Abstract
Background
Acute respiratory distress syndrome (ARDS) is a serious complication of sepsis and sepsis-associated ARDS is associated with significant morbidity and mortality. To date, no study has directly examined the epidemiology of ARDS in severe sepsis from the earliest presentation to the health care system, the Emergency Department (ED).
Methods
Single-center retrospective, observational cohort study of 778 adults with severe sepsis presenting to the ED. The primary outcome was the development of ARDS requiring mechanical ventilation during the first 5 hospital days. ARDS was defined using the Berlin definition. We used multivariable logistic regression to identify risk factors associated independently with ARDS development.
Results
The incidence of ARDS was 6.2% (48 of 778 patients) in the entire cohort. ARDS development varied across the continuum of care: 0.9% of patients fulfilled criteria for ARDS in the ED, 1.4% admitted to the ward developed ARDS, and 8.9% admitted to the ICU developed ARDS. ARDS developed a median of 1 day after admission and was associated with a four-fold higher risk of in-hospital mortality (14% vs. 60%, p<0.001). Independent risk factors associated with increased risk of ARDS development included: intermediate (2–3.9 mmol/L) (p=0.04) and high (≥ 4) serum lactate levels (p=0.008), lung injury prediction score (LIPS) (p<0.001) and microbiologically-proven infection (p=0.01).
Conclusions
In patients presenting to the ED with severe sepsis, the rate of sepsis-associated ARDS development varied across the continuum of care. ARDS developed rapidly and was associated with significant mortality. Elevated serum lactate levels in the ED and a recently validated clinical prediction score were independently associated with the development of ARDS in severe sepsis.
Keywords: severe sepsis, clinical prediction, lactic acid, acute respiratory distress syndrome
Introduction
Severe sepsis, a systemic disease caused by overwhelming infection, develops in as many as 3 million adults in the United States annually and results in substantial morbidity and mortality (1). Acute respiratory distress syndrome (ARDS) is a devastating complication of severe sepsis. Severe sepsis is the most common etiology of ARDS (2–3) and is associated with the highest case-fatality rate (3–4).
Once ARDS develops, lung protective ventilation is the only intervention known to improve mortality (5). Consequently, it is critical to identify patients at greatest risk of ARDS development early in their clinical course (6). The Lung Injury Prediction score (LIPS), a recently validated clinical prediction score to identify patients at-risk for development of ALI, has not been applied to the severe sepsis population directly (7). Further, while prior studies have examined the epidemiology of ARDS in severe sepsis (2–4, 8–15), predominantly from the ICU perspective, to our knowledge, no study has focused their examination on the epidemiology of ARDS in severe sepsis from the earliest presentation to the health care system, the Emergency Department (ED).
It is estimated that two out of three patients with severe sepsis enter the healthcare system through the ED (16); therefore, direct study of this population is justified. The primary aim of our study was to examine the epidemiology of ARDS in patients presenting to the ED with severe sepsis. The secondary aim was to identify risk factors associated with the development of ARDS.
Materials and Methods
Study Setting and Population
We conducted a retrospective, single-center, observational cohort study to examine the epidemiology of severe sepsis-associated ARDS. We began with a well-phenotyped cohort of severe sepsis patients admitted through an academic ED between January 2005 and December 2006 (17). We screened all ED visits to enroll cases of severe sepsis and septic shock in accordance with the International Sepsis Definitions Conference criteria (18). Sepsis was defined as suspected infection (administration of antibiotics in the ED) in the presence of two or more systemic inflammatory response syndrome (SIRS) criteria (18). Severe sepsis was defined as sepsis associated with organ dysfunction, hypoperfusion, or hypotension, and septic shock was defined as sepsis associated with refractory hypotension (18).
Serum lactate levels, drawn with the initial venous blood draw, were measured to assess for hypoperfusion (18–19). We used a serum-based assay, catalyzed by lactate oxidase, for venous lactate level measurements (mmol/L). The severe sepsis protocol in place during the study period recommended the use of protocol-directed resuscitation in patients with hyperlactatemia (≥ 4 mmol/L) and/or septic shock, consistent with the Rivers trial (19). However, resuscitation for each patient enrolled in the study was at the discretion of the clinical team providing care in the ED.
We excluded subsequent visit(s), trauma patients, patients who were discharged or left against medical advice, and patients with a care-limiting, do-not-intubate order. We reviewed the medical record for the hospitalization, including antibiotic administration and the discharge summary, to ensure the validity of severe sepsis during the hospitalization. We recently validated this approach to case selection (20).
Study Protocol
The study was approved by the Institutional Review Board of the University of Pennsylvania with an informed consent exemption and HIPAA waiver. Trained investigators abstracted clinical data from the medical record using a pre-drafted case report form. The data recorded from the ED included sociodemographics, comorbidities, vital signs, laboratory results, ED interventions, and admission service and location (ward or intensive care unit (ICU)) (see Table 1). We calculated an ED-based Acute Physiology and Chronic Health Evaluation (APACHE) II score (21) based on baseline vital signs and laboratory values recorded in the ED. We recorded whether mechanical ventilation was initiated in the ED or during the hospitalization and recorded all arterial blood gas measurements for intubated patients. Survival status was determined by review of the medical record and the Social Security Death Index and clinical details at the time of death were abstracted from the medical record, including the discharge summary.
