Abstract
Nanotechnology has often been applied in the development of targeted drug-delivery systems for the treatment of cancer. An ideal nanoscale system for drug delivery should be able to selectively deliver and rapidly release the carried therapeutic drug(s) in cancer cells and, more importantly, not react to off-target cells so as to eliminate unwanted toxicity on normal tissues. To reach this goal, a selective chemotherapeutic is formulated using a hollow gold nanosphere (HAuNS) equipped with a biomarker-specific aptamer (Apt), and loaded with the chemotherapy drug doxorubicin (DOX). The formed Apt-HAuNS-Dox, approximately 42 nm in diameter, specifically binds to lymphoma tumor cells and does not react to control cells that do not express the biomarker. Through aptamer-mediated selective cell binding, the Apt-HAuNS-Dox is internalized exclusively into the targeted tumor cells, and then released the DOX intracellularly. Of note, although the formed Apt-HAuNS-Dox is stable under normal biological conditions (pH 7.4), it appears ultrasensitive to pH change and rapidly releases 80% of the loaded DOX within 2 h at pH 5.0, a condition seen in cell lysosomes. Functional assays using cell mixtures show that the Apt-HAuNS-Dox selectively kills lymphoma tumor cells, but has no effect on the growth of the off-target cells in the same cultures, indicating that this ultra pH-sensitive Apt-HAuNS-Dox can selectively treat cancer through specific aptamer guidance, and will have minimal side effects on normal tissue.
Keywords: aptamers, hollow gold nanospheres, targeted therapies, pH sensitive, drug delivery
1. Introduction
Nanoparticles can be formed using polymers, metals, protein/peptide, and lyposomes.[1–4] As an emerging delivery approach, nanoparticles are able to carry therapeutic drugs and deliver them into tumor cells.[4–12] Recently, we reported on a hollow gold nanosphere (HAuNS) that could carry an exceptionally high payload of doxorubicin (DOX) to induce cytotoxicity in tumor cells.[13] However, many of the reported nanoparticle delivery systems are not tumor cell-selective, and need to be administered at high concentrations, which may result in non-specific binding and also cause toxicity to off-target, normal cells and tissues. For in vivo therapeutic application, an ideal nanoscale molecule for drug delivery should be: 1) stable for transportation under normal biological conditions and be able to rapidly release the carried therapeutic drug at the destination, and 2) tumor cell-selective and not react to normal cell/tissues to minimize or eliminate unwanted toxicity. To reach this end, investigators have developed and tested various nanoparticles using targeting ligands, antibodies, peptides, oligonucleotide aptamers, and other small molecules to achieve delivery selectivity.[14–17] In contrast to protein antibodies, aptamers are small-molecule probes composed of short, single-stranded oligonucleotides (RNA or ssDNA) ranging from 30 to 60 bases.[18–20] Our previous studies revealed that the synthetic aptamer probe could specifically bind to tumor cells and more efficiently penetrate tumor tissue than antibodies.[21–23] Notably, since they are small oligonucleotides, the aptamers are not immunogenic and are more suitable for in vivo use. Moreover, although drug release from nanoparticles could be triggered by external forces, such as near-infrared light,[13] the cellular condition-induced drug release through natural biologic mechanisms, such as low pH within lysosomes, appears more promising. Thus, in this study, we formulated a novel HAuNS drug delivery system that was equipped with an aptamer (Apt) for selective cell targeting and loaded with DOX for killing tumor cells. The biochemical features, drug release potential, and cell-selective toxicity of the drug delivery system were carefully investigated.
2. Results and Discussion
2.1. Formulation of an Aptamer-Equipped and Doxorubicin-Loaded Hollow Gold Nanosphere Drug-Delivery System (Apt-HAuNS-Dox)
For selective targeting of tumor cells, the surface of the HAuNS was chemically conjugated with 39-mer RNA aptamers specific for CD30 (Figure 1A), a diagnostic biomarker for Hodgkin’s lymphoma and anaplastic large cell lymphoma.[22] To enhance biostability, surface modification of the Apt-HAuNS was subsequently performed using polyethylene glycol (PEG) as described in the Experimental Section. Finally, DOX was loaded through charge force as reported previously.[13] DOX loading into the Apt-HAuNS was monitored by quantifying residual-free DOX in reaction with a US-vis absorption assay,[13] which indicated that aptamer conjugation had no effect on DOX loading efficiency (>90%, approximately 30% (w/w)). Dynamic light scattering measurement revealed that the fabricated Apt-HAuNS-Dox had a peak hydrodynamic diameter of 42 nm, with approximately 80% of them being 25 to 55 nm in diameter (Figure 1B), consistent with the findings of transmission electron microscope (TEM) imaging (Figure 1C).
