Abstract
Schizophrenia is a severe mental illness that often co-occurs with and can be exacerbated by other psychiatric conditions. There have not been adequate efforts to examine schizophrenia and psychiatric comorbidity beyond pairwise examination using clusters of diagnoses. This study used latent class analysis to characterize patterns of 5-year psychiatric comorbidity among a national sample of adults with schizophrenia. Baseline data from 1446 adults with schizophrenia across 57 sites in the United States were analyzed. Three latent classes were identified labeled Solely Schizophrenia, Comorbid Anxiety and Depressive Disorders with Schizophrenia, and Comorbid Addiction and Schizophrenia. Adults in the Solely Schizophrenia class had significantly better mental health than those in the two comorbid classes, but poorer illness and treatment insight than those with comorbid anxiety and depressive disorders. These results suggest that addiction and schizophrenia may represent a separate latent profile from depression, anxiety, and schizophrenia. More research is needed on how treatment can take advantage of the greater insight possessed by those with schizophrenia and comorbid anxiety and depression.
Keywords: Substance-Related Disorders, Diagnosis, Classification, Methods, Depression, Anxiety
1.0 Introduction
Psychiatric comorbidity is recognized as an important clinical problem in the diagnosis, treatment, and prevention of mental illness. Among adults with schizophrenia, numerous studies have shown an increased prevalence of anxiety, depressive, and substance use disorders greater than that found in the general population (Buckley et al., 2009). Most studies have focused on individual comorbid diagnoses with schizophrenia even though comorbidities are often far more complex than single dualities (Kessler et al., 1994; Ginzburg et al., 2010; Weich et al., 2011). There have been few efforts to characterize patterns of psychiatric comorbidity beyond pairwise examination of individual diagnoses among adults with schizophrenia.
For adults with schizophrenia, psychiatric comorbidity has most often been conceptualized in terms of “dual diagnoses”, referring to a co-occurring substance use disorder (RachBeisel et al., 1999; Drake et al., 2007). Epidemiological and clinical studies have consistently found that nearly half of all adults with schizophrenia have a lifetime substance use disorder (Regier et al., 1990; Kessler et al., 1997; O'Daly et al., 2005). Alcohol and drug use disorders have long been recognized to interfere with schizophrenia as comorbid substance abuse is related to greater positive symptoms, higher rate of relapse, and worse physical and mental health in schizophrenia (Buckley et al., 2009). One prominent hypothesis to account for comorbid substance abuse among those with schizophrenia is the “self-medication” hypothesis, which postulates that individuals abuse substances to help relieve painful affects, emotions, states of distress, or other mental health symptoms (Khantzian, 1997), although the theory remains debatable (Mueser et al., 1992).
Depression is another psychiatric disorder that often co-occurs with schizophrenia. An estimated 23–57% of adults with schizophrenia have comorbid depression (Buckley et al., 2009). Schizophrenia with comorbid depression imports a poorer clinical outcome and lower overall quality of life than those with no depression (Sim et al., 2004; Buckley et al., 2009). Yet interestingly, adults with schizophrenia with comorbid depression have been found to have greater awareness of their mental illness than those with no comorbid depression, suggesting comorbidity may be related to a greater degree of insight but a poorer quality of life (Sim et al., 2004).
Anxiety disorders are a third category of psychiatric diagnoses that have been examined in schizophrenia. The prevalence of different anxiety disorders among adults with schizophrenia vary from an estimated 10–15% with a comorbid panic disorder to an estimated 12–29% with a comorbid posttraumatic stress disorder, and an estimated 12–23% with comorbid obsessive compulsive disorder (Buckley et al., 2009; Achim et al., 2011). Studies have shown that adults with schizophrenia and a comorbid anxiety disorder report greater general psychopathology, but few other clinical differences (Tibbo et al., 2003) and some have even found more positive outcomes associated with comorbidity (Garvey et al., 1991; Emsley et al., 1999). Particular attention has been given to schizophrenia with a comorbid obsessive compulsive disorder as considerable evidence suggests a “schizo-obessive” subtype (Poyurovsky et al., 2003; Bottas et al., 2005; Buckley et al., 2009).