Table 1.
Univariate comparisons of patient-specific factors and the development of ARDS.
| Baseline Characteristics | Non-ARDS Group (n=730) | ARDS Group (n=48) | p-value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 57 (45 – 70) | 55 (46 – 70) | 0.95 |
| Gender (female), n (%) | 333 (46) | 24 (50) | 0.56 |
| Race, n (%) * | |||
| White | 316 (45) | 23 (48) | 0.32 |
| Black | 353 (50) | 25 (52) | |
| Other | 32 (5) | 0 (0) | |
| ED Vital Signs | |||
| Temperature, ° C | 37.4 (36.6 – 38.7) | 37.0 (36.5 – 38.4) | 0.48 |
| Heart Rate | 111 (98 – 125) | 128 (111 – 135) | 0.002 |
| Systolic blood pressure | 114 (93 – 133) | 120 (97 – 136) | 0.36 |
| Respiratory Rate | 18 (16 – 22) | 24 (18 – 28) | <0.001 |
| Oxyhemoglobin saturation, % | 97 (95 – 99) | 95 (89 – 97) | <0.001 |
| ED Laboratory Values | |||
| White Blood Cell count | 12.6 (7.6 – 17.7) | 12.1 (5.5 – 18.5) | 0.65 |
| Hematocrit | 35 (30 – 40) | 37 (29 – 41) | 0.55 |
| Platelet count | 229 (156 – 326) | 191 (110 – 251) | 0.005 |
| Creatinine (mg/dL) | 1.3 (0.9 – 2.1) | 1.6 (1.1 – 2.9) | 0.06 |
| Glucose (mg/dL) | 120 (96 – 168) | 120 (96 – 140) | 0.56 |
| Total Bilirubin † (mg/dL) | 0.7 (0.4 – 1.4) | 1.2 (0.6 – 2.0) | 0.006 |
| Protime (sec) † | 14.0 (12.9 – 16.2) | 14.5 (13.3 – 19.6) | 0.12 |
| Lactate (mmol/L) | 2.8 (1.9 – 4.2) | 5.3 (2.9 – 7.7) | <0.001 |
| Comorbid Conditions, n(%) * | |||
| Coronary artery disease | 72 (10) | 3 (6) | 0.41 |
| Chronic renal insufficiency | 114 (16) | 4 (8) | 0.17 |
| Congestive heart failure | 80 (11) | 6 (12) | 0.74 |
| COPD | 45 (6) | 4 (8) | 0.55 |
| Diabetes mellitus | 219 (30) | 6 (12) | 0.01 |
| End-stage renal disease | 48 (7) | 2 (4) | 0.51 |
| HIV | 31 (4) | 5 (10) | 0.05 |
| Hypertension | 290 (41) | 14 (31) | 0.21 |
| Chronic liver failure | 53 (7) | 5 (10) | 0.42 |
| Oncology | 224 (31) | 16 (33) | 0.70 |
| Transplant | 78 (11) | 6 (12) | 0.70 |
| Etiology of Sepsis | |||
| Bacteremia | 105 (14) | 5 (10) | 0.44 |
| Pulmonary | 184 (25) | 24 (48) | <0.001 |
| Urosepsis | 156 (21) | 5 (10) | 0.07 |
| Gastrointestinal | 110 (15) | 10 (21) | 0.28 |
| Soft-tissue infection | 70 (10) | 1 (2) | 0.08 |
| Microbiology-proven Infection | |||
| Culture-positive | 417 (57) | 35 (73) | 0.03 |
| Gram positive | 199 (48) | 19 (54) | |
| Gram negative | 160 (38) | 10 (29) | |
| Viral | 12 (3) | 1 (3) | 0.70 |
| Fungal | 11 (3) | 1 (3) | |
| Mixed | 35 (8) | 4 (11) | |
| ED Interventions | |||
| Time to antibiotics (minutes) | 142 (81 – 231) | 114 (73 – 206) | 0.21 |
| Intravenous fluids (cc) | 2500 (1500 – 3500) | 2850 (1500 – 4400) | 0.16 |
| Vasoactive agents | 59 (8) | 15 (31) | <0.001 |
| Blood transfusion | 55 (8) | 8 (17) | 0.02 |
| Initiation of mechanical ventilation | 57 (8) | 26 (54) | <0.001 |
| Severity of illness at Presentation | |||
| ED APACHE II (baseline) | 15 (11 – 19) | 18 (14 – 26) | <0.001 |
| Shock developed in ED, n (%)‡ | 160 (22) | 22 (46) | <0.001 |
| Lung Injury Prediction Score | |||
| LIPS | 2 (1 – 4) | 6 (3 – 8) | <0.001 |
| Admission Type | |||
| Medical, n (%) | 595 (82) | 43 (90) | 0.16 |
| Mortality | |||
| In-hospital mortality | 102 (14) | 29 (60) | <0.001 |
| 28-day mortality | 126 (17) | 30 (62) | <0.001 |
| 60-day mortality | 165 (23) | 31 (65) | <0.001 |
Definition of abbreviation: APACHE=acute physiology and chronic health evaluation II score; ED=emergency department; COPD=chronic obstructive pulmonary disease; HIV=human immunodeficiency virus; LIPS=lung injury prediction score.