Figure 1.
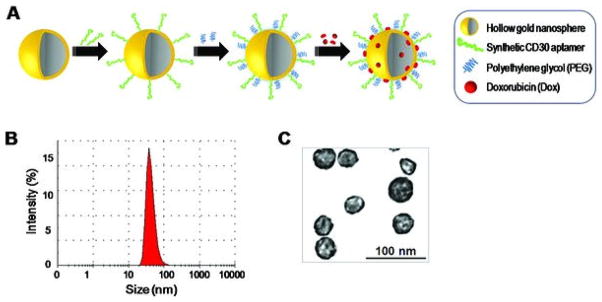
Formulation of the Apt-HAuNS-Dox nanoscale drug-delivery system. (a) Schematic illustration for the synthesis of the Apt-HAuNS-Dox. Aptamers and PEG were conjugated to the surface of HAuNS sequentially via covalent S-Au bonds, followed by loading with doxorubicin through a charge force. (b) Dynamic light scattering measurement indicated the peak size of the formed Apt-HAuNS-Dox, 42 nm. (c) Transmission electron micrograph of the Apt-HAuNS-Dox with scale bar.
2.2. Aptamer-Mediated, Specific Cell Binding with No Reaction to Off-Target Cells
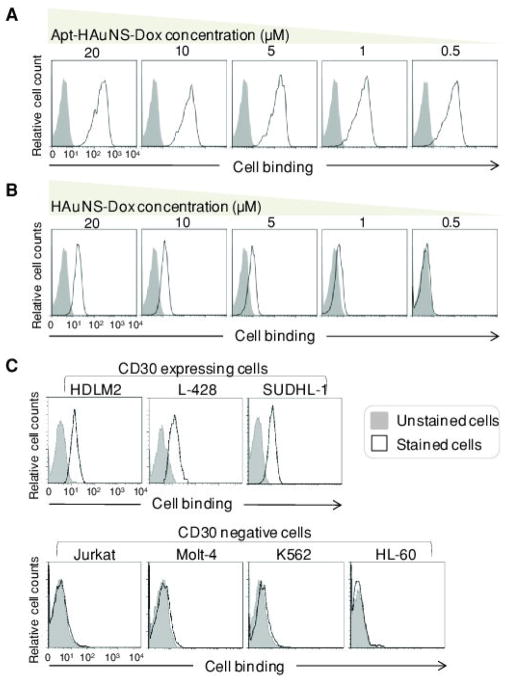
First, to test specific cell binding, the formulated Apt-HAuNS-Dox was incubated with Karpas 299 cells (a CD30-expressing anaplastic large cell lymphoma cell line) for 30 min at different concentrations as indicated in Figure 2A. Since the loaded DOX is fluorescent, resultant cell binding of the Apt-HAuNS-Dox was monitored by flow cytometry analysis. When compared to unstained cells (gray peaks), cell binding of Apt-HAuNS-Dox (open peaks) was detected and showed few changes with concentrations ranging from 0.5 to 20 μM (calculated as the loaded Dox concentration). To validate that the observed cell binding was indeed aptamer-mediated and not through a non-specific mechanism, Karpas 299 cells were also treated with HAuNS-Dox without aptamer conjugation[13] as a control under the same conditions. In contrast to the aptamer-mediated strong cell binding (Figure 2A), a weak, non-specific cell binding was seen in a concentration-dependent fashion, and disappeared when the HAuNS-Dox reached a ≤0.5 μM final concentration (Figure 2B). Since no non-specific cell binding was observed at ≤0.5 μM concentration, this optimal condition was used throughout the study. For further confirmation, 0.5 μM Apt-HAuNS-Dox was incubated with different CD30-expressing lymphoma cells (HDLM2, L-428, and SUDHL-1) and CD30-negative cells (Jurkat, Molt-4, and K562). Flow cytometry revealed that the Apt-HAuNS-Dox specifically bound only to lymphoma cells and did not react to CD30-negative cells (Figure 2C). Additional cultured cells were also tested, including Hodgkin’s lymphoma cells (L540 and KMH2), multiple myeloma cells (RPMI8226), acute myeloid leukemia cells (HL-60 cells), and Burkitt’s lymphoma cells (ST486 and Ramos), and results were summarized in Table 1. Notably, under the same conditions, the HAuNS-Dox did not bind to any of cells tested in Table 1. Moreover, for direct observation, scanning electron microscopy was performed on the cultured cells that were treated with 0.5 μM Apt-HAuNS-Dox for 30 min. After repeated washings to remove free Apt-HAuNS-Dox, the cells were fixed as described in the Experimental Section. Scanning electron microscopy showed that Apt-HAuNS-Dox specifically bound to CD30-expressing HDLM2 lymphoma cells (Figure 3A, arrows), but did not react to CD30-negative HL-60 cells (Figure 3B). It is notable that, in addition to targeting cells of interest, a desired nanoscale drug delivery system also needs to refrain from off-target cell binding. When compared to the ligand-mediated, high-affinity binding, non-specific cell binding of nanoparticles requires a relatively high concentration. Dose-response studies demonstrated that at a ≤0.5 μM final concentration, the Apt-HAuNS-Dox specifically bound to CD30-expressing tumor cells, but did not non-specifically bind to control cells (the far right panels of Figures 2A and 2B, and Table 1). These findings indicate that using Apt-HAuNS-Dox at its optimal concentration (≤0.5 μM) can completely eliminate off-target reactions, resulting in no side effects on normal tissues.