In the current study, we used latent class analysis to examine patterns of psychiatric comorbidity among a national sample of U.S. adults diagnosed with schizophrenia using data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study. The comorbid psychiatric diagnoses examined included depression, anxiety disorders, and substance use disorders. Latent class analysis represents a unique analytic approach to examine multiple comorbidities. Unlike simple descriptive or cluster analytic approaches, latent class analysis characterizes individuals based on probabilistic models of subgroup membership (which is more reflective of reality) instead of needing to specify subgroups beforehand and allows quantitative comparison models of fit across several possible diagnostic combinations (of which there are a possible 32 combinations in this study). This study aimed to use this advanced statistical technique to not only characterize common patterns of comorbidity among adults with schizophrenia, but relate them to differences in symptomatology, illness and treatment insight, and quality of life as these have been identified as important outcomes in schizophrenia (Andreasen et al., 1990; Pini et al., 2001; Hofer et al., 2004).
2.0 Methods
2.1 Sample
Baseline data from a total of 1446 adults participating in CATIE (Lieberman et al., 2005a) were reanalyzed for this study. CATIE was conducted between January 2001 and December 2004 at 57 U.S. sites (16 university clinics, 10 state mental health agencies, 7 Veterans Affairs medical centers, 6 private nonprofit agencies, 4 private-practice sites, and 14 mixed system sites) and is one of the largest randomized controlled drug trials of adults with schizophrenia to date. Details of CATIE have been described elsewhere (Stroup et al., 2003; Lieberman et al., 2005b). All participants were over the age of 18, had received a diagnosis of schizophrenia as determined by the Structured Clinical Interview of the Diagnosis (SCID; First et al., 1997) based on criteria from the Diagnostic and Statistical Manual of Mental Disorders-IV (American Psychiatric Association, 2000) and were judged to be able to take oral antipsychotic medications. Patients were excluded if they had a diagnosis of schizoaffective disorder, mental retardation, or other cognitive disorders; had a history of serious adverse reactions to the proposed treatments; had had only one schizophrenic episode; had a history of treatment resistance; were pregnant or breast-feeding; or had a serious and unstable medical condition. But in general, there were few exclusion criteria as CATIE was an effectiveness trial and efforts were made to draw from a representative sample of adults with schizophrenia as they are treated in “real world” settings.
2.2 Measures
Comorbid psychiatric diagnoses were made using the SCID (First et al., 1997) administered by trained research assistants and were based on 5-year histories.
The Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987) is widely used 30-item measure, administered through a semi-structured interview, that assesses the severity of positive and negative symptoms of schizophrenia along with global psychopathology. The three subscales that correspond to these domains are the Positive scale, Negative scale, and General Psychopathology scale.
The Short Form-12 (SF-12; Ware et al., 1996) health survey is an internationally used 12-item measure that assesses overall health-related quality of life. Scores range from 0 to 100, with a score of 50 representing the average level of functioning in the general population and each 10-point interval representing one standard deviation above or below the population mean. Scores are calculated for a physical component and a mental health component summary sub-score.
The Calgary Depression Scale (CDS; Addington et al., 1992) is a 9-item structured interview specifically developed to assess the level of depression in schizophrenia. Each item is rated on a 4-point measure from 0 (absent) to 3 (severe) on symptoms such as depressed mood, hopelessness, guilt, insomnia, and suicide.
The Insight and Treatment Attitudes Questionnaire (ITAQ; McEvoy et al., 1989) is a semi-structured interview consisting of 11-items rated on a 11-item scale from 0 (no insight) to 2 (good insight) designed for individuals with schizophrenia to reflect their awareness of their illness and need for care. Items are summated for a total scale score.