Continuous data presented as medians with interquartile ranges (25th, 75th percentile). Categorical data presented as counts and percentiles.
Comorbidities, race, intravenous fluids received in the ED not reported in all patients (<5% missing).
Reported in those in whom a measurement was obtained.
Defined as systolic blood pressure < 90 mm Hg despite intravenous fluid resuscitation (more than 1500 mL) or the use of vasoactive agents.
Definition of ARDS
The primary outcome was the development of ARDS requiring mechanical ventilation during the first 5 hospital days, in accord with prior studies examining disease-specific association with ARDS (22, 23). ARDS was defined based on the Berlin definition as acute hypoxemia (ratio of partial pressure of arterial oxygen to fractional concentration of inspired oxygen (P:F) ≤ 300) and the presence of bilateral pulmonary infiltrates on chest radiograph not due exclusively to congestive heart failure or fluid overload (24). ARDS was defined as mild (200 < P:F ≤ 300), moderate (100 < P:F ≤ 200), and severe (P:F ≤ 100) (24). We defined the time of onset as the time that the last of the criteria were fulfilled.
We used a valid, automated electronic system as an initial screening tool to identify ARDS cases requiring mechanical ventilation (25). In addition, an investigator blinded to the results of the electronic screening tool reviewed the case of the 169 subjects who received mechanical ventilation during their hospitalization to determine whether ARDS criteria were met. Specifically, all chest radiographs and arterial blood gas measures during the hospitalization were reviewed for each case. Inter-rater reliability between the automated system and the investigator was measured using the Kappa statistic (K), and was found to be 0.75 (95% CI: 0.64, 0.86), indicating a moderate degree of reliability. Adjudication, required in 16 cases, was performed by a third investigator blinded to prior ARDS determinations.
We report the rate of ARDS development in the overall cohort, as well as by location (ED, ward or ICU admission). Further, to contextualize prior studies, we report the rate of ARDS development in patients intubated in the ED and during the hospitalization.
Risk Factors for ARDS Development
Based on clinical plausibility and/or a relationship with the development of ARDS, we examined the following variables as factors which may be associated with ARDS development: age, gender, race, comorbid conditions (e.g., diabetes mellitus), ED therapy (e.g., time to antibiotics, blood transfusion, fluid resuscitation), cause of ALI (pulmonary vs. non-pulmonary), whether the infection was microbiologically-proven, severity of illness (e.g., APACHE II, shock), and the LIPS (3–15). The LIPS incorporates predisposing conditions associated with ARDS development (e.g., shock, aspiration) and risk modifiers (e.g., alcohol abuse as risk factor, diabetes as protective factor) (7). We did not include the initiation of mechanical ventilation as a candidate risk factor, as we considered this intervention to be in the causal pathway towards ARDS development.
We a priori hypothesized that serum lactate levels, given their association with central components in the pathophysiology of ARDS (inflammation, coagulation and endothelial dysfunction, and neutrophil activation), would be associated with ARDS development (17, 26–29). We categorized serum lactate levels as low (<2 mmol/L), intermediate (2–3.9 mmol/L) and high (≥ 4 mmol/L) (25, 34–35).
Data Analysis
We used the Student’s t-test or Wilcoxon rank-sum test to compare continuous variables and the chi-squared statistic or Fisher’s exact test to compare categorical variables between ARDS cases and non-cases. We used multivariable logistic regression to identify patient-level factors associated independently with ARDS after adjustment for potential covariates. We used variance inflation factors to assess for multicollinearity. Variables found to be collinear with APACHE II which are constituent variables of the APACHE II score were not included separately (e.g., heart rate, respiratory rate, oxygenation). Emergency department shock state and use of vasoactive agents were found to be collinear; the latter was not included separately. We added potential covariates associated with the development of ARDS at a significance of < 0.20 one-at-a-time to the base model, which included candidate risk factors associated with the development of ALI at a significance of < 0.20. We maintained the potential confounder in the model if its inclusion altered the point estimate for the odds ratio (OR) of a risk factor by >10% (30). As several important variables (e.g., shock) are incorporated in the LIPS calculation, we first created a model without its inclusion. We then included the LIPS to determine whether the identified factors were associated with ARDS development independent of the LIPS. In sensitivity analyses, given the potential for overfitting the model, we removed those variables which were not significantly associated with ARDS development.
In secondary analyses, we calculated the area under the receiver operating characteristic curve (AUC) to assess for model discrimination in the ability of the LIPS and serum lactate levels to predict ARDS development and the Hosmer-Lemeshow test statistic to assess for model calibration. We compared the predictive ability of LIPS, the baseline APACHE II score, and initial serum lactate levels. We excluded ED ARDS patients in these analyses to examine the ability to predict the development of ARDS. Finally, we used a fractional polynomial regression to depict the fitted relationship between the development of ARDS and initial serum lactate levels as a continuous variable (31). We used Stata 10.0 software for statistical analyses (Stata Datacorp, College Station, TX).
