, we have demonstrated that by fusing hEPO into the ultralong CDR3H region of BLV1H12, one can generate a long-lived Ab-hEPO fusion protein that has sustained erythropoiesis stimulating activity in mice. Unlike the selection of antibodies with agonist activity, which requires significant binding- and/or functional-based screening (18, 19), the direct grafting of agonist polypeptides into the ultralong CDR3 region allows efficient generation of functional chimeric fusions with enhanced pharmacokinetic properties. Ongoing studies include humanization of the Ab-hEPO scaffold and application of this strategy to polypeptide hormones and ion channel blockers.
Methods
Cloning of BLV1H12 Full-length IgG, BLV1H12-hEPO Fusion Protein and hEPO
The mammalian expression vector for BLV1H12 full-length IgG heavy chain was generated by in-frame ligation of the amplified BLV1H12 Fab heavy chain (VH and CH1) to the pFuse-hIgG1-Fc backbone vector (InvivoGen, CA). The gene encoding hEPO was synthesized by Genscript (NJ, USA), and amplified by polymerase chain reaction (PCR). To optimize the folding and stability of the BLV1H12-hEPO (Ab-hEPO) fusion protein, flexible GGGGS linker was added at each end of the hEPO gene fragment. The Ab-hEPO fusion protein was then created by replacing the knob domain (Cys108–Tyr146) in CDR3H region of BLV1H12 (Figure 1) with hEPO through overlap extension PCR. Genes encoding antibody BLV1H12 light chain and hEPO with a C-terminal 6×His-tag were amplified and cloned into the pFuse vector without the hIgG1 Fc fragment. The resulting mammalian expression vectors were verified by DNA sequencing. Primers used for generating expression vetors are listed in Table S1.
Expression and Purification of BLV1H12 IgG, BLV1H12-hEPO Fusion Protein and hEPO
BLV1H12 full-length IgG (Ab), Ab-hEPO and hEPO were expressed through transient transfection of freestyle HEK293 cells growing in shaker flasks (125 rpm) with freestyle 293 expression medium (Life Technologies) at 37°C with 5% CO2. Culture medium containing secreted proteins was harvested every 48 hours twice after transfection. Ab and Ab-hEPO were purified by Protein A/G chromatography (Thermo Fisher Scientific, IL), while hEPO was purified by Ni-NTA affinity chromatography. Briefly, collected supernatant was loaded onto a Protein A/G or Ni-NTA column twice, which was pre-equilibrated in binding buffer (PBS, pH 7.4 for Protein A/G column, and TBS (20 mM Tris-HCl, 136 mM NaCl, pH 7.4) with 10 mM imidazole for Ni-NTA column). Columns were washed with 10 column volumes of binding buffer, followed by 10 column volumes of washing buffer (PBS, pH7.4 for Protein A/G column, and TBS with 30 mM imidazole, pH 7.4 for Ni-NTA column). Proteins bound to the columns were eluted with 100 mM glycine, pH 2.7, for Ab and Ab-hEPO, or TBS with 400 mM imidazole, pH 7.4, for hEPO. Immediately after elution, Tris-HCl (100 mM final concentration) was added to the Ab and Ab-hEPO to adjust pH to ~8. Eluted proteins were then concentrated using Amicon centrifugal filters (Millipore) and exchanged into PBS (pH 7.4). Purified proteins were analyzed by SDS-PAGE gels and mass spectrometry.