One item was used from the Lehman Quality of Life Interview (Lehman, 1988) that asks participants to rate their subjective quality of life on a 7-point scale from 1 (terrible) to 7 (delighted).
2.3 Data Analysis
Latent class analyses were conducted fitting two to five-class solutions to the psychiatric diagnostic data. Following recommendations by Jung and Wickrama (2008) to address problems with nonconvergence and local solutions, 100 random sets of starting values with 10 iterations were specified and best loglikelihood values were replicated. Several fit indices were generated, including the Akaike Information Criterion (AIC; Akaike, 1987), the Bayesian Information Criterion (BIC; Schwarz, 1978), the sample size-adjusted BIC, and entropy values. The final number of classes was determined by these fit indices (lower AIC, BIC, adjusted BIC scores and higher entropy values indicating better fit), along with the Lo-Mendell-Rubin (LMR; Lo et al., 2001) test and the bootstrapped likelihood ratio (LR; McLachlan, 1987; Nylund et al., 2007) test which compares n classes to n-1 classes with a non-significant value indicating the n-1 class model should be accepted. All of these fit indices were considered in determining the best fitting model as suggested by experts (Muthen, 2004; Nylund et al., 2007) and done in recent studies (e.g., Wolf et al., 2012). Mplus version 6.12 was used for latent class analyses.
Participants were assigned to the latent class to which they had the highest probability of being a member (average latent class probabilities were 0.96. 0.90, and 0.84, for Class 1 to 3, respectively). Analyses of variance and chi-square tests were conducted to compare participants in different latent classes on demographic and clinical characteristics. Post-hoc tests were conducted with the Hochberg’s GT2 test and pairwise chi-square tests. Significance for all statistical analyses were conducted at the p<.01 level. SPSS version 17.0 was used to test differences between the latent classes.
3.0 Results
Of a sample of 1446 adults with schizophrenia, 400 (27.7%) had comorbid major depression, 355 (24.6%) had comorbid alcohol abuse/dependency, 419 (29.0%) had comorbid drug abuse/dependency, 73 (5.0%) had comorbid obsessive compulsive disorder, and 197 (13.6%) had some other comorbid anxiety disorder in the past 5 years. Overall, 810 (56.0%) of the total sample had at least one comorbid mental health disorder with schizophrenia.
As shown in Table 1, latent class analysis revealed that three classes provided the best fit statistics. A three-class model provided a significantly better fit than a two-class model, as evidenced by lower AIC and BIC values, higher entropy values, and significant Lo-Mendell-Rubin and bootstrapped likelihood ratio tests. Models with more than four-classes did not provide better fit than the three-class model as AIC and BIC values were not lower, entropy values were not higher, and neither likelihood ratio test was significant.
Table 1.