Figure 2.
The Apt-HAuNS-Dox specifically bound to lymphoma cells and did not react to off-target cells not expressing the biomarker. (a) Through the aptamer-mediated guidance, the Apt-HAuNS-Dox specifically bound to Karpas 299 lymphoma cells at a concentration as low as 0.5 μM (calculated by the loaded DOX) and cell-binding affinity showed little change when reduced from 20 to 0.5 μM. (b) In contrast, under the same conditions, the non-specific cell binding of the HAuNS-Dox (containing no aptamer) gradually decreased as concentrations reduced. No non-specific cell binding was observed at a concentration ≤0.5 μM, which was used throughout the study. (c) Under the optimal concentration (0.5 μM), in addition to Karpas cells (a), the formed Apt-HAuNS-Dox also specifically bound to HDLM2, L-428, and SUDHL-1 lymphoma cells, but did not react to off-target cells, Jurkat, Molt-4, K562, and HL-60 that did not express CD30-biomarkers.
Table 1.
Cell binding profile of Apt-HAuNS-Dox.
| Cell lines | CD30 expression | Apt-HAuNS-Dox | HAuNS-Dox |
|---|---|---|---|
| HDLM2 (Hodgkin lymphoma) | + | + | − |
| KMH2 (Hodgkin lymphoma) | + | + | − |
| L-428 (Hodgkin lymphoma) | + | + | − |
| L-540 (Hodgkin lymphoma) | + | + | − |
| SUDHL-1 (ALCL)a) | + | + | − |
| Kapas 299 (ALCL)a) | + | + | − |
| Jurkat (T cell lymphoma) | − | − | − |
| ST486 (Burkitt’s lymphoma) | − | − | − |
| Ramos (Burkitt’s lymphoma) | − | − | − |
| Molt-4 (Leukemia) | − | − | − |
| K562 (Leukemia) | − | − | − |
| HL-60 (Leukemia) | − | − | − |
| RPMI 8226 (Myeloma) | − | − | − |
Anaplastic large cell lymphoma.
Figure 3.

Scanning electron microscopy study of specific cell binding by the Apt-HAuNS-Dox. Cells were treated with the Apt-HAuNS-Dox, fixed, and examined by scanning electron microscopy as described in the Experimental Section. (a) Specific binding of the Apt-HAuNS-Dox to HDLM2 lymphoma cells was confirmed (arrows). (b) Under the same conditions, the Apt-HAuNS-Dox did not react to HL-60 cells that did not express CD30 biomarkers.
2.3. pH Ultrasensitive Release of the Loaded Doxorubicin within the Targeted Cells
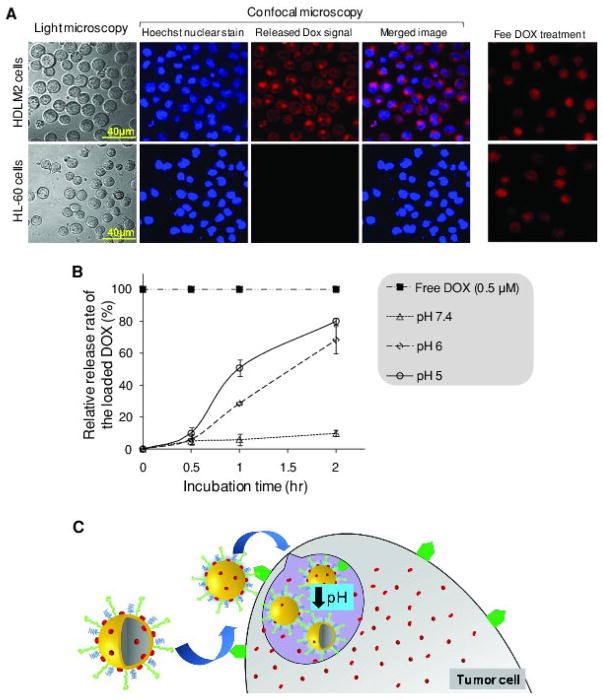
In addition to selective delivery, a desired nanoscale drug delivery system must also efficiently release the carried drug within the targeted tumor cells. To confirm intracellular delivery, CD30-expressing HDLM2 cells were incubated with Apt-HAuNS-Dox (0.5 μM final concentration) at 37 °C. In a control group, CD30-negative HL-60 cells were treated under the same conditions. After culture for 2 h, cells were harvested, nuclei-stained with Hoechst 33342, and examined by confocal fluorescent microscopy (Figure 4A). It is notable that because the DOX was incorporated into the nanoparticle, the DOX-emitted fluorescence was significantly reduced by the quencher effect of HAuNS.[13] Thus, the detected fluorescent signals (red) reflected intracellular free DOX released from Apt-HAuNS-Dox. In contrast, the DOX-emitted signal was not observed in CD30-negative control cells, although they received the same treatment and cell nuclei stain (blue). These findings confirm cell-specific delivery of Apt-HAuNS-Dox and intracellular release of DOX.