Results
Study Cohort
We studied 778 adults who were admitted through the ED with severe sepsis (see Figure 1). In the ED, sepsis was associated with acute organ dysfunction in 544 of 778 patients (69.9%), hypoperfusion (≥ 2 mmol/L) in 588 of 778 (75.6%), and hypotension (systolic blood pressure < 90 mm Hg or use of vasoactive agents) in 360 of 778 patients (46.3%), to qualify as severe sepsis. The majority of patients (n=413, 53.1%) were admitted to an Intensive Care Unit (ICU). The most common sources of infection in the cohort were: respiratory (26.7%), urologic (20.7%), gastrointestinal (15.4%), bacteremia (14.1%), and soft tissue-related infections (9.1%). Microbiologically-proven infection was identified in 58.1% (n=452) of the cohort. The 28-day all-cause mortality for the cohort was 20.0% (156 of 778).
Figure 1.
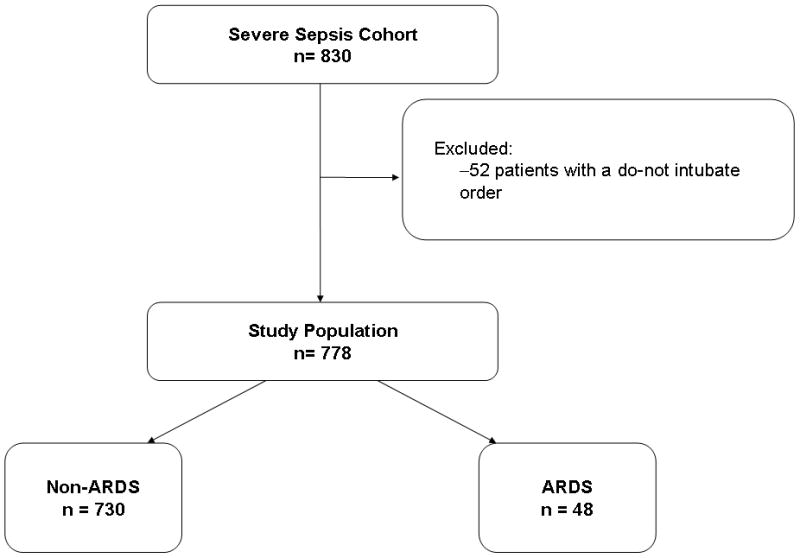
Enrollment and outcomes (ARDS) for severe sepsis cohort.
Incidence of ARDS
The incidence of ARDS was 6.2% (95% CI: 4.6 – 8.1%) in the entire cohort (48 of 778 patients). ARDS developed a median of 1 day after admission (IQR 1 to 2 days). At the time of ARDS development, the median P:F was 136 (IQR 114 to 220). Based on the initial measures available at the time of ARDS development, 14 patients began with mild ARDS (29.2%), 24 with moderate ARDS (50.0%), and 10 with severe ARDS (20.8%).
Across the continuum of care, 7 of 778 patients fulfilled criteria for ARDS in the ED (0.9%, 95% CI: 0.3, 1.8%), 5 of 364 patients admitted to a general medical or surgical ward developed ARDS requiring mechanical ventilation subsequently (1.4%, 95% CI: 0.5, 3.2%), and 36 of 407 patients admitted to an ICU developed ARDS requiring mechanical ventilation subsequently (8.9%, 95% CI: 6.3, 12.0%). The ICU admissions were significantly more likely to develop incident ARDS (p<0.001).
Of the 82 patients in whom ventilator support was initiated in the ED, 7 fulfilled ARDS criteria in the ED (8.5%, 95% CI: 3.5, 16.8%) and, ultimately, 25 fulfilled criteria during the hospitalization (30.5%, 95% CI: 20.8 – 41.6%). In the 18 subjects who were intubated in the ED but did not fulfill ARDS criteria until later in the hospitalization, 11 did not fulfill the radiographic criteria for ALI, 5 had a P:F > 300, and 2 did not have an ABG in the ED and therefore may have fulfilled criteria for ARDS if additional data had been available. The incidence of ARDS was 26.4% (95% CI: 17.6 – 37.0%) in 87 patients who required ventilator support post-ED.
In-hospital, 28-day, and 60-day all-cause mortality were significantly greater in those who developed ARDS (p<0.001, Table 1). ARDS-related in-hospital deaths occurred, on average, early (median 4 days after hospitalization, IQR 1 to 8 days). Multi-system organ failure was present prior to death in each of the 29 in-hospital, ARDS-related deaths, resulting in 13 cases of in-hospital cardiac arrest.
Association between Clinical and Physiologic Variables and ALI
In univariate analyses, higher initial serum lactate levels, higher severity of illness (e.g., APACHE II scores, shock, organ dysfunction), greater intensity of care in the ED (e.g., use of vasoactive agents, initiation of mechanical ventilation and transfusion), pulmonary cause of sepsis, culture-positive severe sepsis, no past medical history of diabetes mellitus, and higher LIPS were associated with the development of ARDS (see Table 1). The ARDS incidence within each lactate stratum was: 3 of 190 (1.6%, 95% CI: 0.3, 4.5) in the low stratum; 17 of 353 (4.8%, 95% CI: 2.8, 7.6) in the intermediate stratum, and 28 of 233 (12.0%, 95% CI: 8.1, 16.9) in the high stratum (Figure 3).
