Abstract
Background
Adults with congenital heart disease (CHD) listed for heart transplantation infrequently are supported with ventricular assist devices (VADs). This may disadvantage their priority for organ allocation. We sought to determine the relationship between VAD implantation and successful transplantation among patients listed for heart transplant.
Methods
Adults with CHD patients (N=1,250) were identified in the United Network for Organ Sharing (UNOS) database from 1985 – 2010 and compared to patients without congenital etiology for heart failure (N=59,606). VAD use at listing, listing status, status upgrades, and reasons for upgrade prior to transplant were trended at 5 year intervals and appropriate statistical comparisons were made between groups.
Results
Since 1985, VAD use prior to transplant has increased significantly in patients without CHD but not in CHD patients (17% vs. 3% in 2006–2010, p<0.0001). CHD patients were more likely to be listed as status 2, compared to those without (66% vs. 40%, p<0.001 for 2006–2010), and less likely to be upgraded to status 1 after listing (43% vs. 55%, p=0.03). Among those upgraded to status 1, CHD patients were less likely to have a VAD at transplant than those without (3% vs. 18%, p=0.005). VAD use was more likely to result in death in CHD patients.
Conclusions
VAD use is less common in CHD patients than patients without CHD, both at the time of listing and transplantation. Reduced VAD use appears to contribute to lower listing status and organ allocation. These differences have grown more disparate over time. Separate criteria for organ allocation for CHD patients may be justified.
Background
Adult congenital heart disease (CHD) patients are a growing population because of improved survivorship through childhood. They remain vulnerable to myocardial dysfunction and clinical heart failure,1 a major cause of death in these individuals.2, 3 Because of this, adult CHD patients are a growing proportion of patients referred for heart transplantation.4 Despite the anatomic and physiologic challenges,5 favorable longterm transplant outcomes have been reported.6, 7
The use of ventricular assist devices (VAD) as a bridge to transplant has become more commonplace, particularly since the introduction of continuous flow pumps.8, 9 Extension of this practice to CHD patients, however, has been slower. Data from the United Network for Organ Sharing (UNOS) Standard Transplant and Research dataset has demonstrated that, compared to those without CHD, listed CHD patients are less likely to have a VAD or other mechanical support, and longer wait times in status 2.10, 11 Consequently, cardiovascular mortality on the heart transplant wait list is higher in CHD patients.
In 2006, a universal policy change was made in US organ allocation, such that status 1 patients outside the local referral area were offered organs before local status 2 patients. This change decreased the number of overall transplants for status 2 patients.4, 12, 13 Because VAD implantation is a qualification for status 1 listing, lower VAD implantation rates in listed patients with CHD may result in lower priority status and reduced organ allocation for these patients.12 We sought to determine the impact of VAD utilization on listing and heart allocation for CHD heart transplant candidates by following trends over time.
Methods
Patient Population
Patient-level data were obtained from UNOS, a US registry of transplant listing, organ allocation, and outcomes maintained continuously since 1985. Our Institutional Review Board approved our use of these data, and the requirement for individual consent was waived. We excluded patients who were <18 years old at the time of listing, listed for double organ transplantation, or listed for re-transplantation. The remaining patients were classified as CHD or without CHD patients based on the stated etiology of their heart failure.
Variables obtained included age, sex, listing status, inotrope use, and VAD implant at the time of listing. Patients who were status 2 at listing but status 1 (1, 1A or 1B) at the time of organ offering were considered to have had a status upgrade. Patients in whom inotropic support was provided at time of transplant but not at listing were considered upgraded on the basis of inotrope use. Similarly, patients in whom VAD was present at transplant but not at listing were considered upgraded on the basis of VAD placement. Both were expressed as a percentage of patients upgraded. These were not mutually exclusive, nor did they account for all upgrades. All VADs were included together regardless of designation as “right” or “left,” given the potential incongruity of nomenclature for systemic vs. sub-pulmonic ventricles. Missing values for VAD fields were assumed to indicate no VAD support was present.
Data were analyzed by 5-year incremental eras starting from 1985 and were based on the listing date. Groups were compared using SPSS for Mac (Version 18) using Chi-square testing for categorical variables, and Students t-test for continuous variables. P<0.05 was considered statistically significant. All authors have had access to the primary UNOS data, have read and agree to the manuscript as written, and take full responsibility for the integrity of the reported findings.