Figure 4.
Specific intracellular delivery of the Apt-HAuNS-Dox and ultra pH-sensitive release of DOX. (a) Intracellular delivery of the Apt-HAuNS-Dox. Cells were treated with the Apt-HAuNS-Dox for 2 h and nuclei were then stained by Hoechst 33342, and examined by confocal microscope as described in the Experimental Section. Cells were observed under light microscope and the fluorescent signals from the nuclear stain (Hoechst in blue, DOX in red) were detected by confocal microscope. The merged images indicated that the Apt-HAuNS-Dox specifically bound to HDLM2 lymphoma cells and released the loaded DOX intracellularly (upper row). Notably, the detected red fluorescent signals were emitted from the released/free DOX since it was quenched by the intact Apt-HAuNS-Dox. In contrast, the Apt-HAuNS-Dox did not react to HL-60 cells that did not express CD30 biomarkers and no DOX signals were detected within these control cells. In the control group (far right column), cells were treated with free DOX under the same condition, showing equal drug diffusion into HDLM2 and HL-60 cells in a non-cell-specific fashion. (b) pH-triggered DOX release. The Apt-HAuNS-Dox was incubated in cultured media with pH 7.4, 6.0, and 5.0 as described in the Experimental Section. Relative release rates of DOX were monitored by a fluorescent microplate reader at 0, 0.5, 1.0, 1.5, and 2.0 h. The releasing rate (%) of DOX was calculated by comparing the fluorescent signal from free DOX (100%) at the same concentration as that loaded into the Apt-HAuNS-Dox. (c) It is expected that the Apt-HAuNS-Dox is able to selectively target lymphoma cells via the aptamer-mediated biomarker interaction, which results in internalization and intracellular delivery into lysosomes. Since the Apt-HAuNS-Dox is ultra pH sensitive, the low pH condition in lysosomes will trigger rapid release of the loaded DOX, killing the targeted cells.
To examine pH effect on DOX release, the Apt-HAuNS-Dox was added into culture medium with different pH levels (5.0, 6.0, and 7.4) in a microplate and incubated for 2 hours at room temperature. In control wells, the same dose of free DOX was added as a reading control of fluorescent signals (100%) and incubated under the same conditions. Free Dox from Apt-HAuNS-Dox was detected by microplate reader at 490 nm and release rates were quantified (%) by using the free DOX control. Figure 4B showed that Apt-HAuNS-Dox was ultrasensitive to pH changes and rapidly released the incorporated DOX up to 80% at pH 5.0 and 68% at pH 6.0 within 2 h. In contrast, Apt-HAuNS-Dox was stable at pH 7.4 with 7.5% release of DOX in 2 h. Notably, the DOX releasing rate of Apt-HAuNS-Dox was 20-fold higher than that previously observed in HAuNS-Dox, 80% versus less than 4% release within 2 h at pH 5.0.[13] Although the exact molecular mechanism is unknown, the ultra-pH sensitivity may be a result of surface conjugation of oligonucleotide aptamers, which might interrupt the homogenous PEG coating of the HAuNS and facilitate release of the loaded DOX in low pH. It is reasonable to believe that this pH ultrasensitive Apt-HAuNS-Dox will be more useful for treating tumors in deep body sites, where drug release triggers, such as near-infrared light, cannot reach the nanoparticles for the induction of DOX release. As shown in Figure 1A, DOX was incorporated into Apt-HAuNS through a charge force at pH 7.4 and, therefore, the formed Apt-HAuNS-Dox is stable under normal physiological conditions. However, we hypothesized that after being delivered into lysosomes, which have an acidic environment (low pH), the positive charge of the DOX may be neutralized and lead to release of the DOX from the Apt-HAuNS-Dox (Figure 4C).