In Vitro Proliferative Activity Assay on TF-1 Cells
Human TF-1 cells were cultured at 37°C with 5% CO2 in RPMI-1640 medium containing 10% fetal bovine serum (FBS), penicillin and streptomycin (50 U/mL), and 2 ng/ml human granulocyte macrophage colony stimulating factor (GM-CSF). To test the proliferative activity of Ab-hEPO, cells were washed three times with RPMI-1640 medium plus 10% FBS, resuspended in RPMI-1640 medium with 10% FBS at a density of 1.5×105 cells/ml, plated in 96-well plates (1.5×104 cells per well) with various concentrations of hEPO, Ab-hEPO and Ab, and then incubated for 72 hours at 37°C with 5% CO2. Cells were then treated with Alamar Blue (Invitrogen) for 4 hours at 37°C. Fluorescence intensity measured at 595 nm is proportional to cell viability and plotted versus protein concentration. The EC50 values were determined by fitting data into a logistic sigmoidal function: y = A2 + (A1−A2)/(1 + (x/x0)p), where A1 is the initial value, A2 is the final value, x0 is the inflection point of the curve, and p is the power.
Pharmacokinetics in Mice
hEPO (0.18 mg/kg) and Ab-hEPO (1.5 mg/kg) in PBS (pH 7.4) were administrated by intravenous (i.v.) injection into three CD1 mice per group. Blood was collected from day 0 to day 14 and analyzed by ELISA using anti-human IgG Fc (Abcam) and anti-hEPO (R&D systems) antibodies. Data were normalized by taking the maximal concentration at the first time point (30 minutes). The percentage of maximal concentration was plotted versus time, and the half-lives were determined by fitting data into the first-order equation, A=A0e−kt, where A0 is the initial concentration, t is the time, and k is the first order rate constant.
Pharmacodynamics in Mice
Vehicle (PBS, pH 7.4), Ab (810 µg/kg), hEPO (90 µg/kg) and Ab-hEPO (810 µg/kg) were administrated by subcutaneous (s.c.) injection into CD1 mice (three per group) at day 0 and day 2. Blood was collected at different time points and the hematocrit levels were measured by centrifugation in micro-hematocrit capillary tubes.
Supplementary Material
Acknowledgment
The authors thank D. Caballero and J. Gonzalez in the Department of Pharmacology of California Institute for Biomedical Research (Calibr) for help on animal study and H. Zhang and J. Xie in the Department of Molecular Biology of The Scripps Research Institute for helpful discussion. This work was supported by National Institutes of Health (NIH) Grant R01 GM062159 (to P.G.S.). This manuscript is number 24082 of The Scripps Research Institute.
Footnotes
Supporting Information
Supplemental Table S1 and Figures S1–S7. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Wang F, Ekiert DC, Ahmad I, Li W, Zhang Y, Bazirgan O, Torkamani A, Raudsepp T, Mwangi W, Criscitiello MF, Wilson IA, Schultz PG, Smider VV. Reshaping antibody diversity. Cell. 2013;153:1379–1393. doi: 10.1016/j.cell.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berens SJ, Wylie DE, Lopez OJ. Use of a single VH family and long CDR3s in the variable region of cattle Ig heavy chains. Int. Immunol. 1997;9:189–199. doi: 10.1093/intimm/9.1.189. [DOI] [PubMed] [Google Scholar]
- 3.Lopez O, Perez C, Wylie D. A single VH family and long CDR3s are the targets for hypermutation in bovine immunoglobulin heavy chains. Immunol. Rev. 1998;162:55–66. doi: 10.1111/j.1600-065x.1998.tb01429.x. [DOI] [PubMed] [Google Scholar]
