Abstract
Objective
Cooling to electrocerebral inactivity (ECI) by electroencephalography (EEG) remains the gold-standard to maximize cerebral and systemic organ protection during deep hypothermic circulatory arrest (DHCA). We sought to determine predictors of ECI to help guide cooling protocols when EEG monitoring is unavailable.
Methods
Between July 2005 and July 2011, 396 patients underwent thoracic aortic operation with DHCA; EEG monitoring was utilized in 325 (82%) of these cases to guide the cooling strategy and constituted the study cohort. EEG monitoring was utilized for all elective cases and when available for non-elective cases. Multivariable linear regression was used to assess predictors of the nasopharyngeal temperature and cooling time required to achieve ECI.
Results
Cooling to a nasopharyngeal temperature of 12.7°C or for a duration of 97 minutes was required to achieve ECI in > 95% of patients. Only 7% and 11% of patients achieved ECI by 18°C or 50 minutes of cooling, respectively. No independent predictors of nasopharyngeal temperature at ECI were identified. Independent predictors of cooling time included body surface area (+18 minutes/m2), white race (+7 minutes), and starting nasopharyngeal temperature (+3 minutes/°C). Low complication rates were observed (1.5% ischemic stroke, 1.5% permanent paraparesis/paraplegia, 2.2% new onset dialysis, and 4.3% 30 day/in-hospital mortality).
Conclusion
Cooling to a nasopharyngeal temperature of 12.7°C or for a duration of 97 minutes achieved ECI in > 95% of patients in our study population. However, patient-specific factors were poorly predictive of the temperature or cooling time required to achieve ECI, necessitating EEG monitoring for precise ECI detection.
INTRODUCTION
Deep hypothermic circulatory arrest (DHCA) is commonly used to provide a bloodless surgical field and facilitate replacement of the aortic arch or descending/thoracoabdominal aorta. Although all end-organs are at risk of ischemic injury during circulatory arrest, neuronal tissues may be most sensitive to oxygen deprivation. Neurologic injury during ischemia can be minimized by using hypothermia to lower the cerebral metabolic rate of oxygen consumption (CMRO2). Pre-clinical studies have shown maximal suppression of CMRO2 occurs at electroencephalographic (EEG) isoelectricity, or electrocerebral inactivity (ECI) (1, 2), and cooling to deep hypothermia (≤ 18°C) has evolved as the preferred technique for cerebral and systemic organ protection during DHCA (3, 4). However, the cooling time and temperature required to achieve ECI are highly variable among patients (5). As a result, intraoperative EEG monitoring has become a valuable method for guiding temperature selection with DHCA, to allow for confirmation of ECI without over or under cooling.
Intraoperative EEG monitoring requires an advanced team of specially trained neurologists, anesthesiologists, and EEG technologists, and these services are not available at all times or at all institutions. Thus, strategies for predicting ECI in the absence of EEG monitoring are needed. In 2001, Stecker and colleagues reviewed their monitored DHCA experience and found that cooling to a nasopharyngeal (NP) temperature of 12.5°C or for a duration of 50 minutes achieved ECI in 100% of patients (n = 47) (5). However, these data have not been independently validated in the decade since they were published. In the current study, we sought to report the NP temperatures and cooling times required to achieve ECI in a larger modern cohort of patients undergoing thoracic aortic operation with DHCA and EEG monitoring. In addition, we hypothesized that patient-specific factors would be predictive of the temperature or cooling time required to achieve ECI and could be used to help guide cooling protocols when EEG monitoring services are unavailable.
METHODS
Patient Population and Data Collection
This study was approved by the Institutional Review Board of Duke University and the need for individual patient consent was waived. The Duke Thoracic Aortic Surgery Database is a prospectively maintained electronic clinical registry of all patients who have undergone a thoracic aortic procedure at Duke University Medical Center (Durham, NC) since July 2005 (3, 6). A query of the database identified 396 consecutive thoracic aortic operations with DHCA performed between July 2005 and July 2011; EEG monitoring was utilized in 325 (82%) of these cases to guide the cooling strategy and constituted the study cohort. EEG was utilized for all elective cases and when available for non-elective cases (31 urgent and 12 emergent). Data on intraoperative cooling parameters were ascertained from anesthesia and perfusion records. For data collection purposes, the time point of ECI was considered to be equivalent to the time of initiation of DHCA, consistent with institutional practice protocols. Cooling time was defined as the duration between the start of cooling and the time of ECI. The NP temperature was recorded at the start of cooling and at ECI. The lowest NP temperature achieved prior to rewarming was also recorded. The baseline hemoglobin concentration was recorded prior to surgery and the first PaCO2 concentration was recorded after cooling onset. Comorbid conditions and postoperative complications were defined using Society of Thoracic Surgeons definitions (www.sts.org).