2.4. Selective Tumor Cell Killing by Apt-HAuNS-Dox with no Effect on Off-Target Cells
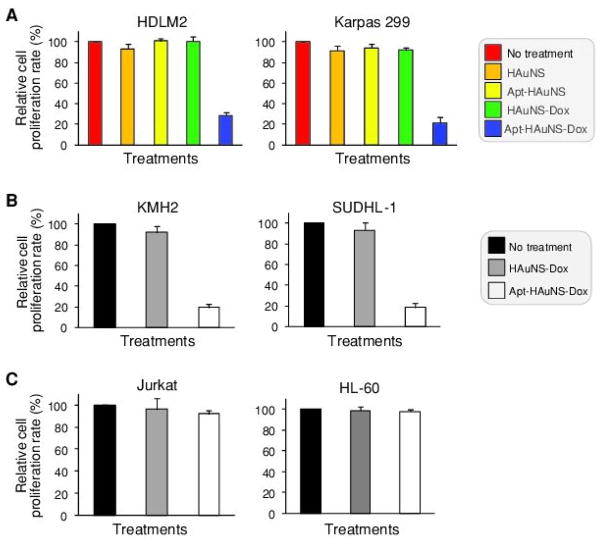
After confirmation of specific cell targeting, intracellular delivery, and pH-ultrasensitive DOX release, we investigated the potential therapeutic role of the Apt-HAuNS-Dox on tumor cells. Cultured CD30-expressing lymphoma cells, HDLM-2 and Karpas 299, were treated with Apt-HAuNS-Dox (0.5 μM final concentration) as described in the Experimental Section. In control groups, the cells were exposed to the HAuNS-Dox that had no aptamer conjugation, the Apt-HAuNS that had no DOX loading, the HAuNS alone, or non-treatment under the same conditions. After culture for 48 hours, viable cells were counted and relative cell proliferation rates (%) were calculated using non-treatment control cultures as 100%. Figure 5A shows that proliferation rates of lymphoma cells were significantly inhibited by exposure to the Apt-HAuNS-Dox (78–83% decrease) when compared to control cultures with non-treatment. Treatments of the HAuNS alone, the Apt-HAuNS, or the HAuNS-Dox that contained the same concentration of DOX and no Apt conjugation, showed no inhibitory effect on lymphoma cell proliferation, confirming the Apt-mediated, specific cell effect. In addition, specific growth inhibition induced by the Apt-HAuNS-Dox was also observed in the additional CD30-expressing lymphoma cells, KMH2 and SUDHL-1 (Figure 5B). Moreover, CD30-negative cells (Jurkat and HL-60) were also cultured with the same set of treatments and change in cell proliferation was examined. When compared to the non-treatment control, neither the Apt-HAuNS-Dox nor the HAuNS-Dox had an effect on the control cell proliferation (Figure 5C), indicating the Apt-HAuNS-Dox was cell specific.
Figure 5.
Specific cell growth inhibition by the Apt-HAuNS-Dox, but not the HAuNS-Dox. (a) The CD30-expressing lymphoma cells, HDLM2 and Karpas 299, were exposed to the Apt-HAuNS-Dox, the HAuNS-Dox (no aptamer), the Apt-HAuNS (no DOX), the HAuNS alone, or no treatment. After culture for 48 h, viable cells under each treatment condition were counted and relative cell proliferation rates (%) were calculated using non-treatment control cultures as 100%. (b) The additional CD30-expressing lymphoma cells, KMH2 and SUDHL-1, were also treated with the Apt-HAuNS-Dox, the HAuNS-Dox or no treatment. The relative cell proliferation was examined as described above. (c) In contrast, under the same treatment conditions, the Apt-HAuNS-Dox had no inhibitory effect on Jurkat and HL-60 control cells that did not express CD30-biomarkers
For further validation, cell mixtures composed of both CD30-expressing lymphoma cells and CD30-negative control cells were prepared at a 1:1 ratio and treated with a single dose of the Apt-HAuNS-Dox (0.5 μM final concentration). After culture for 48 h, the cells were collected and stained with CD30 antibody to distinguish lymphoma cells from control cells in the same cultures. The cells were then counted. Our previous study showed that aptamer probes and antibodies could simultaneously target CD30 biomarkers and did not interrupt each other, probably due to different binding sites.[22] Viable cells were then gated (side scatter vs. forward scatter) and a relative number of each cell population was counted by flow cytometry analysis. To eliminate artificial bias on cell number counting, cell mixtures were also treated with free DOX or vehicle alone. Cultures showed that the paired cells had similar growth rates without treatment (Figure 6A, vehicle alone). Notably, since chemotherapy drugs are not tumor cell-specific, the presence of free DOX in the cultures killed lymphoma cells as well as control cells, indicating that both cells were equally sensitive to DOX (Figure 6B). In contrast, treatment of Apt-HAuNS-Dox selectively killed the CD30-expressing lymphoma cells and had no effect on the off-target control cell growth within the mixed cultures (Figure 6C). These findings demonstrate that, under the optimal conditions, our Apt-HAuNS-Dox is able to selectively kill tumor cells without unwanted toxicity on off-target cells, which is critical for in vivo application of a nanoscale drug-delivery system.