Fit indices for 2, 3, and 4 class-models of comorbid mental health diagnoses among adults with schizophrenia
| # of classes | AIC | BIC | Adjusted BIC | Entropy | LMR test | Bootstrapped LR test |
|---|---|---|---|---|---|---|
| 2 | 6457.93 | 6515.97 | 6481.03 | 0.68 | p< 0.001 | p< 0.001 |
| 3 | 6352.01 | 6441.71 | 6387.71 | 0.80 | p< 0.001 | p< 0.001 |
| 4 | 6352.05 | 6473.41 | 6400.35 | 0.76 | p= 0.30 | p= 0.12 |
| 5 | 6359.63 | 6512.65 | 6420.53 | 0.72 | p= 0.27 | P= 0.50 |
| 6 | 6368.29 | 6552.97 | 6441.79 | 0.87 | p= 0.45 | p= 0.43 |
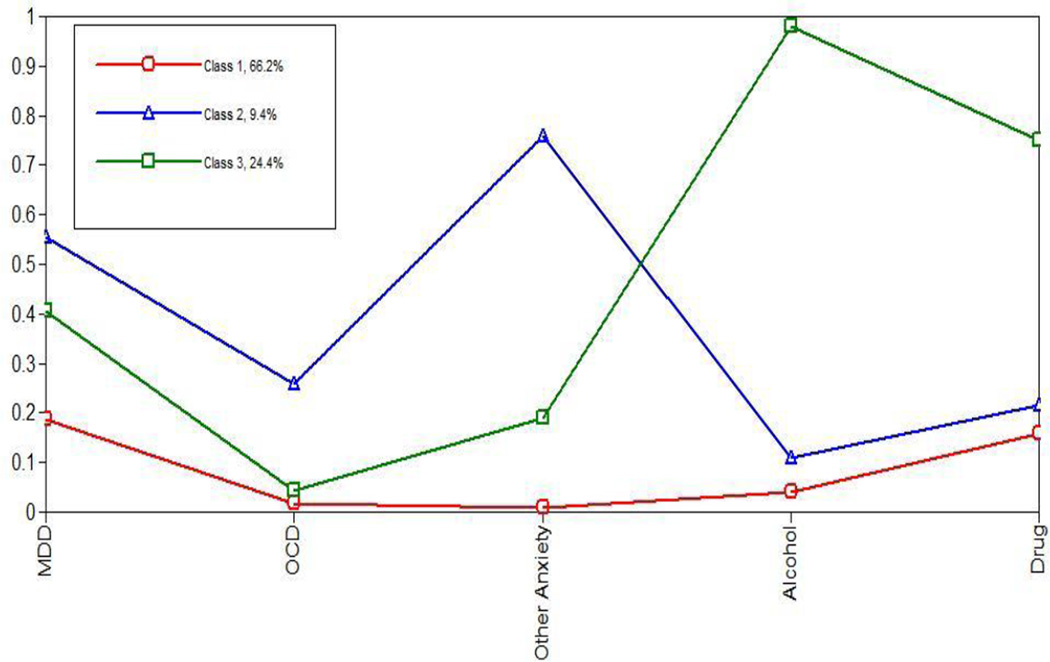
A three-class model illustrated meaningful differences between individuals (Figure 1). Class 1 (labeled Solely Schizophrenia) consisted of adults who tended to have schizophrenia but low probabilities (less than 20%) of having any other psychiatric diagnoses. Class 2 (labeled Comorbid Anxiety and Depressive Disorders with Schizophrenia) consisted of adults who had schizophrenia and high probabilities (greater than 50%) of having a comorbid anxiety and depressive disorder. Class 3 (labeled Comorbid Addiction and Schizophrenia) consisted of adults who had schizophrenia and high probabilities (greater than 70%) of having a comorbid alcohol abuse/dependency and drug abuse/dependency diagnoses.
Figure 1. Three classes of comorbid diagnoses among adults with schizophrenia.
Note: MDD= Major Depressive Disorder, OCD= Obsessive Compulsive Disorder; Class 1= Solely Schizophrenia, Class 2= Comorbid Anxiety and Depressive Disorders with Schizophrenia, Class 3- Comorbid Addiction and Schizophrenia
As shown in Table 2, there were a few significant demographic differences between individuals in the three classes. Those in Class 1 (Solely Schizophrenia) were significantly older than those in Class 3 (Comorbid Addiction and Schizophrenia); those in Class 3 were more likely to be male than those in Class 1 and 2. Those in Class 2 were more educated and were more likely to be married than those in Class 3. Although those in Class 1 reported the longest duration of illness, there were no significant differences on Positive and Negative subscale scores on the PANSS; but they did have lower scores on the General Psychopathology subscale of the PANNS compared to those in Class 3.
Table 2.