EEG Monitoring
EEG monitoring was performed with gold disc electrodes that were applied to the scalp according to the International 10/20 electrode application system. All electrodes except Fp1 and Fp2 were applied; these electrodes were omitted due to placement of other probes on the frontal scalp region. The baseline EEG was recorded after anesthetic induction but before initiation of cooling. Continuous EEG monitoring was performed from the onset of cardiopulmonary bypass (CPB) and continued during rewarming until the termination of CPB. The EEG sensitivity was typically 5 – 7 μV/mm at the onset of CPB and was gradually increased as the EEG activity diminished in amplitude. After anesthesia was discontinued, the EEG sensitivity was increased to 2 μV/mm. ECI was not assessed until 15 minutes after cessation of anesthetics to remove any confounding effects of anesthetics on the EEG. ECI was then defined as the absence of EEG activity greater than 2 μV/mm for a period of at least 3 minutes. Once this criterion was met, the surgeon was alerted and circulatory arrest was initiated. The presence of artifact did not prevent the determination of ECI if the underlying EEG could still be interpreted. No quantitative EEG analysis was used to determine ECI. The surgeon was alerted if the EEG had asymmetry that had not been noted in the baseline recording.
Anesthetic and Perfusion Technique
Cerebral oximetry was initiated prior to induction of anesthesia. Temperature was monitored at the NP and bladder. Anesthesia was induced using a combination of intravenous fentanyl and propofol. Anesthesia was maintained using a combination of propofol and remifentanil/fentanyl infusion. Neuromuscular blockade was achieved using standard non-depolarizing agents at the anesthesiologist's discretion. Propofol and remifentanil/fentanyl infusion rates were reduced by 50% at the onset of CPB and discontinued at 28°C NP. Infusions were restarted during rewarming upon resumption of electrocerebral activity. Body temperature was maintained during the rewarming and post-CPB phases with surface warming pads (Arctic Sun, Medivance Inc., Louisville, CO). Pharmacological neuroprotection was provided with methylprednisolone (1 gm) given intravenously prior to induction of anesthesia, and magnesium (4 gm) and lidocaine (200mg) given intravenously at the start of CPB (7). Lidocaine (200 mg) administration was repeated at the start of rewarming.
Non-pulsatile CPB was conducted using a membrane oxygenator following a crystalloid and mannitol prime and using an arterial line filter. Porcine heparin was administered as a bolus of 300 U/kg and supplemented to maintain an activated clotting time of greater than 480 seconds. Cooling and re-warming were performed using a Stockert 3T (Sorin Group USA Inc, Arvada, CO) or Hemotherm 400MR (Cincinnati Sub-Zero, Cincinnati, OH) heater cooler, with a 10°C gradient maintained between the blood inflow temperature and the NP temperature. A 5000 unit bolus of heparin was given prior to circulatory arrest. During CPB, temperature adjusted flow rates of 2.5 L/min/m2 were used and mean arterial pressure was maintained between 50-70 mm Hg. Alpha stat management for maintenance of normal pH, pO2 and pCO2 values was used. Transfusion of blood and blood products were directed by point-of-care tests (6, 8).
Operative Technique
Prior to the portion of the aortic reconstruction requiring circulatory arrest, the patient was cooled on CPB until ECI was detected on EEG. Concomitant procedures, such as coronary artery bypass grafting or valve procedures, were typically performed at 26°C, with full cooling resumed near completion of the concomitant procedure. Once ECI was achieved, the circulation was stopped, and the open aortic reconstruction portion of the case carried out. For proximal aortic repairs including hemi- or total arch replacement, antegrade cerebral perfusion (ACP) via the right axillary artery was typically used for adjunctive cerebral perfusion during the period of systemic DHCA; retrograde cerebral perfusion (RCP) was used in lieu of ACP in select hemi-arch cases in which the right axillary artery was not suitable for cannulation, generally because of a diameter less than 6 mm on preoperative imaging or dissection of the artery (3). For distal arch, descending, and thoracoabdominal repairs utilizing DHCA via the left chest, no adjunctive cerebral perfusion was employed given the short, usually < 20 minutes, cerebral DHCA times required (9). Cannulation for left chest repairs typically involved use of a percutaneous multistage venous cannula placed via the right common femoral vein; arterial cannulation was most commonly in the descending aorta, with the femoral artery being used much less often. The arterial cannulation site was then moved to the Dacron graft, usually via an integral graft sidearm, after completion of the open proximal aortic anastomosis. Following the portion of the aortic reconstruction requiring DHCA, CPB was reinstituted, and the patient gradually re-warmed following a 5-minute period of cold reperfusion for free radical washout (10, 11). The remainder of the aortic repair was completed during the rewarming phase.