Results
Of 78,470 individuals in the database, 40,785 were excluded (including 13,177 pediatric patients, 4,068 listed for multiple organs simultaneously, and 2,389 listed for redo transplant, not mutually exclusive), leaving a study population of 60,856. From these, we identified 1,250 CHD patients (36% female), and 59,606 without CHD (22% female). CHD patients in general were younger at listing (33.5±12.5 years vs. 51.4±11.2 years, p<0.001), as expected from previous studies.10, 11 Peak VO2 was not significantly different (12.6±3.2 vs. 11.6±3.5 ml/kg/min). The majority of CHD patients were classified as “CHD with unknown surgery” (N=828), with 372 identified as “CHD with surgery” and 47 as “CHD without surgery.”
Adults listed for transplantation for both groups is given by era (table 1). The percentage of listed patients who are transplanted has declined over time, with a larger drop in CHD patients to 50% (95% CI 45 – 56%) vs. 62% (95% CI 61 – 63%) of patients without CHD for the most recent era (p<0.001).
Table 1.
Adults listed for heart transplantation by era.
| Era | CHD Listed (N) |
CHD Transplanted (%) |
95%CI | Without CHD Listed (N) |
Without CHD Transplanted (%) |
95%CI |
|---|---|---|---|---|---|---|
| 1985–1990 | 69 | 100 | (100-100) | 6,712 | 85 | (85 – 86) |
| 1991–1995 | 204 | 77 | (71 – 82) | 14,004 | 70 | (69 – 71) |
| 1996–2000 | 293 | 57 | (52 – 63) | 15,414 | 61 | (60 – 62) |
| 2001–2005 | 366 | 67 | (62 – 72) | 11,825 | 67 | (66 – 67) |