Demographic and clinical differences between three classes of adults with schizophrenia (N= 1,446)
| Class 1: Solely Schizophrenia (n= 957) |
Class 2: Comorbid Anxiety and Depressive Disorders with Schizophrenia (n= 136) |
Class 3: Comorbid Addiction and Schizophrenia (n= 353) |
Test of Difference (F or X2) |
Post-hoc tests | |
|---|---|---|---|---|---|
| Age | 41.39 (11.46) | 40.28 (9.32) | 38.22 (10.47) | 10.65** | 1>3 |
| Race- White | 570 (60.00%) | 89 (65.44%) | 208 (58.92%) | 5.14 | ns |
| Gender- male | 581 (72.99%) | 64 (52.89%) | 264 (84.89%) | 50.76** | 3>1>2 |
| Education (years) | 12.16 (2.30) | 12.44 (2.41) | 11.76 (1.98) | 6.09* | 2>3 |
| Married | 101 (12.69%) | 24 (19.83%) | 23 (7.40%) | 9.46* | 2>3 |
| Illness duration | 15.13 (10.87) | 12.08 (9.35) | 13.24 (10.50) | 7.23* | 1>2 |
| PANSS- Positive | 18.25 (5.77) | 18.17 (5.36) | 19.15 (5.34) | 3.51 | ns |
| PANSS- Negative | 20.28 (6.45) | 20.23 (6.65) | 19.86 (6.23) | 0.57 | ns |
| PANSS- General | 36.34 (9.28) | 38.73 (9.68) | 38.21 (9.09) | 7.79** | 3>1 |
| CDS1 score | 12.94 (4.10) | 16.07 (5.25) | 14.22 (4.33) | 37.30** | 2>3>1 |
| ITAQ2 score | 17.77 (5.24) | 19.39 (4.22) | 18.61 (4.73) | 8.27** | 2>1 |
| SF-123- Physical | 48.66 (9.83) | 45.66 (11.76) | 47.95 (10.31) | 5.31* | 1>2 |
| SF-12- Mental | 42.15 (11.85) | 36.59 (10.76) | 39.29 (10.83) | 18.42** | 1>2,3 |
| Subjective QOL4 | 4.45 (1.39) | 4.04 (1.25) | 4.10 (1.41) | 11.42** | 1>2,3 |
p< 0.01,
p< 0.001
CDS= Calgary Depression Scale
ITAQ= Insight and Treatment Attitudes Questionnaire
SF-12= Medical Outcomes Short Form-12 item survey
QOL= Quality of Life
Notably, on clinical differences, individuals in Class 1 (Solely Schizophrenia) had significantly higher SF-12 Mental Component Summary scores, higher subjective quality of life scores, and lower CDS scores than those in Class 2 and 3, all reflecting better mental health. However, those in Class 1 also had significantly lower ITAQ scores than those in Class 2 indicating poorer insight into their illness and need for treatment. When these analyses were repeated controlling for demographic differences, these clinical differences remained.
4.0 Discussion
Among a national sample of adults with schizophrenia, the majority (56%) had a comorbid anxiety, depression, or substance use disorder diagnosis in the past 5 years, consistent with previous studies on people with severe mental illness (RachBeisel et al., 1999; Drake et al., 2007; Buckley et al., 2009; Achim et al., 2011). It appears there are three distinct latent profiles of psychiatric comorbidity among adults with schizophrenia: those without any comorbidity, those with comorbid anxiety and depressive disorder, and those with substance use disorders. These latent profiles contributes to ongoing discussions about the nosology of mental illness by showing the common overlap between anxiety and depressive disorders (Watson, 2005) and their separation from substance use disorders.