Figure 3.
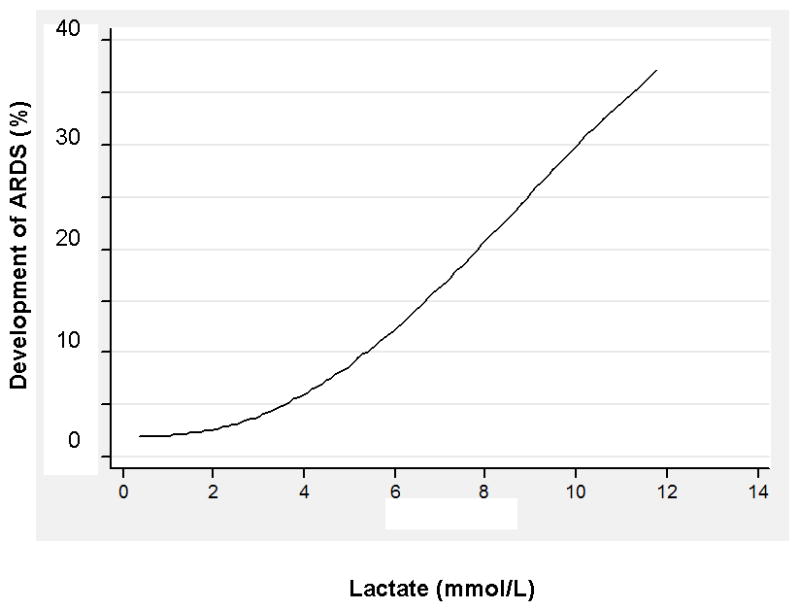
Fitted relationship between serum lactate levels and predicted probability of ARDS, using a fractional polynomial regression model.
Independent risk factors associated with increased risk of ARDS development included: pulmonary source of sepsis (p<0.001), microbiologically-proven infection (p=0.01), and higher severity of illness, as measured by the baseline APACHE II score (p=0.02) and serum lactate levels (Table 2). Specifically, we found that intermediate (p=0.04) and high serum lactate levels (p=0.003), when compared to low serum lactate levels, were significantly associated with ARDS development. The presence of diabetes was confirmed to be a protective factor for ARDS development (p=0.01). In the model which included the LIPS, the LIPS, intermediate and high serum lactate levels, and microbiologically-proven infection were found to be associated independently with ARDS development (see Table 3). Finally, when the non-significant variables were removed given the potential for overfitting the model, these three identified factors remained significantly associated with ALI development.
Table 2.
Multivariable logistic regression models demonstrating adjusted odds ratio for development of acute respiratory distress syndrome.
| Complete Model (N=778) | Adjusted Odds Ratio (95% CI) | p-value |
|---|---|---|
| Initial Serum Lactate Strata | ||
| Low | Reference | Reference |
| Intermediate | 3.76 (1.06 – 13.31) | 0.04 |
| High | 6.42 (1.86 – 22.23) | 0.003 |
| APACHE II (baseline) * | 1.06 (1.01 – 1.11) | 0.02 |
| Diabetes mellitus | 0.31 (0.13 – 0.77) | 0.01 |
| Pulmonary source of sepsis | 3.97 (2.06 – 7.67) | <0.001 |
| Microbiologically-proven infection | 2.47 (1.20 – 5.08) | 0.01 |
| Shock at presentation | 1.74 (0.87 – 3.49) | 0.12 |
| Transfusion at presentation | 1.70 (0.69 – 4.21) | 0.25 |
Definition of abbreviation: APACHE=Acute physiology and chronic health evaluation score; CI=confidence interval.
An adjusted odds ratio of greater than 1 indicates that the factor is associated with greater odds of developing ARDS. The following variables did not alter the odds ratio estimates of the candidate risk factors significantly, nor were they significantly associated with ARDS development: serum creatinine, platelet count, chronic renal insufficiency, human immunodeficiency virus, urosepsis, soft-tissue infection, or admission type (medical vs. surgical).
Odds ratio for each 1-unit increase in baseline APACHE II score.
Table 3.
Multivariable logistic regression models demonstrating adjusted odds ratio for development of acute respiratory distress syndrome including the Lung Injury Prediction Score.
| Variable | Adjusted Odds Ratio (95% CI) | p-value |
|---|---|---|
| Initial Serum Lactate Strata | ||
| Low | Reference | Reference |
| Intermediate | 3.88 (1.08 – 13.99) | 0.04 |
| High | 5.56 (1.57 – 19.64) | 0.008 |
| Diabetes mellitus | 0.45 (0.18 – 1.13) | 0.09 |
| Pulmonary source of sepsis | 1.70 (0.78 – 3.70) | 0.18 |
| Microbiologically-proven infection | 2.57 (1.23 – 5.36) | 0.01 |
| Shock at presentation | 0.95 (0.44 – 2.05) | 0.90 |
| Transfusion at presentation | 1.83 (0.74 – 4.51) | 0.19 |
| Lung Injury Prediction Score | 1.37 (1.19 – 1.58) | <0.001 |
Definition of abbreviation: CI=confidence interval.