| 2006–2010 | 318 | 50 | (45 – 56) | 11,651 | 62* | (61 – 63) |
Number of adults listed for transplantation by era, together with percent transplanted with 95 confidence limits), for adults with congenital heart patients (CHD) vs. those wtihout.
denotes p<0.001 for comparison of patients CHD vs. without.
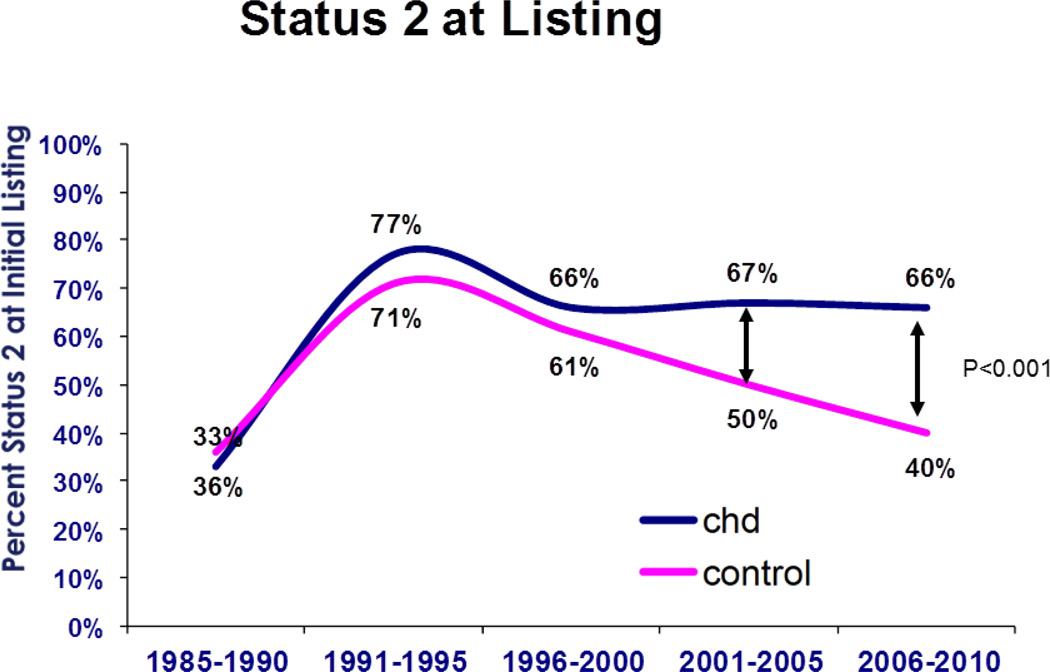
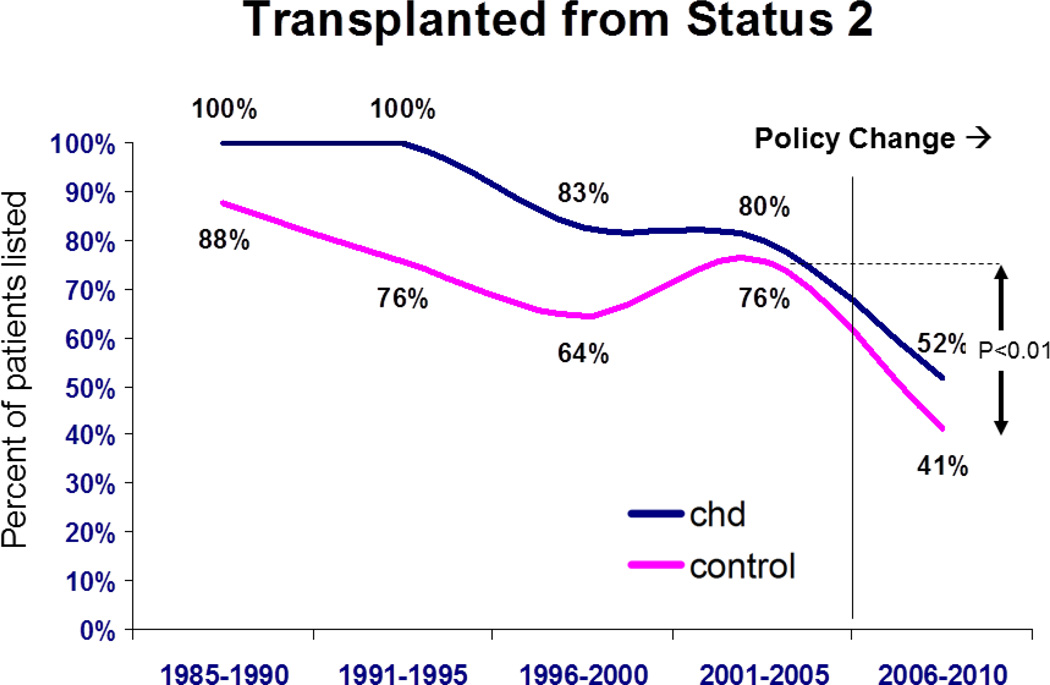
The percentage of patients initially listed as status 2 has not changed in the CHD group for the past three eras (Figure 1). However, this percentage has gradually dropped for those without CHD to 40% in the most recent era (95% CI 39 – 41%, p<0.001 vs. CHD). The number of patients transplanted after initial listing in status 1 has not changed significantly over time for either group. Yet there has been a decline in transplantation from status 2 since 2006 for both groups (Figure 2). Currently the proportion of patients transplanted from status 2 is 33% (95% CI 24 – 42%) for CHD, and 41% (95% CI 39 – 44%) for those without CHD (p<0.0001 for both compared to prior era).
Figure 1.
Percentage of patients from each group listed in status 2 for both groups as a function of era. CHD = congenital heart disease. Controls are adult patients without CHD.
Figure 2.
Percentage of patients from each group transplanted from status 2 as a function of era. CHD = congenital heart disease, control are those without CHD. P values are for comparison between eras for both patient groups.