Statistical Analysis
Multivariable models were used to assess patient-specific predictors of the NP temperature and cooling time required to achieve ECI. All 325 patients were included in the analysis of predictors of NP temperature at ECI. Patients undergoing a concomitant procedure (n=97, 30%) were excluded from the analysis of predictors of cooling time given that the concomitant procedure was usually performed at an intermediate point during cooling, leading to an artificially prolonged cooling time. Twelve candidate variables were preselected a priori for investigation, based on a review of the literature and expert opinion. These variables were: age, sex, race (white vs. non-white), height, weight, body mass index (BMI), body surface area (BSA), history of prior stroke, peripheral vascular disease, baseline hemoglobin, NP temperature at the start of cooling, and PaCO2 during cooling (5). Four of these potential predictors were highly correlated with each other (height, weight, BMI, BSA). Therefore, prior to inclusion in multivariable models, the univariate association between these variables and the outcome variables were investigated. Based on the strength of association with NP temperature at ECI and cooling time, BSA alone was chosen from this group for inclusion in the multivariable models. Univariate associations were tested by Pearson correlation coefficients for continuous variables and by t-tests for categorical variables. Multivariable linear regression models were created using the final nine preselected candidate predictors. Significance was assessed at a P value of less than 0.05. All analyses were performed with SAS statistical software version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
Operations and Outcomes
Baseline patient characteristics and comorbidities are shown in Table 1, procedural characteristics and cooling parameters are shown in Table 2, and 30-day/in-hospital outcomes are shown in Table 3. The majority of cases were elective, although EEG monitoring was used in 43 (13%) non-elective cases when staff and resources were available and patient stability allowed time for lead placement. Two-hundred and ninety one (90%) operations involved the proximal thoracic aorta and 34 (10%) involved the descending or thoracoabdominal aorta. Adjunctive antegrade (n=259, 80%) and/or retrograde (n=34, 10%) cerebral perfusion was used in 290 (89%) operations. Overall, 13 (4.0%) patients experienced stroke, of which 5 (1.5%) were considered ischemic in origin. Five (1.5%) patients experienced permanent paraparesis/paraplegia, and new onset dialysis was required by 7 (2.2%) patients. There were 14 (4.3%) 30-day/in-hospital deaths.
Table 1.
Clinical features
| Variable | |
|---|---|
| Patient characteristics | |
| - Age (years) | 58 ± 14 (19-85) |
| - Male | 227 (70%) |
| - White race | 256 (79%) |
| - Height (cm) | 175 ± 11 (140-208) |
| - Weight (kg) | 87 ± 21 (42-170) |
| - Body mass index (kg/m2) | 28.3 ± 6.0 (16.8-53.4) |
| - Body surface area (m2) | 2.0 ± 0.3 (1.3-2.9) |
| Patient comorbidities | |
| - Hypertension | 263 (81%) |
| - Hyperlipidemia | 168 (52%) |
| - Active or recent tobacco use | 160 (49%) |
| - Diabetes | 30 (9%) |
| - Coronary artery disease | 84 (26%) |
| - History of prior stroke | 28 (9%) |
| - Chronic obstructive pulmonary disease | 50 (15%) |
| - Renal insufficiency (preoperative creatinine > 1.5 mg/dl) | 29 (9%) |
| - Peripheral vascular disease | 22 (7%) |
| - Prior aortic surgery | 87 (27%) |
Data represented as mean ± standard deviation (range), or number (percent).
Table 2.