An adjusted odds ratio of greater than 1 indicates that the factor is associated with greater odds of developing ARDS. APACHE II was found to be collinear with the LIPS; when APACHE II was included in the model, it did not alter the odds ratio estimates for the other risk factors and it was not significantly associated with ARDS development (p=0.75).
Odds ratio for each 1-unit increase in Lung Injury Prediction score score.
We found that the LIPS model discriminated those who did and did not develop ARDS with an AUC of 0.76 (95% CI: 0.69, 0.84) and was well calibrated (p=0.72). The LIPS model predicted ARDS development with greater accuracy than ED APACHE II (AUC 0.63, 95% CI: 0.54, 0.72, p=0.01). In addition, serum lactate, which demonstrated good discrimination (AUC 0.73, 95% CI: 0.65, 0.81) and was well calibrated (p=0.27), also predicted ALI development with greater accuracy than APACHE II (p=0.04). In Figure 3, we present the fitted relationship between initial serum lactate levels and ALI development.
Discussion
To enhance our understanding of the epidemiology of sepsis-associated ARDS and our ability to risk-stratify at-risk patients, we examined a cohort of severe sepsis patients from the earliest presentation to the health care system, the ED. We found that the rate of sepsis-associated ARDS development varied across the continuum of care. We found that when ARDS developed, it developed rapidly, was associated with significant mortality, and progressed rapidly to multi-system organ failure in those in whom it was fatal. We identified factors which can be used to risk-stratify patients with severe sepsis at high risk for development of ARDS, including factors present at hospital presentation (i.e., serum lactate levels and a validated clinical prediction score).
The morbidity and mortality associated with ARDS is significant, resulting in high case-fatality rates and demonstrable impairment in neuropsychological and physical function in many survivors (32–33). Because interventions to improve outcomes are extremely limited once ARDS develops (5), intense focus is being shifted toward prevention and treatment prior to its development (6).
By focusing our examination on a cohort of severe sepsis patients, beginning at hospital presentation, our study reinforces and enhances our understanding of the epidemiology of sepsis-associated ARDS. We found that progression to ARDS was rapid and conferred a significantly increased risk of in-hospital death. Our findings regarding incidence (7, 14), time to ARDS (7, 13–15), and sepsis-associated ARDS mortality (11–13) are consistent with prior studies. Two recent studies, which investigated heterogeneous groups of patients at-risk for development of ALI, each found that 7% of the sepsis patient subgroup developed ARDS (7, 14). Our study, therefore, validates the fact that the vast majority of patients admitted with severe sepsis will not develop ARDS. Because in-hospital death was four-fold higher in patients who developed ARDS, these findings also emphasize the need to identify those at greatest risk of ARDS development and to elucidate strategies to prevent ARDS beyond preventive ventilatory approaches (34–35). Although death occurred relatively early in the hospitalization, culminating in multi-system organ failure complicated by refractory shock, further investigation is required to determine if care delivery in the ED or initial hospital course could be optimized to improve outcomes.
We found that the rate of development differs across time and location. In the ED, approximately 1% of patients presenting with severe sepsis fulfill criteria for ARDS. Consistent with a prior study which examined the prevalence of ARDS in a heterogeneous group of critically ill adults receiving mechanical ventilation in the ED (36), ARDS existed in 8.5% of severe sepsis patients receiving mechanical ventilation in the ED. While our study confirmed that approximately 50% of severe sepsis patients are cared for on the general ward (37–38), we found that the burden of ARDS development was confined to patients admitted directly to the ICU. These observations contextualize the findings from prior studies (2–15) which detailed the incidence and outcomes of sepsis-associated ARDS from the perspective of the ICU.
We found that the LIPS, initial serum lactate levels, and the presence of a microbiologically-proven infection were independently associated with ARDS development. As such, our study confirms the ability of the LIPS to predict ARDS development in severe sepsis patients and identifies serum lactate at initial presentation as a novel, simple risk-stratification tool to predict ARDS development. In contrast to microbiologic data, which requires time for processing and growth, often yielding a culture diagnosis after ARDS has developed, LIPS and lactate are available at hospital admission.
Recently, Agrawal and colleagues found that inflammatory (IL-8) and endothelial (angiopoietin-2 (Ang-2)) biomarkers predict the development of ARDS independent of sepsis and illness severity in critically ill patients (39). In contrast to IL-8 and Ang-2, serum lactate levels are routinely measured as part of protocolized sepsis care. As such, serum lactate levels appear to be a useful, clinically-available tool to predict ARDS development in addition to their better characterized ability to identify patients at-risk of death (17, 26), irrespective of hemodynamic status (17, 40).