Nearly a quarter of the sample was classified as a group with schizophrenia and a high probability of a comorbid substance use disorder. This “dual diagnosis” group is known to be associated with poor clinical functioning and treatment attrition (RachBeisel et al., 1999; Drake et al., 2007), which is consistent with our findings when compared to those in the “solely schizophrenia” group. No direct support can be provided from this cross-sectional study on the “self-medication” hypothesis, i.e., substances are often used to medicate psychiatric symptoms (Khantzian, 1985; Markou et al., 1998; Swendsen et al., 2000), but those with comorbid substance use disorders tended to reported more general psychopathology (on the PANSS) and depressive symptoms than those with no comorbid disorders suggesting substance use may be related to efforts to self-medicate. Nonetheless, our finding underscores the need for continued efforts to focus on addiction problems among adults with schizophrenia (Mueser et al., 1992; Fazel et al., 2009; Volkow, 2009).
Adults with schizophrenia and comorbid anxiety and depressive disorders constituted the smallest group and represented a separate profile from those with comorbid substance use disorders. We did not find a particular “schizo-obsessive” subtype as others have suggested (Poyurovsky et al., 2003; Bottas et al., 2005; Buckley et al., 2009), even though we treated obsessive compulsive disorder separate from other anxiety disorders. However, this does not mean such a schizophrenia subtype does not exist, but merely suggests it is not a major latent profile and may be subsumed by other comorbid psychiatric conditions.
Overall, the results illustrated the potential effect of psychiatric comorbidity on clinical functioning among adults with schizophrenia. Adults with only schizophrenia and low probability of comorbid mental disorders were older and reported better mental health and greater quality of life than those with comorbid mental disorders, regardless if disorders were anxiety, depression, and/or substance abuse. This finding is consistent with a large body of literature documenting the negative effects of comorbidity in schizophrenia (Mueser et al., 1992; Tibbo et al., 2003; Buckley et al., 2009).
Interestingly, adults with only schizophrenia reported less insight about their illness and need for treatment compared to those with schizophrenia and comorbid anxiety and depression, consistent with several previous studies (Sim et al., 2004; Lincoln et al., 2007). These findings may reflect the idea that although those with psychiatric comorbidity report lower quality of life, they are also more aware and accepting of their illness possibly because they have multiple mental health problems. Further research is needed to investigate the relation between comorbidity, insight, treatment adherence, and quality of life, especially as some studies have shown that impaired insight is amenable to interventions (Pijnenborg et al., 2013).
There were several limitations of the study worth discussion. Because the sample was from a large randomized controlled drug trial, participants may not be representative of all adults with schizophrenia. The analysis was cross-sectional so the stability of these findings cannot be determined and further study is needed on how these groups may change over time. There has not been universal agreement yet on the best fit indices for latent class analyses, although most agree a combination of indices guided by parsimony and theory is best (Muthen, 2004; Nylund et al., 2007). Additionally, there may have been other unmeasured variables in this study that may have accounted for differences between classes, such as comorbid medical conditions.
In conclusion, this study contributes to the literature by illustrating the patterns of psychiatric comorbidity among a national sample of adults with schizophrenia and providing greater specificity and quantitative validity than previous characterizations of dual diagnoses. Comorbid anxiety and depressive disorders appeared to be part of a separate profile from those with comorbid substance use disorders. Psychiatric comorbidity with schizophrenia in general was associated with poorer mental health and lower quality of life, but better insight into their illness and need for care, particularly for those with comorbid anxiety and depression. Interventions that address comorbidity in schizophrenia should be supported and novel ways to harness the insight that individuals with comorbid disorders may have should be explored.