Procedural characteristics
| Variable | |
|---|---|
| Case status | |
| - Elective | 282 (87%) |
| - Urgent | 31 (10%) |
| - Emergent | 12 (4%) |
| ASA class | |
| -II | 7 (2%) |
| -III | 232 (71%) |
| -IV | 86 (26%) |
| Redo sternotomy | 64 (20%) |
| Principle procedure | |
| - Root or ascending aorta replacement only | 2 (1%) |
| - Root/ascending + hemi-arch | 256 (79%) |
| - Root/ascending + total arch | 33 (10%) |
| - Descending or thoracoabdominal | 34 (10%) |
| Concomitant procedure | 97 (30%) |
| Operative characteristics | |
| - Maximum aortic diameter (cm) | 5.8 ± 1.1 (3.7-13.9) |
| - Cross-clamp time (min, n=295) | 140 ± 43 (19-255) |
| - Cardiopulmonary bypass time (min) | 220 ± 52 (116-655) |
| - Systemic DHCA time w/out perfusion (min) | 27 ± 22 (9-158) |
| - Cerebral DHCA time w/out perfusion (min) | 5 ± 5 (0-28) |
| - Antegrade cerebral perfusion time (min, n=259) | 16 ±7 (1-50) |
| - Retrograde cerebral perfusion time (min, n=34) | 16 ± 6 (2-29) |
| Cooling parameters | |
| - Baseline hemoglobin (g/dl) | 13.3 ± 1.9 (7.4-18.1) |
| - NP temperature at start of cooling (°C) | 35.3 ± 0.9 (30.0-37.4) |
| - Cooling time to ECI* (min, n=228) | 69 ± 17 (29-136) |
| - Cooling rate* (°C/min, n=228) | 0.30 ± 0.07 (0.16-0.56) |
| - PaCO2 during cooling (mm Hg) | 43 ± 5 (28-56) |
| - NP temperature at ECI (°C) | 15.5 ± 1.9 (10.4-24.9) |
| - Nadir NP temperature (°C) | 14.1 ± 1.9 (10.1-24.9) |
Data represented as mean ± standard deviation (range), or number (percent). ASA = American Society of Anesthesiologists; DHCA = deep hypothermic circulatory arrest; ECI = electrocerebral inactivity; NP = nasopharyngeal; PaCO2 = arterial carbon dioxide tension.
excludes patients with concomitant procedure.
Table 3.
30-day/in-hospital adverse events
| Complication | |
|---|---|
| Acute renal failure (Creatinine > 2.0 and > 2x baseline) | 25 (7.7%) |
| New onset dialysis | 7 (2.2%) |
| Prolonged ventilation (> 24 hours) | 22 (6.8%) |
| Tracheostomy | 9 (2.8%) |
| Stroke (neurologic deficit lasting > 72 hours) | 13 (4.0%) |
| - Embolic stroke | 6 (1.8%) |
| - Ischemic stroke | 5 (1.5%) |
| - Intracerebral hemorrhage | 2 (0.6%) |
| Permanent paraparesis/paraplegia | 5 (1.5%) |
| Death | 14 (4.3%) |
Data represented as number (percent).
Predictors of NP Temperature at ECI
The mean NP temperature at ECI was 15.5°C ± 1.9°C (range 10.4°C to 24.9°C). The cumulative proportion of patients achieving ECI as a function of NP temperature is shown in Figure 1A. Cooling to a NP temperature of 12.7°C was required to achieve ECI in > 95% of patients, whereas only 7% of patients achieved ECI by 18°C NP. On univariate and multivariable analysis, no patient-specific factors were predictive of the NP temperature at ECI (Table 4). The r2 value of the full model was 0.04, indicating that the nine variables accounted for 4% of the variability in NP temperature at ECI.
Figure 1.
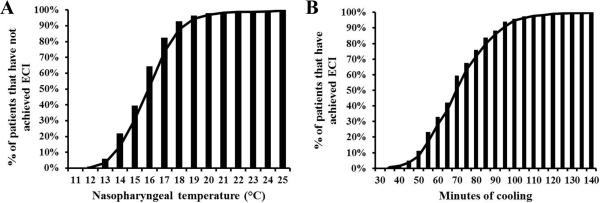
(A) Nasopharyngeal temperature (n=325) and (B) cooling time required to achieve electrocerebral inactivity (ECI) (n=228, excludes patients undergoing a concomitant procedure).
Table 4.