Our study, which focused on risk factors present in the most proximal phase of the hospitalization, confirms the notion that severity of illness and a pulmonary source of infection are risk factors for the development of ARDS (3, 10, 14, 41–42), while diabetes is protective (19). As constituent variables of the LIPS, these risk factors are incorporated in the clinical risk prediction score to identify patients at high risk of ARDS development. Importantly, the presence of shock in the ED was not independently associated with ARDS development. Prior ICU-based studies report an incidence of ARDS development in septic shock patients of approximately 40% (11–12, 15). In contrast, we found that 12% of patients fulfilling hemodynamic criteria for septic shock in the ED developed ARDS. Collectively, these findings suggest that the prognostic utility of shock is muted when present in the ED, as compared to shock which persists or develops in the ICU.
Our study has several potential limitations. First, ARDS misclassification is a potential limitation based on our retrospective study design and our reliance on available radiographs and blood gas measurements. To minimize this potential bias, we based our determination of ARDS on established criteria (24), used a valid, electronic screening tool (25), and verified the accuracy of our determination through a separate case review by an independent physician investigator. Second, our decision to limit our cases to ARDS requiring mechanical ventilation to identify those most at-risk of subsequent death may have resulted in misclassification as some non-ventilated patients may have met criteria for ARDS; however, this non-differential misclassification would bias our results towards the null. Third, we acknowledge the potential for ascertainment bias. Despite the use of an established protocol to measure serum lactate in patients with suspected infection, we acknowledge that a potential delay exists between sepsis recognition and the serum lactate measurement. Fourth, although limited to two cases, it is possible that these patients would have fulfilled criteria in the ED for ARDS if a blood gas had been obtained. Fifth, we focused our observational study design on clinical details present on ED arrival. As a result, we are unable to comment on the trajectory of other longitudinal organ failure measures (e.g., SOFA scores) during the hospitalization, the potential impact of ED length of stay on care delivery and outcomes, or the impact of initial ventilator settings, as this data was not available. Furthermore, whether different initial resuscitation strategies or late fluid-management strategies would have altered the rate of ARDS development and ARDS-related outcomes remains unclear and requires further investigation (43). Finally, as a single-center study, our findings warrant external validation.
Conclusions
We found that the rate of ARDS development in a cohort of patients admitted with severe sepsis was low overall, yet differed significantly across time and location. When ARDS developed, it developed rapidly and was associated with a high case-fatality rate. Finally, we found that initial serum lactate measurements and a validated clinical prediction score (LIPS) at hospital presentation can be used to risk-stratify patients with severe sepsis at high risk of ARDS development.
Figure 2.
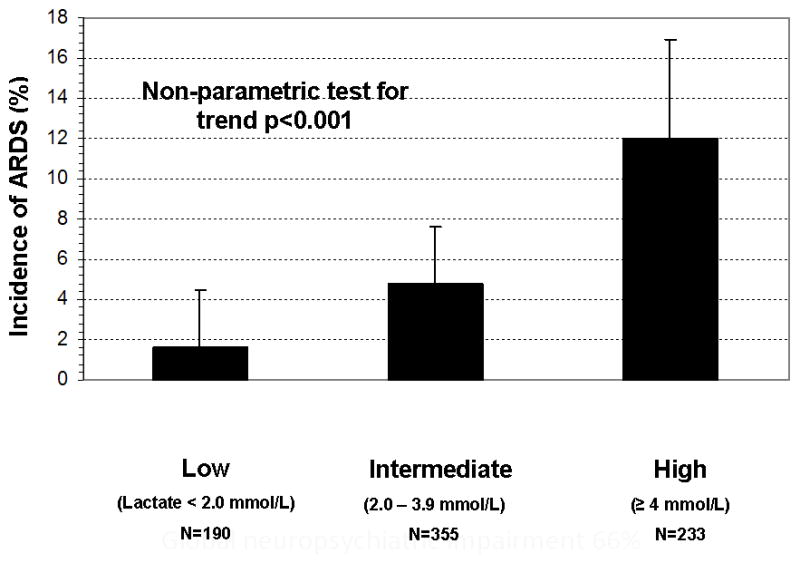
The incidence of ARDS by initial serum lactate level measured in the emergency department. The incidence and upper bound of the 95% confidence interval are presented by categorized serum lactate levels. Serum lactate categorized as: Low = 0 – 1.9 mmol/L, Intermediate = 2 – 3.9 mmol/L, and High = ≥4 mmol/L.
Acknowledgments
Funding: The study was supported in part by T32 HL07891 training grant and NIH Loan Repayment Program, National Institutes of Health, National Heart, Lung and Blood Institute, Bethesda, MD.
Footnotes
Disclosures: For each of the above authors, no financial or other potential conflicts of interest exist related to this work. Presented as an abstract at the American Thoracic Society International Conference, May 2009, San Diego, California.