Figure 6.
The Apt-HAuNS-Dox selectively killed lymphoma cells, but had no effect on the growth of off-target cells in the same cultures. The cell mixtures, composed of paired lymphoma cells and control cells at a 1:1 ratio, were treated with vehicle alone (no treatment) (a), the free DOX (b), or the Apt-HAuNS-Dox (c), respectively. After culture for 48 h, the cells were harvested and stained with CD30 antibody. Viable lymphoma cells and control cells in the same cultures were then simultaneously counted by flow cytometry analysis. The relative cell counts of each cell population were calculated by using total cells as 100%.
3. Conclusion
For targeted tumor therapy, we fabricated an aptamer-equipped and ultra pH-sensitive nanoscale drug delivery system, Apt-HAuNS-Dox, which could specifically target lymphoma tumor cells and rapidly release up to 80% of the loaded DOX within 2 hours at low pH. More importantly, in mixed cell cultures, the Apt-HAuNS-Dox was able to selectively kill lymphoma tumor cells and had no side effects on the off-target cells. These findings provide solid evidence for the development of a safe nanoscale drug delivery system for in vivo therapy.
4. Experimental Section
Reagents
Hollow gold nanospheres (HAuNS) with an average diameter of 42 nm were synthesized by sacrificial galvanic replacement of cobalt nanoparticles according to previously reported procedures.[13] Sodium citrate was used as a stabilizer and absorbed on the surface of the gold nanoparticles to prevent the aggregation of nanoparticles. The 39-mer RNA aptamer specific for CD30, a lymphoma biomarker for diagnosis of Hodgkin’s lymphoma and anaplastic large cell lymphoma, was synthesized by Bio-Synthesis (Lewisville, TX), as previously described, by using the sequence: 5′-mGmAmUUCGUAUGGGUGGGAUCGGGAAGGGCUACGAACAmCmCmG-[Thiol]-3′ (mN represents 2′-O-Methyl RNA). Doxorubicin and methoxy-PEG-SH (MW 5000) were obtained from Sigma-Aldrich (St. Louis, MO). Trisodium citrate dehydrate (>99%), cobalt chloride hexahydrate (99.99%), sodium borohydride (99%), and chloroauric acid trihydrate (ACS reagent grade) were purchased from Fisher Scientific (Pittsburgh, PA). Methylene chloride (ACS grade) was obtained from Baxter Healthcare Corp (Deerfield, IL).
Formulation of the Aptamer-Equipped and Doxorubicin-Loaded Hollow Gold Nanosphere (Apt-HAuNS-Dox)
For activation, the HAuNS (50 OD) were washed twice with sodium citrate solution, and then re-suspended in 0.4 mL sodium citrate solution. The synthetic thiolated CD30 aptamer (0.5 OD) was added into HAuNS solution and mixed for 4 h by stirring. Subsequently, 0.04 μmol PEG5000-SH was added into the solution and mixed with overnight stirring to form the aptamer-equipped and PEGylated HAuNS (Apt-HAuNS). Next, free DOX (5 mg) in nuclease-free water (5 mL) was added to 5 mL of sodium citrate solution containing Apt-HAuNS (40 OD), and the mixture was stirred at room temperature for 24 h. Finally, the formulated Apt-HAuNS-Dox was purified by repeated washes with nuclease-free PBS and centrifugation at 8000 rpm (7000 g) for 10 min, re-suspended in PBS, and stored at 4 °C until use (Figure 1A). Similarly, for control purposes, the HAuNS-Dox was also prepared without the addition of aptamers. The size of Apt-HAuNS-Dox was measured using dynamic light scattering on a Zetasizer nano detector (Malvern Instruments Ltd, Worcestershire, UK)[24] and also examined by a JEM-1400 transmission electron microscope operated at 80 kV (JEOL Ltd., Tokyo, Japan).