Selected predictors of nasopharyngeal temperature (°C) at electrocerebral inactivity (multivariable linear regression model, n=325, r2=0.04).
| Variable | Parameter estimate | Standard error | T Value | P Value |
|---|---|---|---|---|
| Age (years) | −0.01 | 0.01 | −1.72 | 0.09 |
| Male | 0.38 | 0.28 | 1.33 | 0.19 |
| White race | −0.08 | 0.28 | −0.28 | 0.78 |
| Body surface area (m2) | −0.003 | 0.46 | −0.01 | 0.99 |
| History of prior stroke | −0.01 | 0.38 | −0.02 | 0.98 |
| Peripheral vascular disease | 0.50 | 0.43 | 1.18 | 0.24 |
| Baseline hemoglobin (g/dl) | 0.01 | 0.07 | 0.21 | 0.83 |
| NP temperature at start of cooling (°C) | 0.05 | 0.07 | 0.73 | 0.47 |
| PaCO2 during cooling (mm Hg) | 0.04 | 0.02 | 1.74 | 0.08 |
NP = nasopharyngeal; PaCO2 = arterial carbon dioxide tension.
Predictors of Cooling Time
The mean cooling time required to achieve ECI was 69 ± 17 minutes (range 29 to 136 minutes). The cumulative proportion of patients achieving ECI as a function of cooling time is shown in Figure 1B. Cooling for a duration of 97 minutes was required to achieve ECI in > 95% of patients. Only 11% of patients achieved ECI after 50 minutes of cooling. On univariate analysis, higher NP temperature at the start of cooling (r = 0.25; p = 0.0001), male gender (72 minutes vs. 63 minutes; p = 0.0003), larger BSA (r = 0.37; p = < 0.0001), higher baseline hemoglobin (r = 0.14; p = 0.04), and white race (71 minutes vs. 63 minutes; p = 0.002) were associated with longer cooling time. On multivariable analysis, independent predictors of longer cooling time were: BSA (+18 minutes/m2; p = < 0.0001), white race (+7 minutes; p = 0.01), and NP temperature at the start of cooling (+3 minutes/°C; p = 0.01) (Table 5). The r2 value of the full model was 0.21, indicating that the nine variables accounted for 21% of the variability in cooling time. Given the novel association between race and cooling time, candidate covariates were compared between patients of white and non-white race. Patients of white race were found to have larger BSA (2.1 m2 vs. 2.0 m2; p = 0.006), higher baseline hemoglobin (13.8 g/dl vs. 12.0 g/dl; p < 0.0001), and higher NP temperature at the start of cooling (35.3°C vs. 35.0°C; p = 0.03) when compared to patients of non-white race.
Table 5.
Selected predictors of the cooling time required to achieve electrocerebral inactivity (multivariable linear regression model, n=228, r2=0.21).
| Variable | Parameter estimate | Standard error | T Value | P Value |
|---|---|---|---|---|
| Age (years) | 0.05 | 0.08 | 0.60 | 0.55 |
| Male | 3.57 | 2.82 | 1.26 | 0.21 |
| White race | 7.21 | 2.89 | 2.50 | 0.01 |
| Body surface area (m2) | 18.46 | 4.52 | 4.09 | < 0.0001 |
| History of prior stroke | −5.93 | 3.79 | −1.57 | 0.12 |
| Peripheral vascular disease | −1.64 | 5.23 | −0.31 | 0.75 |
| Baseline hemoglobin (g/dl) | −0.87 | 0.72 | −1.21 | 0.23 |
| NP temperature at start of cooling (°C) | 2.77 | 1.08 | 2.57 | 0.01 |
| PaCO2 during cooling (mm Hg) | −0.28 | 0.24 | −1.17 | 0.24 |
NP = nasopharyngeal; PaCO2 = arterial carbon dioxide tension.
DISCUSSION
In the present study we report patient outcomes and predictors of the NP temperature and cooling time required to achieve ECI in the largest reported series of adult patients undergoing thoracic aortic surgery with DHCA and EEG monitoring. Our data reaffirm that excellent patient outcomes can be achieved with DHCA when patients are cooled to ECI with EEG surveillance. In our series, cooling to a NP temperature of 12.7°C or for a duration of 97 minutes was required to achieve ECI in > 95% of patients. However, no patient-specific factors were predictive of the NP temperature at ECI, but BSA, race, and starting temperature were predictive of the cooling time required to achieve ECI.