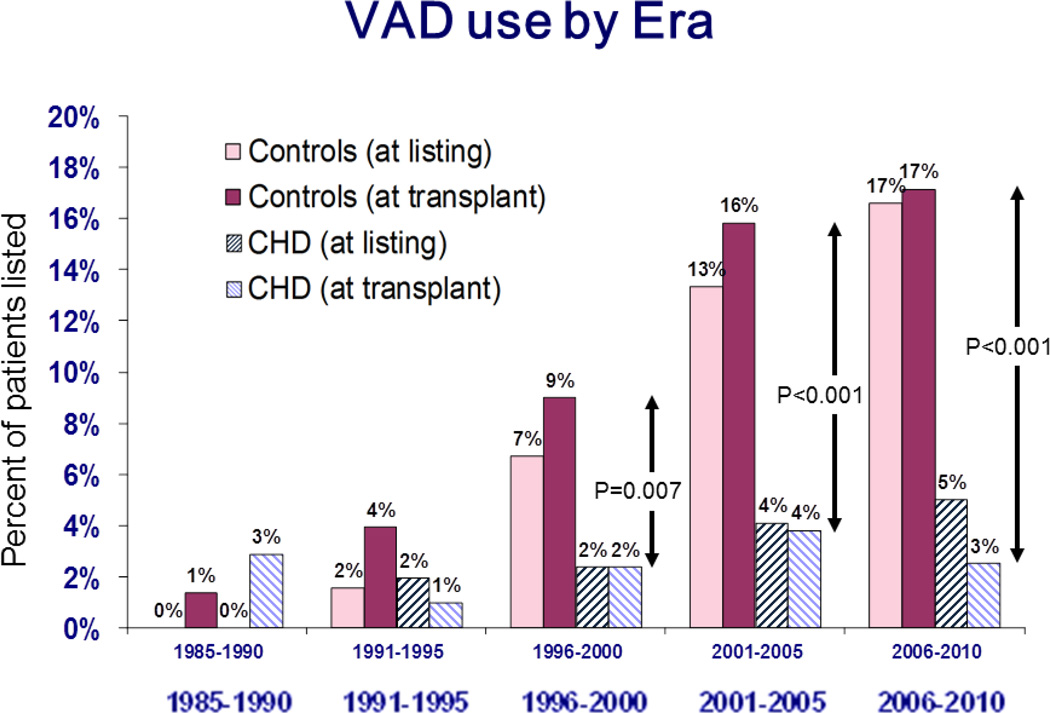
For patients without CHD, VAD use rose steadily to 17% (95% CI 16–17%) at listing and to 17% (95% CI 16.5 – 17.8%) at transplant over the study period (p < 0.001 for change from 1985–1990 for both listing and transplant, Figure 3). Strikingly, there has not been a significant change in VAD utilization in CHD patients over this same time period.
Figure 3.
Percentage of patients with implanted ventricular assist devices at listing and transplant for both congenital heart patients (CHD) and those without (controls), shown by era. P values are for comparison between patient groups for VAD use at transplant.
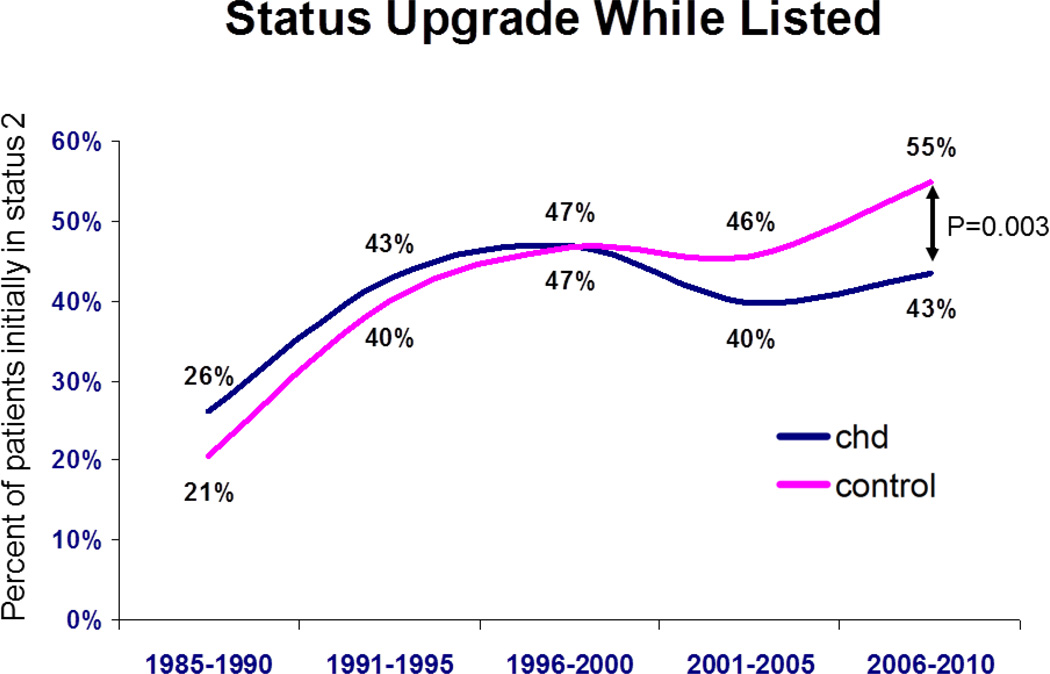
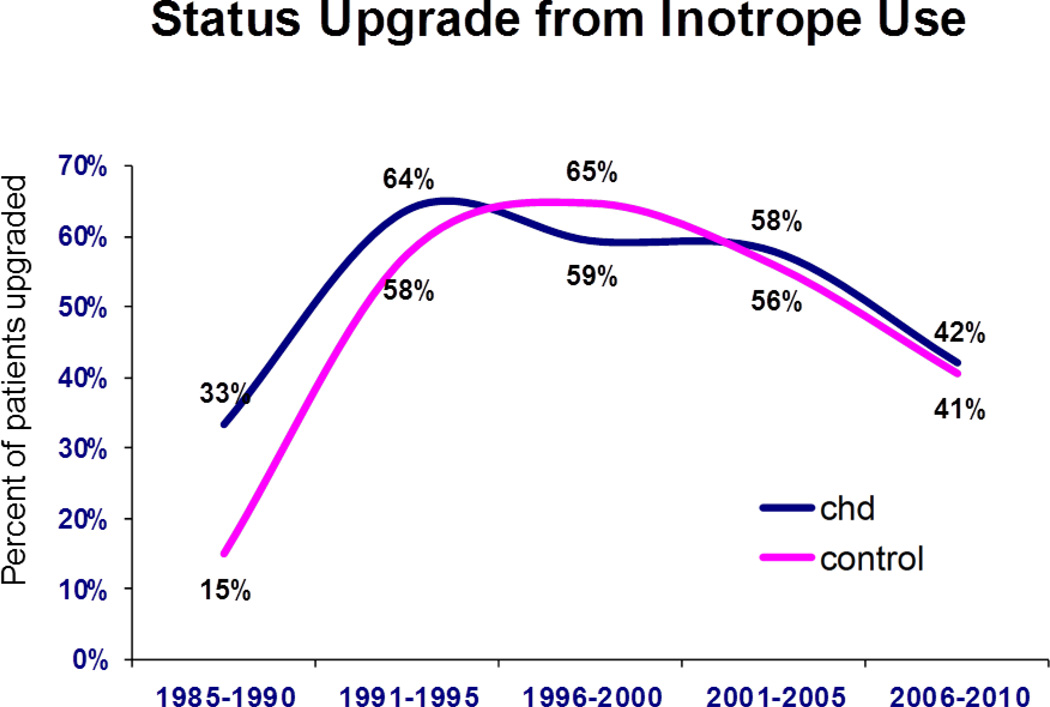
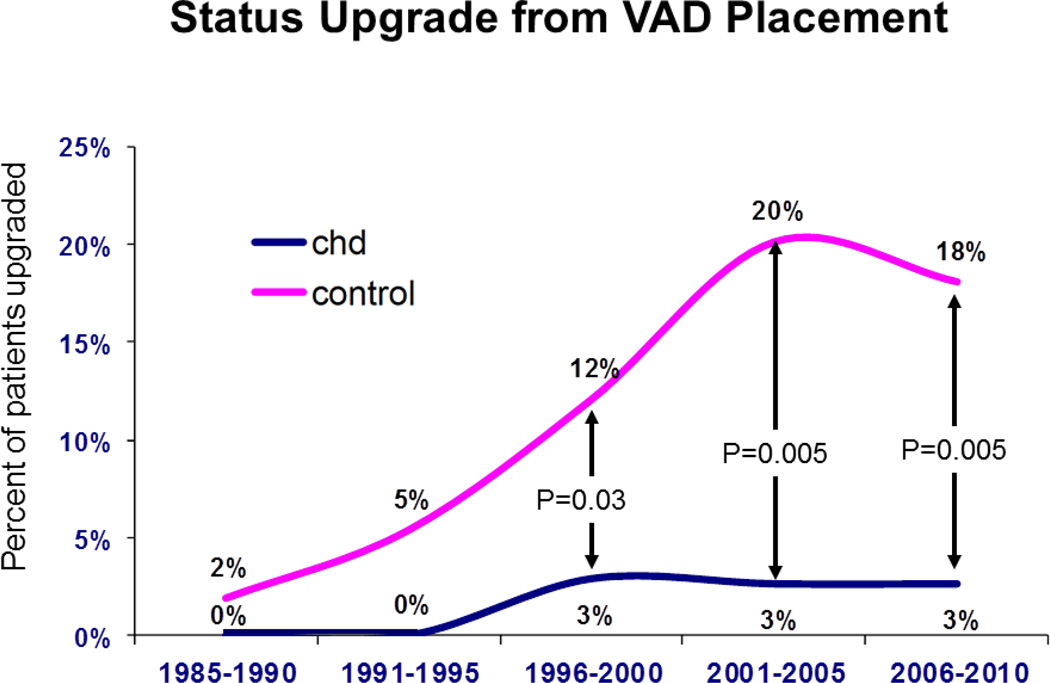
The frequency of status upgrades while listed is shown for both groups (Figure 4). A similar percentage of patients were upgraded from status 2 at listing to status 1 at transplant for both CHD vs. those without CHD during all eras except 2006 – 2010, when there was a significantly higher percentage upgraded among patients without CHD group (55%, 95% CI 53 --56%) compared to the CHD group (43%, 95% CI 36 – 51%, p=0.003 vs. those without). Of those upgraded, the percent of patients upgraded with interval institution of inotrope support did not differ between groups (Figure 5). This contrasts with the percent of patients upgraded because of interval VAD implantation, which was significantly higher in those without CHD for each era from 1996 (Figure 6). For 2006 – 2010, 18% of patients without CHD upgraded had a VAD at the time of transplant (95% CI 16 -- 20%) vs. only 3% in CHD patients (95% CI 0-- 6%, p=0.005).