Cell Culture
Human anaplastic large cell lymphoma cell lines, Karpas 299 and SUDHL-1, were used in collaboration with Dr. Mark Raffeld at the National Cancer Institute/National Institutes of Health. Acute T-cell leukemia cell lines, Jurkat and Molt-4, human myeloid leukemia cell lines K562 and HL-60, Burkitt’s lymphoma cell lines ST486 and Ramos, and myeloma cell line RPMI8226, were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Hodgkin’s lymphoma cell lines, L540, HDLM2, L428, and KMH2, were used in collaboration with Dr. Barbara Savoldo at the Baylor College of Medicine (Houston, TX). Cells were routinely cultured in RPMI-1640 medium (Mediatech, Manassas, VA), supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 100 units/mL penicillin, and 100 μg/mL streptomycin (Atlanta Biologicals, Lawrenceville, GA) at 37 °C in a 5% CO2 atmosphere.
Cell Binding Assays
Cultured cells (2 × 105) were incubated with Apt-HAuNS-Dox or HAuNS-Dox control in 0.1 mL of PBS at room temperature for 30 min. To determine the optimal concentration at which the Apt-HAuNS-Dox can specifically target CD30-expressing lymphoma cells without non-specific binding to CD30-negative control cells, a dosing experiment was performed at concentrations of 0.5–20 μM as indicated in Figure 2A. The concentration was calculated based on the final concentration of the loaded DOX. A standard concentration of 0.5 μM Apt-HAuNS-Dox was used for the rest of the cell binding assays. After washing once with PBS buffer containing 0.1% BSA, cells were re-suspended in PBS buffer. Finally, resultant cell binding was analyzed by a LSRII flow cytometer (BD Biosciences, San Jose, CA) with FlowJo (v7.0) software by detecting fluorescent signals (667 nm) emitted from the Apt-HAuNS-Dox.[13] All experiments were performed three times with similar results. To validate binding specificity of the Apt-HAuNS-Dox, various cultured lymphoma and leukemia cell lines were tested and results are summarized in Table 1.
Scanning Electron Microscopy
The cultured CD30-expressing HDLM2 cells (a Hodgkin’s lymphoma cell line) and CD30-negative HL-60 cells (an acute myeloid leukemia cell line) were incubated with Apt-HAuNS-Dox for 30 minutes at room temperature. After washing with PBS buffer, cells were fixed with 2.5% glutaraldehyde at 4 °C for 2 h, followed by sequential dehydration for 10 min each in 20%, 30%, 50%, 70%, 90%, and 100% ethanol. The fixed cells were sputter-coated with PtPd using a Cressington 208 HR Sputter Coater (Cressington Scientific, Cranberry Twp, PA) and examined by scanning electron microscopy (Nova NanoSEM 230, FEI Co., Hillsboro, OR). All experiments were performed three times with similar results.
Confocal Fluorescence Microscopy
For selective intracellular cell delivery assays, cells (2 × 105) were treated with the Apt-HAuNS-Dox in 0.1 mL RPMI 1640 medium at 37 °C for 4 h. After washing twice with PBS, cell nuclei were stained with Hoechst 33342 (5 μg/mL, Thermo Scientific, Rockford, IL) for 15 min, cytospined onto slides, and examined by confocal microscope (Olympus FluoViewTM 1000, Olympus America, Center Valley, PA) at 400 × magnification. All experiments were performed three times with similar results.
pH-Induced, Drug-Releasing Assay
To study DOX release in different pH conditions, the Apt-HAuNS-Dox was added into RPMI 1640 medium with pH 5.0, 6.0, or 7.4 and incubated at room temperature. The reaction solutions were collected post-incubation for 0, 0.5, 1.0, and 2.0 h, and centrifuged at 14 000 rpm (11 000g) for 20 min. The supernatants were separated and fluorescent signals from the free DOX released from the Apt-HAuNS-Dox were analyzed spectrophotometrically.[13] Notably, due to the quenching effect of the gold nanoparticles, the loaded DOX was optically silent within the intact Apt-HAuNS-Dox delivery system. The releasing rate of DOX was calculated (%) by comparing the fluorescent signal in control wells, which contain free DOX (100%) at the same concentration as that loaded into the Apt-HAuNS-Dox. All experiments were performed three times with similar results.
Cell Growth Rate and Selective Cell Inhibition Assays
Cell growth rate was investigated by counting viable cells post treatment as described previously.[24] Briefly, Cells (2 × 105) were treated by Apt-HAuNS-Dox in RPMI 1640 medium for 2 h. After replacement with fresh medium containing 10% serum, cells were cultured for an additional 48 h and then harvested. The cells were stained with 0.1% trypan blue in PBS for 15 min and viable cells were counted using a hemocytometer under a light microscope. To rule out a non-specific effect, the HAuNS-Dox without aptamer conjugations, Apt-HAuNS without loaded DOX, and HAuNS alone were used in control groups. Finally, the relative cell growth rate was calculated by the ratio of the number of viable cells in treated samples to the number of viable cells in the non-treatment controls. All experiments were performed three times with similar results.