References
- 1.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Hudson LD, Steinberg KP. Epidemiology of acute lung injury and ARDS. CHEST. 1999;116:74S–82S. doi: 10.1378/chest.116.suppl_1.74s-a. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. CHEST. 2005;128:525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 5.ARDSnet Investigators. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Levitt JE, Matthay MA. Clinical review: early treatment of acute lung injury – paradigm shift toward prevention and treatment prior to respiratory failure. Critical Care. 2012;16:223. doi: 10.1186/cc11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, et al. Early identification of patients at risk of acute lung injury: evaluation of the lung injury prediction score in a multicenter cohort study. Amer J Respir Crit Care Med. 2011;183:462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan RL, Sahn SA, Petty TL. Incidence and outcome of the respiratory distress syndrome in gram-negative sepsis. Arch Intern Med. 1979;139:867–869. [PubMed] [Google Scholar]
- 9.Fein AM, Lippmann M, Holtzman H, Eliraz A, Goldberk SK. The risk factors, incidence, and prognosis of ARDS following septicemia. Chest. 1983;83:40–42. doi: 10.1378/chest.83.1.40. [DOI] [PubMed] [Google Scholar]
- 10.Fowler AA, Hamman RF, Good JT, Benson KN, Baird M, Eberle DJ, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98:593–597. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- 11.Moss M, Parsons E, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 12.Moss M, Guidot DM, Steinberg KP, Duhon GF, Treece P, Wolken R, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28:2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Eggimann P, Harbath S, Ricou B, Hugonnet S, Ferriere K, Suter P, et al. Acute respiratory distress syndrome after bacteremic sepsis does not increase mortality. Am J Respir Crit Care Med. 2003;167:1210–1214. doi: 10.1164/rccm.200210-1196OC. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson Niall D, Frutos-Vivar F, Esteban A, Gordo F, Honrubia T, Penuelas O, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Critical Care. 2007;11:R96. doi: 10.1186/cc6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36:1518–1522. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 16.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35:1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 17.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–7. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 18.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 19.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 20.Whittaker SA, Mikkelsen ME, Gaieski DF, Koshy S, Kean C, Fuchs BD. Severe sepsis cohorts derived from claims-based strategies appear to be biased toward a more severely ill patient population. Crit Care Med. 2013 doi: 10.1097/CCM.0b013e31827466f1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 22.Shah CV, Localio AR, Lanken PN, Gallop R, Bellamy S, Ma SF, et al. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Crit Care Med. 2008;36:2309–2315. doi: 10.1097/CCM.0b013e318180dc74. [DOI] [PubMed] [Google Scholar]
- 23.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 24.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 25.Azzam HC, Khalsa SS, Urbani R, Shah CV, Christie JD, Lanken PN, et al. Validation study of an automated electronic acute lung injury screening tool. JAMIA. 2009;16:503–508. doi: 10.1197/jamia.M3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trzeciak S, Dellinger RP, Chansky ME, Arnold RC, Schorr C, Milcarek B, et al. Serum lactate as a predictor of mortality in patients with infection. Int Care Med. 2007;33:970–977. doi: 10.1007/s00134-007-0563-9. [DOI] [PubMed] [Google Scholar]
- 27.Vary TC, Hazen SA, Maish G, Cooney RN. TNF binding protein prevents hyperlactatemia and inactivation of PDH complex in skeletal muscle during sepsis. J Surg Res. 1998;80:44–51. doi: 10.1006/jsre.1998.5324. [DOI] [PubMed] [Google Scholar]
- 28.Dixon B, Santamaria JD, Campbell DJ. Plasminogen activator inhibitor activity is associated with raised lactate levels after cardiac surgery with cardiopulmonary bypass. Crit Care Med. 2003;31:1053–9. doi: 10.1097/01.CCM.0000055390.97331.DB. [DOI] [PubMed] [Google Scholar]
- 29.Kellum JA, Kramer DJ, Lee K, Mankad S, Bellomo R, Pinsky MR. Release of lactate by the lung in acute lung injury. CHEST. 1997;1111:1301–1350. doi: 10.1378/chest.111.5.1301. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Amer J of Epid. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 31.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 32.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 33.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neto AS, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Damasceno MC, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308(16):1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 35.Roy S, Habashi N, Sadowitz B, Andrews P, Ge L, Wang G, et al. Early airway pressure release ventilation prevents ARDS—a novel preventive approach to lung injury. Shock. 2013;39(1):28–38. doi: 10.1097/SHK.0b013e31827b47bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goyal M, Houseman D, Johnson NJ, Christie J, Mikkelsen ME, Gaieski DF. Prevalence of acute lung injury among medical patients in the emergency department. Acad Emerg Med. 2012;19(9):E.1011–1018. doi: 10.1111/j.1553-2712.2012.01429.x. [DOI] [PubMed] [Google Scholar]
- 37.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Esteban A, Frutos-Vivar F, Ferguson ND, Penuelas O, Lorente JA, Gordo F, et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289. doi: 10.1097/01.CCM.0000260960.94300.DE. [DOI] [PubMed] [Google Scholar]
- 39.Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, et al. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187(7):736–742. doi: 10.1164/rccm.201208-1460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sterling SA, Puskarich MA, Shapiro NI, Trzeciak S, Kline JA, Summers RL, et al. Characteristics and outcomes of patients with vasoplegic versus tissue dysoxic septic shock. Shock. 2013;40(1):11–14. doi: 10.1097/SHK.0b013e318298836d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sevransky JE, Martin GS, Shanholtz C, Mendez-Tellez PA, Pronovost P, Brower R, et al. Mortality in sepsis versus non-sepsis induced acute lung injury. Critical Care. 2009;13:R150. doi: 10.1186/cc8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: Potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 43.Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]