Acknowledgements
Funding for CATIE was provided by NIMH Grant# N01MH90001. The NIMH had no further role in study design, data collection, analysis, interpretation, writing, or decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Maziade M, Raymond E, Olivier D, Merette C, Roy MA. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophrenia Bulletin. 2011;37(4):811–821. doi: 10.1093/schbul/sbp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophrenia Research. 1992;6:201–208. doi: 10.1016/0920-9964(92)90003-n. [DOI] [PubMed] [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text rev. ed. Washington, DC: Author; 2000. [Google Scholar]
- Andreasen NC, Flaum M, Swayze VW, Tyrrell G, Arndt S. Positive and negative symptoms in schizophrenia: A critical reappraisal. Journal of the American Medical Association. 1990;47(7):615–621. doi: 10.1001/archpsyc.1990.01810190015002. [DOI] [PubMed] [Google Scholar]
- Bottas A, Cooke RG, Richter MA. Comorbidity and pathophysiology of obsessive-compuslive disorder in schizophrenia: Is there evidence for a schizo-obsessive subtype of schizophrenia? Journal of Psychiatry and Neuroscience. 2005;30(3):187–193. [PMC free article] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophrenia Bulletin. 2009;35(2):383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RE, Mueser KT, Brunette MF. Management of persons with co-occurring severe mental illness and substance use disorder: Program implications. World Psychiatry. 2007;6(3):131–136. [PMC free article] [PubMed] [Google Scholar]
- Emsley RA, Oosthuizen PP, Joubert AF, Roberts MC, Stein DJ. Depressive and anxiety symptoms in patients with schizophrenia and schizophreniform disorder. Journal of Clinical Psychiatry. 1999;60(11):747–750. doi: 10.4088/jcp.v60n1105. [DOI] [PubMed] [Google Scholar]
- Fazel S, Langstrom N, Hjern A, Grann M, Lichtensein P. Schizophrenia, substance abuse, and violent crime. Journal of the American Medical Association. 2009;301(19):2016–2023. doi: 10.1001/jama.2009.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorder-Patient Edition. New York: New York State Psychiatric Institute; 1997. [Google Scholar]
- Garvey M, Noyes R, Anderson D, Cook B. Examination of comorbid anxiety in psychiatric inpatients. Comprehensive Psychiatry. 1991;32(4):277–282. doi: 10.1016/0010-440x(91)90075-n. [DOI] [PubMed] [Google Scholar]
- Ginzburg K, Ein-Dor T, Solomon Z. Comorbidity of posttraumatic stress disorder, anxiety and depression: A 20-year longitudinal study of war veterans. Journal of Affective Disorders. 2010;123(1–3):249–257. doi: 10.1016/j.jad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Hofer A, Kemmler G, Eder U, Edlinger M, Hummer M, Fleischhacker WW. Quality of life in schizophrenia: The impact of psychopathology, attitude toward medication, and side effects. Journal of Clinical Psychiatry. 2004;65(7):932–939. [PubMed] [Google Scholar]
- Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2(1):302–317. [Google Scholar]
- Kay SR, Fiszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry. 1997;54(4):313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson C, Hughes M, Eschleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the US: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorder: Focus on heroin and cocaine dependence. American Journal of Psychiatry. 1985;142(11):1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4(5):231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Lehman A. A quality of life interview for the chronically mentally ill. Evaluation and Program Planning. 1988;11:51–62. [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK Investigators, C.A.T.o.I.E.C. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005a;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM, Davis CE, Lebowitz BD, Severe JS, Hsiao JK CATIE Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine. 2005b;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Lullmann E, Rief W. Correlates and long-term consequences of poor insight in patients with schizophrenia: A systematic review. Schizophrenia Bulletin. 2007;33(6):1324–1342. doi: 10.1093/schbul/sbm002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropsychopharmacology. 1998;18(3):135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Apperson J, Appelbaum PS, Ortlip P, Brecosky J, Hammill K, Geller JL, Roth L. Insight in schizophrenia: Its relationship to acute psychopathology. Journal of Nervous and Mental Disease. 1989;177:43–47. doi: 10.1097/00005053-198901000-00007. [DOI] [PubMed] [Google Scholar]