Figure 4.
Percentage of patients from either group who were upgraded from status 2 at listing to status 1 at time of organ offering as a function of era. CHD = congenital heart disease.
Figure 5.
Percentage of patients upgraded from status 2 with interval requirement of inotropic support. CHD = congenital heart disease.
Figure 6.
Percentage of patients upgraded from status 2 with interval ventricular assist device implant (C). CHD = congenital heart disease.
The outcome of VAD patients at listing for the entire study period is shown (Table 2). For non-CHD, 68% (95% CI 67 – 70%) were transplanted, and 26% (95% CI 24 – 27%) died or were unstable. The remaining patients (6%) were removed for other reasons including transfer out of area, refusal, clinical improvement, or other. By comparison, for CHD patients, 48% (95% CI 33 – 63%) were eventually transplanted, 41% (95% CI 26 – 55%) had died or were too unstable for transplant, and 12% were removed from listing (p=0.015 for CHD vs. without CHD).
Table 2.
Outcome of all patients with VADs at the time of listing
| CHD | Without CHD | |||||
|---|---|---|---|---|---|---|
| N | % | 95% CI | N | % | 95% CI | |
| Transplanted | 20 | 48 | (33–63) | 3001 | 68 | (67–70) |
| Died/unstable | 17 | 40 | (26–55) | 1128 | 26 | (24–27) |
| Removed/other | 5 | 12 | (2–22) | 255 | 6 | (5–7) |
CHD = congenital heart disease. Removed/other category includes those with clinical improvement, transfer away from the transplant center, refusal of transplant, or removed in error.
Discussion
Our analysis of the UNOS data demonstrates that CHD patients are: 1) less likely to have a status upgrade while listed, 2) less likely to receive a VAD after being listed, 3) less likely to receive an allograft while listed, and 4) more likely to die or become too unstable rather than be transplanted if a VAD is present. Additionally, since 2006, there has been a considerable decline in transplantation from status 2 among patients without CHD and especially with CHD, reflecting the allocation policy shift.
The observed low utilization of VAD among CHD patients can be interpreted in several ways. One possibility is that less severe heart failure is present in CHD patients listed for transplant. This seems unlikely since CHD patients had similar exercise capacity and worse cardiovascular outcomes while listed.11 A second more likely explanation is that increased anatomic complexity limits implantation. Based on single-center publications, the majority of congenital heart transplants are done in patients with transposition of the great arteries with a systemic right ventricle,14–16 whereas most VADs are designed for implantation in a morphologic left ventricle. An alternative explanation is that CHD patients suffer severe circulatory failure, but not from ventricular systolic dysfunction. The patients most likely to fit this category are single-ventricle patients palliated with a Fontan procedure, who comprise a considerable portion of transplanted CHD individuals.14–16 Many single ventricle patients develop significant morbidity from chronically elevated systemic venous pressure, including protein-losing enteropathy (PLE), malnutrition, ascites, coagulopathy, liver cirrhosis and desaturation through veno-venous collaterals. All of these occur in the context of normal systolic function of the systemic single ventricle. VAD use in the systemic circulation is therefore not a suitable solution for “Fontan failure.”17, 18 To support the pulmonic circulation, VAD implant requires complex cannulation,19 and is currently reported only anecdotally,20, 21 often without successful outcome.22 Novel pump designs have been proposed specifically for Fontan patients to address this problem,19, 23 with the expectation that successful utilization may favorably impact transplant candidacy and outcome.