For the cell inhibition assay, the Apt-HAuNS-Dox (0.5 μM final concentration) was added into a cell mixture that contained CD30-expressing HDLM2 and CD30-negative HL-60 cells or CD30-expressing KMH2 and HL-60 cells at an initial ratio of 1:1. After 2 h of treatment, the mixed cells were seeded in fresh RPMI 1640 medium containing 10% serum and continuously cultured for 48 h. Cells were then harvested and stained by PE-conjugated anti-CD30 antibody (BD Biosciences, San Jose, CA). The mixed cells were separated by flow cytometry based on their CD30 expression, and each cell population was counted separately. All experiments were performed three times with similar results.
Acknowledgments
This project was supported in part by NIH grants R01CA151955 (Y.Z.), R33CA173382 (Y.Z.), 5P50CA126752 (Y.Z.), U54CA151668 9 (C.L.), and the John S. Dunn Foundation (C.L).
Contributor Information
Prof. Chun Li, Email: CLi@mdanderson.org.
Prof. Youli Zu, Email: yzu@tmhs.org.
References
- 1.Deepa G, Kumar NA, Pillai JJ, Kumar GS. Curr Med Chem. 2012 [PubMed] [Google Scholar]
- 2.Panda T, Deepa K. J Nanosci Nanotechnol. 2011;11:10279. doi: 10.1166/jnn.2011.5021. [DOI] [PubMed] [Google Scholar]
- 3.Lehto T, Ezzat K, Langel U. Science. 2012;104:397. doi: 10.1016/B978-0-12-416020-0.00010-3. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. J Drug Target. 2012 doi: 10.3109/1061186X.2012.716845. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Liao L, Bian X, Kong J, Yang P, Liu B. Small. 2012;8:2715. doi: 10.1002/smll.201200217. [DOI] [PubMed] [Google Scholar]
- 6.Brannon-Peppas L, Blanchette JO. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Brigger I, Dubernet C, Couvreur P. Adv Drug Deliv Rev. 2012 doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 8.Sultana S, Khan MR, Kumar M, Kumar S, Ali M. J Drug Target. 2012 doi: 10.3109/1061186X.2012.712130. [DOI] [PubMed] [Google Scholar]
- 9.Wahajuddin S, Arora Int J Nanomed. 2012;7:3445. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiwari M. J Cancer Res Ther. 2012;8:19. doi: 10.4103/0973-1482.95168. [DOI] [PubMed] [Google Scholar]
- 11.Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, Fu T, et al. Nanomedicine. 2012;7:1253. doi: 10.2217/nnm.12.87. [DOI] [PubMed] [Google Scholar]
- 12.Panyam J, Labhasetwar V. Adv Drug Deliv Rev. 2012 doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 13.You J, Zhang G, Li C. ACS Nano. 2010;4:1033. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith B, Lyakhov I, Loomis K, Needle D, Baxa U, Yavlovich A, Capala J, Blumenthal R, Puri A. J Control Release. 2011;153:187. doi: 10.1016/j.jconrel.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gormley AJ, Malugin A, Ray A, Robinson R, Ghandehari H. J Drug Target. 2011;19:915. doi: 10.3109/1061186X.2011.623701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S, Lee J, Kumar P, Lee KY, Lee SK. J Control Release. 2011;152:e9. doi: 10.1016/j.jconrel.2011.08.089. [DOI] [PubMed] [Google Scholar]
- 17.Estevez MC, Huang YF, Kang H, O’Donoghue MB, Bamrungsap S, Yan J, Chen X, Tan W. Methods Molec Biol. 2010;624:235. doi: 10.1007/978-1-60761-609-2_16. [DOI] [PubMed] [Google Scholar]
- 18.Oliphant AR, Brandl CJ, Struhl K. Molec Cell Biol. 1989;9:2944. doi: 10.1128/mcb.9.7.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellington AD, Szostak JW. Nature. 1990;346:818. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 20.Tuerk C, Gold L. Science. 1990;249:505. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P, Zhao N, Zeng Z, Chang CC, Zu Y. Am J Clin Pathol. 2010;134:586. doi: 10.1309/AJCP55KQYWSGZRKC. [DOI] [PubMed] [Google Scholar]
- 22.Zhang P, Zhao N, Zeng Z, Feng Y, Tung CH, Chang CC, Zu Y. Lab Investigation. 2009;89:1423. doi: 10.1038/labinvest.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng Z, Zhang P, Zhao N, Sheehan AM, Tung CH, Chang CC, Zu Y. Mod Pathol. 2010;23:1553. doi: 10.1038/modpathol.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao N, Bagaria HG, Wong MS, Zu Y. J Nanobiotechnol. 2011;31:2. doi: 10.1186/1477-3155-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]