Debate continues within the cardiothoracic surgical community regarding the optimal temperature and cooling strategy for hypothermic circulatory arrest. Cooling protocols vary widely between institutions, with end-points of cooling ranging from ECI determined by EEG, fixed temperature targets between 15°C and 28°C measured at the rectum, bladder, or nasopharyngeal area (3, 5), or predetermined cooling durations (12). Cooling to ECI by EEG ensures maximal suppression of metabolic activity prior to circulatory arrest, while minimizing CPB time and hypothermic injury by avoiding excessive cooling, and it is the preferred strategy at our institution and a number of other experienced aortic centers (3, 12, 13). Our study reaffirms the safety of this cooling approach on short-term patient outcomes. The rates of central nervous system injury, visceral organ injury, and death in our cohort were lower than national numbers and lower than the majority of single-institution reports employing lesser degrees of hypothermia (3). While the study population reported included primarily elective cases, 13% of operations were non-elective and 10% involved the descending or thoracoabdominal aorta, patient groups where operative mortality often ranges between 9-25% (14, 15).
Given the personnel and resources required for intraoperative EEG monitoring, these services are usually only available in the elective setting at select centers. For this reason, patient-specific predictors of ECI would be valuable to help tailor cooling protocols for individual patients in the absence of EEG monitoring. We first assessed predictors of the NP temperature required to achieve ECI in 325 EEG monitored patients. Our data from this analysis appears similar to prior reports. In our series, ECI occurred at NP temperatures between 10.4°C and 24.9°C (mean 15.5°C) compared to between 12.5°C and 27.2°C (mean 17.8°C, n = 47) reported by Stecker and between 10.1°C and 24.1°C (mean 16.9°C, n = 56) reported by Coselli (5, 13). In addition, neither our study nor the Stecker study identified any patient-specific variables that were predictive of the temperature at which ECI occurs (5). Though speculative, the mean temperature at which ECI occurred in our study may have been lower than in others due to the avoidance of isofluorane and thiopental and by allowing a 15 minute anesthetic washout period prior to calling ECI. Anesthetic agents can suppress cerebral activity and artificially raise the temperature threshold at which ECI occurs (16).
The cooling time required to achieve ECI ranged from 29 to 136 minutes (mean 69 minutes) amongst 228 monitored patients in our study, and was significantly longer than that reported by Stecker (range 12 to 50 minutes, mean 27.5 minutes, n = 47) or Coselli (range 20 to 107 minutes, mean 41 minutes, n = 56) (5, 13). Correspondingly, the cooling rate reported by Stecker was nearly twice as rapid as in our study (0.61-0.70°C/min versus 0.30°C/min). This discrepancy is not easily explained by disparate perfusion practices, as CPB flow rates during cooling averaged 2.15-2.24 L/min/m2 in the Stecker study and were estimated to be 2.5 L/min/m2 in our study. Patient size was also similar between the two studies (1.86-1.96 m2 versus 2.0 m2). We suspect differences in cooling protocols, such as use of different heater-cooler exchangers or temperature gradients, may account for these differences between centers. In addition, the anesthetic washout period employed at our center may further extend the time to ECI. Regardless of the cause, cooling times do not appear generalizable across institutions, and cooling for a predetermined time period based on literature reports is likely an unreliable method of ensuring ECI.
Patient-specific predictors of the cooling time required to achieve ECI in our study included larger BSA (+18 minutes/m2), white race (+7 minutes versus non-white race), and a higher starting temperature (+3 minutes/°C). Larger BSA was previously identified by Stecker and colleagues as a predictor of the time required to cool to burst suppression (5) and was also recently found to be the most significant determinant of effective cooling during endovascular hypothermia (17). This is presumably secondary to the fact that larger body masses are slower to achieve thermal equilibrium. Identification of starting temperature as a predictor of cooling time appears merely to be a function of the cooling rate, as 3.3 minutes were required on average to cool by 1°C in our study, which is nearly equivalent to the predictive value of starting temperature on cooling time. The effect of race on cooling time is a novel observation and may reflect differences in neurophysiology between patients of different genetic backgrounds (18). Alternatively, white patients were noted to have larger BSA, higher baseline hemoglobin, and higher NP temperature at the start of cooling then non-white patients. These variables may have confounded the association between race and cooling time and driven the effect of race to higher impact. However, overall these variables and others were poorly predictive of cooling time and accounted for only 21% of the variability between patients. In the Stecker study, independent predictors of cooling time that were not validated in our patient cohort included increased hemoglobin concentration and decreased PaCO2 during cooling (5).