- 4.Saini SS, Allore B, Jacobs RM, Kaushik A. Exceptionally long CDR3H region with multiple cysteine residues in functional bovine IgM antibodies. Eur. J. Immunol. 1999;29:2420–2426. doi: 10.1002/(SICI)1521-4141(199908)29:08<2420::AID-IMMU2420>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 5.Saini SS, Farrugia W, Ramsland PA, Kaushik AK. Bovine IgM antibodies with exceptionally long complementarity-determining region 3 of the heavy chain share unique structural properties conferring restricted VH + Vlambda pairings. Int. Immunol. 2003;15:845–853. doi: 10.1093/intimm/dxg083. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Wang D, de Lichtervelde L, Sun SB, Smider VV, Schultz PG, Wang F. Functional antibody CDR3 fusion proteins with enhanced pharmacological properties. Angew. Chem. Int. Ed. 2013;52:8295–8298. doi: 10.1002/anie.201303656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, Finer-Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski JJ, Egrie J, Stroud RM. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998;395:511–516. doi: 10.1038/26773. [DOI] [PubMed] [Google Scholar]
- 8.Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 9.Reichel C, Gmeiner G. Erythropoietin and analogs. Handb Exp Pharmacol. 2010:251–294. doi: 10.1007/978-3-540-79088-4_12. [DOI] [PubMed] [Google Scholar]
- 10.Dalle B, Henri A, Rouyer-Fessard P, Bettan M, Scherman D, Beuzard Y, Payen E. Dimeric erythropoietin fusion protein with enhanced erythropoietic activity in vitro and in vivo. Blood. 2001;97:3776–3782. doi: 10.1182/blood.v97.12.3776. [DOI] [PubMed] [Google Scholar]
- 11.Leroy-Viard K, Rouyer-Fessard P, Beuzard Y. Improvement of mouse beta-thalassemia by recombinant human erythropoietin. Blood. 1991;78:1596–1602. [PubMed] [Google Scholar]
- 12.Kato M, Miura K, Kamiyama H, Okazaki A, Kumaki K, Kato Y, Sugiyama Y. Pharmacokinetics of erythropoietin in genetically anemic mice. Drug Metab. Dispos. 1998;26:126–131. [PubMed] [Google Scholar]
- 13.Hoang T, Paradis E, Brady G, Billia F, Nakahara K, Iscove NN, Kirsch IR. Opposing effects of the basic helix-loop-helix transcription factor SCL on erythroid and monocytic differentiation. Blood. 1996;87:102–111. [PubMed] [Google Scholar]
- 14.Bitonti AJ, Dumont JA, Low SC, Peters RT, Kropp KE, Palombella VJ, Stattel JM, Lu Y, Tan CA, Song JJ, Garcia AM, Simister NE, Spiekermann GM, Lencer WI, Blumberg RS. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9763–9768. doi: 10.1073/pnas.0403235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nett JH, Gomathinayagam S, Hamilton SR, Gong B, Davidson RC, Du M, Hopkins D, Mitchell T, Mallem MR, Nylen A, Shaikh SS, Sharkey N, Barnard GC, Copeland V, Liu L, Evers R, Li Y, Gray PM, Lingham RB, Visco D, Forrest G, DeMartino J, Linden T, Potgieter TI, Wildt S, Stadheim TA, d'Anjou M, Li H, Sethuraman N. Optimization of erythropoietin production with controlled glycosylation-PEGylated erythropoietin produced in glycoengineered Pichia pastoris. J. Biotechnol. 2012;157:198–206. doi: 10.1016/j.jbiotec.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Way JC, Lauder S, Brunkhorst B, Kong SM, Qi A, Webster G, Campbell I, McKenzie S, Lan Y, Marelli B, Nguyen LA, Degon S, Lo KM, Gillies SD. Improvement of Fc-erythropoietin structure and pharmacokinetics by modification at a disulfide bond. Protein engineering, design & selection : PEDS. 2005;18:111–118. doi: 10.1093/protein/gzi021. [DOI] [PubMed] [Google Scholar]
- 17.Im SJ, Yang SI, Yang SH, Choi DH, Choi SY, Kim HS, Jang do S, Jin KS, Chung YK, Kim SH, Paik SH, Park YC, Chung MK, Kim YB, Han KH, Choi KY, Sung YC. Natural form of noncytolytic flexible human Fc as a long-acting carrier of agonistic ligand, erythropoietin. PloS one. 2011;6:e24574. doi: 10.1371/journal.pone.0024574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Wilson IA, Lerner RA. Selection of antibodies that regulate phenotype from intracellular combinatorial antibody libraries. Proc. Natl. Acad. Sci. U.S.A. 2012;109:15728–15733. doi: 10.1073/pnas.1214275109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.