- McLachlan GJ. On bootstrapping the likelihood ratio test statistic for the number of components in a normal mixture. Applied Statistics. 1987;36(3):318–324. [Google Scholar]
- Mueser KT, Bellack AS, Blanchard JJ. Comorbidity of schizophrenia and substance abuse: Implications for treatment. Journal of Consulting and Clinical Psychology. 1992;60(5):845–856. doi: 10.1037//0022-006x.60.6.845. [DOI] [PubMed] [Google Scholar]
- Muthen B. Latent variable analysis: growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of Quantitative Methodology for the Social Sciences. Thousand Oaks, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14(4):535–569. [Google Scholar]
- O'Daly OG, Guillin O, Tsapakis EM, Martinez D, Shergill SS, Murray RM. Schizophrenia and substance abuse comorbidity: A role for dopamine sensitization? Journal of Dual Diagnosis. 2005;1(2):11–40. [Google Scholar]
- Pijnenborg GH, van Donkersgoed RJ, David AS, Aleman A. Changes in insight during treatment for psychotic disorders: A meta-analysis. Schizophrenia Research. 2013;144(1–3):109–117. doi: 10.1016/j.schres.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Pini S, Cassano GB, Dell'Osso L, Amador XF. Insight into illness in schizophrenia, schizoaffective disorder, and mood disorders with psychotic features. American Journal of Psychiatry. 2001;158(1):122–125. doi: 10.1176/appi.ajp.158.1.122. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M, Kriss V, Weisman G, Faragian S, Kurs R, Schneidman M, Fuchs C, Weizman A, Weizman R. Comparison of clinical characteristics and comorbidity in schizophrenia patients with and without obsessive-compulsive disorder: Schizophrenic and OC symptoms in schizophrenia. Journal of Clinical Psychiatry. 2003;64(11):1300–1307. doi: 10.4088/jcp.v64n1104. [DOI] [PubMed] [Google Scholar]
- RachBeisel J, Scott J, Dixon L. Co-occurring severe mental illness and substance use disorders: A review of recent research. Psychiatric Services. 1999;50(11):1427–1434. doi: 10.1176/ps.50.11.1427. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Journal of American Medical Association. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6(2):461–464. [Google Scholar]
- Sim K, Mahendran R, Siris SG, Heckers S, Chong SA. Subjective quality of life in first episode schizophrenia spectrum disorders with comorbid depression. Psychiatry Research. 2004;129(2):141–147. doi: 10.1016/j.psychres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Stroup TS, McEvoy JP, Swartz MS, Byerly MJ, Glick RD, Canive JM, McGee MF, Simpson GM, Stevens MC, Lieberman JA. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizoprhenia trial design and protocol development. Schizophrenia Bulletin. 2003;29:15–31. doi: 10.1093/oxfordjournals.schbul.a006986. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Tennen H, Carney MA, Affleck G, Willard A, Hromi B. Mood and alcohol consumption: An experience sampling test of the self-medication hypothesis. Journal of Abnormal Psychology. 2000;109(2):198–204. [PubMed] [Google Scholar]
- Tibbo P, Swainson J, Chue P, LeMelledo JM. Prevalence and relationship to delusions and hallucinations of anxiety disorders in schizophrenia. Depression and Anxiety. 2003;17(2):65–72. doi: 10.1002/da.10083. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Substance use disorders in schizophrenia-Clinical implications of comorbidity. Schizophrenia Bulletin. 2009;35(3):469–472. doi: 10.1093/schbul/sbp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114(4):522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Weich S, McGride O, Hussey D, Exeter D, Brugha T, McManus S. Latent class analysis of co-morbidity in the Adult Psychiatric Morbidity Survey in England 2007: Implications for DSM-5 and ICD-11. Psychological Medicine. 2011;41(10):2201–2212. doi: 10.1017/S0033291711000249. [DOI] [PubMed] [Google Scholar]
- Wolf EJ, Miller MW, Reardon AF, Ryabchenko KA, Castillo D, Freund R. A latent class analysis of dissociation and posttraumatic stress disorder. Archives of General Psychiatry. 2012;69(7):698–705. doi: 10.1001/archgenpsychiatry.2011.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]