Status upgrades in both groups were also attributable to inotropic support. However, dobutamine does not increase stroke volume in Fontan or systemic RV patients.24–26, 27, 28 Thus inotropic support as a criterion for heart failure severity and heart allocation priority may also not be applicable to this population.
Given the scarcity of organs, should transplant priority be given to patients with lower risk of perioperative complications? CHD patients represent a dilemma; higher risk may be balanced by high potential benefits. While early mortality in specific subsets is higher,29 long-term survival is excellent and comparable to outcome in recipients without CHD. Despite these realistic concerns, in a contemporary cohort from a single center, there was no difference in post heart transplant survival at one month, one year or five years between patients with CHD or without CHD.30 As such, transplantation remains an important therapy for CHD patients. They are generally younger than patients without CHD and their transplant survival benefit may add years to their adulthood. 6, 7 PLE, for example, contributes substantially to perioperative mortality,31 yet transplantation is curative.18, 32, 33 Despite the favorable long-term outcomes, one could argue that VAD use stabilizes the patients prior to transplant, and that patients with higher perioperative risk, such as single ventricle patients,29 should not necessarily have priority. Yet CHD patients in whom a VAD is not an option may improve their perioperative outcomes by earlier transplantation prior to progression of comorbidities and concomitant risk.
Clinical Relevance
The 2006 allocation policy change created a disadvantage for status 2 patients, who now wait longer for an organ. Because VAD implant defines a status 1A or 1B patient, there is a “cutting in line” effect for those with VADs who have a head start in allocation for this precious resource. VAD use offers recipients a period of potential stability making them better suited for eventual transplant.34 Non-VAD candidates do not have this safety net. In light of the disparity, we agree with others that for certain sub-populations such as those with CHD, inapplicable or less-effective therapies such as VAD implantation or inotrope dependence should not be so heavily weighted in the determination of organ allocation. 10–12 It has been recently shown that the current status definitions that favor VAD patients are disproportionate to their actual mortality risk relative to those without VAD.34 Collectively the data support a reappraisal of allocation procedures to render the organ allocation process more equitable. Allocation priorities should balance the risks of mortality without transplant as well as perioperative risks with the relative long-term benefits of transplantation in younger patients.
Limitations
Inherent limitations of this study include the retrospective design from a dataset not necessarily tailored for CHD patients. There is no method to validate the reported CHD diagnosis within the registry. Our methods required assumption that missing data, particularly for VAD use, implied that therapy was not given. While we cannot formally validate this assumption, we know from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) dataset on implanted devices instigated in 2005, that only 21 VADs were registered in CHD patients, which is comparable with the data we reported in this study. We did not differentiate right verses left VAD because of lack of standardized nomenclature for congenital defects and uncertainty as to which circulation was being supported. We did not study children, in whom unique issues are often raised, though bridging to transplant with VAD has been done successfully.21, 35
Conclusions
CHD poses new challenges to heart failure management and transplantation. Use of mechanical support as a bridge to transplantation in the CHD population is currently limited, and highlights the need for VAD innovation and the development of surgical techniques for VAD implantation in anatomically complex CHD patients. Given the significant challenges of VAD use in this population, the feasibility of increasing VAD support in patients with CHD remains in question. Orthotopic heart transplant has been shown to be an effective therapy in these individuals. Given this, current allocation policies that favor patients without CHD may not be fair. Though the proportion of CHD patients undergoing transplant is small, national standards should account for their unique limitations and allow equitable organ allocation based on both risk and potential benefit.
Acknowledgments
Funding Sources:
Dr. Broberg was supported by a Clinical Research Development Grant from the National Heart, Lung, and Blood Institute (NHLBI 1K23HL093024-01). This work was supported in part by Health Resources and Services Administration Contract 234-2005-37011C. This content is the responsibility of the authors alone and does not necessarily reflect the views or policy of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations, imply endorsement by the US Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None of the authors have any relevant financial relationships to disclose.