The lack of predictability of ECI likely reflects the complexity of factors inherently involved in the neurophysiologic reaction to hypothermia. Even minor variations in anesthetic technique alter EEG waveforms, which may affect both the time and temperature at which ECI occurs (19). Further, it may be overly simplistic to assume that only a few patient variables could accurately and reliably predict ECI, and it is more likely that a multitude of factors contribute (16, 20, 21). In the absence of stronger predictive models, monitoring with EEG appears to be the only reliable means of determining ECI prior to DHCA. In the absence of EEG monitoring, our study reaffirms that cooling below 13°C achieves ECI in the majority of patients.
Limitations
Our study contains several important limitations. Anesthetic technique was not included in the analysis as a covariate. However, we believe that our protocol-driven anesthetic management likely minimized the variability in technique. Similarly, surgical technique was not analyzed as a covariate; however, all procedures were performed with standardized techniques by two surgeons. Finally, due to the retrospective nature of some of the outcome measures, the applicability of our dataset to other patient populations may be limited. As the present study is observational, unmeasured confounders not captured by patient medical records could have influenced our findings.
Conclusion
Our study reaffirms that cooling to ECI with DHCA affords excellent neurologic and visceral protection and validates that cooling to a NP temperature between 12-13°C achieves ECI in nearly all patients in a large contemporary series. The cooling time required to achieve ECI appears highly variable between patients and centers and should not be used to reliably predict ECI. Patient-specific factors were poorly predictive of the temperature or cooling time required to achieve ECI, necessitating EEG monitoring for reliable detection of ECI. When EEG monitoring is unavailable, cooling below 13°C NP appears to be the most reliable method of ensuring ECI.
Acknowledgments
Funding source: American Heart Association (Dallas, TX) Scientist Development Grant (to MLJ), Foundation for Anesthesia Education and Research (Rochester, MN) Mentored Research Training Grant (to MLJ), Thoracic Surgery Foundation for Research and Education (Chicago, IL) Research Fellowship (to NDA), and National Institutes of Health (Bethesda, MD) grants T32-CA093245 (to SDB), T32-HL069749 (to JBW) and U01-HL088953 (to JBW).
Footnotes
Presented at the American Association for Thoracic Surgery Aortic Symposium 2012, New York, New York, April 26-27, 2012
Disclosures: None
REFERENCES
- 1.Michenfelder JD, Milde JH. The effect of profound levels of hypothermia (below 14 degrees C) on canine cerebral metabolism. J Cereb Blood Flow Metab. 1992 Sep;12(5):877–80. doi: 10.1038/jcbfm.1992.120. [DOI] [PubMed] [Google Scholar]
- 2.Mezrow CK, Midulla PS, Sadeghi AM, Gandsas A, Wang W, Dapunt OE, et al. Evaluation of cerebral metabolism and quantitative electroencephalography after hypothermic circulatory arrest and low-flow cardiopulmonary bypass at different temperatures. J Thorac Cardiovasc Surg. 1994 Apr;107(4):1006–19. [PubMed] [Google Scholar]
- 3.Lima B, Williams JB, Bhattacharya SD, Shah AA, Andersen N, Gaca JG, et al. Results of proximal arch replacement using deep hypothermia for circulatory arrest: is moderate hypothermia really justifiable? Am Surg. 2011 Nov;77(11):1438–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss AJ, Lin HM, Bischoff MS, Scheumann J, Lazala R, Griepp RB, et al. A propensity score-matched comparison of deep versus mild hypothermia during thoracoabdominal aortic surgery. J Thorac Cardiovasc Surg. 2012 Jan;143(1):186–93. doi: 10.1016/j.jtcvs.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Stecker MM, Cheung AT, Pochettino A, Kent GP, Patterson T, Weiss SJ, et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg. 2001 Jan;71(1):14–21. doi: 10.1016/s0003-4975(00)01592-7. [DOI] [PubMed] [Google Scholar]
- 6.Andersen ND, Bhattacharya SD, Williams JB, Fosbol EL, Lockhart EL, Patel MB, et al. Intraoperative use of low-dose recombinant activated factor VII during thoracic aortic operations. Ann Thorac Surg. 2012 Jun;93(6):1921–8. doi: 10.1016/j.athoracsur.2012.02.037. discussion 8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlon N, Grocott HP, Mackensen GB. Neuroprotection during cardiac surgery. Expert Rev Cardiovasc Ther. 2008 Apr;6(4):503–20. doi: 10.1586/14779072.6.4.503. [DOI] [PubMed] [Google Scholar]
- 8.Williams JB, Phillips-Bute B, Bhattacharya SD, Shah AA, Andersen ND, Altintas B, et al. Predictors of massive transfusion with thoracic aortic procedures involving deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2011 May;141(5):1283–8. doi: 10.1016/j.jtcvs.2010.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gega A, Rizzo JA, Johnson MH, Tranquilli M, Farkas EA, Elefteriades JA. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg. 2007 Sep;84(3):759–66. doi: 10.1016/j.athoracsur.2007.04.107. discussion 66-7. [DOI] [PubMed] [Google Scholar]
- 10.Ehrlich MP, McCullough J, Wolfe D, Zhang N, Shiang H, Weisz D, et al. Cerebral effects of cold reperfusion after hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2001 May;121(5):923–31. doi: 10.1067/mtc.2001.113175. [DOI] [PubMed] [Google Scholar]
- 11.Di Mauro M, Iaco AL, Di Lorenzo C, Gagliardi M, Varone E, Al Amri H, et al. Cold reperfusion before rewarming reduces neurological events after deep hypothermic circulatory arrest. Eur J Cardiothorac Surg. 2012 May 30; doi: 10.1093/ejcts/ezs281. [DOI] [PubMed] [Google Scholar]
- 12.Bavaria JE, Pochettino A, Brinster DR, Gorman RC, McGarvey ML, Gorman JH, et al. New paradigms and improved results for the surgical treatment of acute type A dissection. Ann Surg. 2001 Sep;234(3):336–42. doi: 10.1097/00000658-200109000-00007. discussion 42-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coselli JS, Crawford ES, Beall AC, Jr., Mizrahi EM, Hess KR, Patel VM. Determination of brain temperatures for safe circulatory arrest during cardiovascular operation. Ann Thorac Surg. 1988 Jun;45(6):638–42. doi: 10.1016/s0003-4975(10)64766-2. [DOI] [PubMed] [Google Scholar]
- 14.Rigberg DA, McGory ML, Zingmond DS, Maggard MA, Agustin M, Lawrence PF, et al. Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg. 2006 Feb;43(2):217–22. doi: 10.1016/j.jvs.2005.10.070. discussion 23. [DOI] [PubMed] [Google Scholar]
- 15.Williams JB, Peterson ED, Zhao Y, O'Brien SM, Andersen ND, Miller DC, et al. Contemporary results for proximal aortic replacement in north america. J Am Coll Cardiol. 2012 Sep 25;60(13):1156–62. doi: 10.1016/j.jacc.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blume WT. Drug effects on EEG. J Clin Neurophysiol. 2006 Aug;23(4):306–11. doi: 10.1097/01.wnp.0000229137.94384.fa. [DOI] [PubMed] [Google Scholar]
- 17.Lyden P, Ernstrom K, Cruz-Flores S, Gomes J, Grotta J, Mullin A, et al. Determinants of effective cooling during endovascular hypothermia. Neurocrit Care. 2012 Jun;16(3):413–20. doi: 10.1007/s12028-012-9688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Graaf AS, Lombard CJ, Claassen DA. Influence of ethnic and geographic factors on the classic photoparoxysmal response in the electroencephalogram of epilepsy patients. Epilepsia. 1995 Mar;36(3):219–23. doi: 10.1111/j.1528-1157.1995.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 19.Jameson LC, Sloan TB. Using EEG to monitor anesthesia drug effects during surgery. J Clin Monit Comput. 2006 Dec;20(6):445–72. doi: 10.1007/s10877-006-9044-x. [DOI] [PubMed] [Google Scholar]
- 20.Reilly EL. Electrocerebral inactivity as a temperature effect: unlikely as an isolated etiology. Clin Electroencephalogr. 1981 Apr;12(2):69–71. doi: 10.1177/155005948101200205. [DOI] [PubMed] [Google Scholar]
- 21.Rekand T, Sulg IA, Bjaertnes L, Jolin A. Neuromonitoring in hypothermia and in hypothermic hypoxia. Arctic Med Res. 1991;50(Suppl 6):32–6. [PubMed] [Google Scholar]