References
- 1.Bolger AP, Coats AJ, Gatzoulis MA. Congenital heart disease: The original heart failure syndrome. Eur Heart J. 2003;24:970–976. doi: 10.1016/s0195-668x(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 2.Nieminen HP, Jokinen EV, Sairanen HI. Causes of late deaths after pediatric cardiac surgery: A population-based study. J Am Coll Cardiol. 2007;50:1263–1271. doi: 10.1016/j.jacc.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 3.Oechslin EN, Harrison DA, Connelly MS, Webb GD, Siu SC. Mode of death in adults with congenital heart disease. Am J Cardiol. 2000;86:1111–1116. doi: 10.1016/s0002-9149(00)01169-3. [DOI] [PubMed] [Google Scholar]
- 4.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The registry of the international society for heart and lung transplantation: Twenty-eighth adult heart transplant report-2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Hosseinpour AR, Cullen S, Tsang VT. Transplantation for adults with congenital heart disease. Eur J Cardiothorac Surg. 2006;30:508–514. doi: 10.1016/j.ejcts.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Patel ND, Weiss ES, Allen JG, Russell SD, Shah AS, Vricella LA, Conte JV. Heart transplantation for adults with congenital heart disease: Analysis of the united network for organ sharing database. Ann Thorac Surg. 2009;88:814–821. doi: 10.1016/j.athoracsur.2009.04.071. discussion 821-812. [DOI] [PubMed] [Google Scholar]
- 7.Karamlou T, Hirsch J, Welke K, Ohye RG, Bove EL, Devaney EJ, Gajarski RJ. A united network for organ sharing analysis of heart transplantation in adults with congenital heart disease: Outcomes and factors associated with mortality and retransplantation. J Thorac Cardiovasc Surg. 2010;140:161–168. doi: 10.1016/j.jtcvs.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 8.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Baldwin JT, Young JB. The fourth intermacs annual report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH, HeartMate IICI. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 10.Davies RR, Russo MJ, Yang J, Quaegebeur JM, Mosca RS, Chen JM. Listing and transplanting adults with congenital heart disease. Circulation. 2011;123:759–767. doi: 10.1161/CIRCULATIONAHA.110.960260. [DOI] [PubMed] [Google Scholar]
- 11.Everitt MD, Donaldson AE, Stehlik J, Kaza AK, Budge D, Alharethi R, Bullock EA, Kfoury AG, Yetman AT. Would access to device therapies improve transplant outcomes for adults with congenital heart disease? Analysis of the united network for organ sharing (unos) J Heart Lung Transplant. 2011;30:395–401. doi: 10.1016/j.healun.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Moazami N, Feldman D. Rethinking the terminology of mechanical circulatory support. The Journal of thoracic and cardiovascular surgery. 2012;144:2–3. doi: 10.1016/j.jtcvs.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Nativi JN, Kfoury AG, Myrick C, Peters M, Renlund D, Gilbert EM, Bader F, Singhal AK, Everitt M, Fisher P, Bull DA, Selzman C, Stehlik J. Effects of the 2006 u.S. Thoracic organ allocation change: Analysis of local impact on organ procurement and heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29:235–239. doi: 10.1016/j.healun.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 14.Irving C, Parry G, O'Sullivan J, Dark JH, Kirk R, Crossland DS, Chaudhari M, Griselli M, Hamilton JR, Hasan A. Cardiac transplantation in adults with congenital heart disease. Heart. 2010;96:1217–1222. doi: 10.1136/hrt.2009.184713. [DOI] [PubMed] [Google Scholar]
- 15.Speziali G, Driscoll DJ, Danielson GK, Julsrud PR, Porter CJ, Dearani JA, Daly RC, McGregor CG. Cardiac transplantation for end-stage congenital heart defects: The mayo clinic experience. Mayo cardiothoracic transplant team. Mayo Clinic proceedings. Mayo Clinic. 1998;73:923–928. doi: 10.4065/73.10.923. [DOI] [PubMed] [Google Scholar]
- 16.Carrel T, Neth J, Pasic M, Laske A, Jenni R, Maggiorini M, Turina M. Should cardiac transplantation for congenital heart disease be delayed until adult age? Eur J Cardiothorac Surg. 1994;8:462–468. doi: 10.1016/1010-7940(94)90015-9. discussion 469. [DOI] [PubMed] [Google Scholar]
- 17.Sian Pincott E, Burch M. Indications for heart transplantation in congenital heart disease. Current cardiology reviews. 2011;7:51–58. doi: 10.2174/157340311797484240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies RR, Sorabella RA, Yang J, Mosca RS, Chen JM, Quaegebeur JM. Outcomes after transplantation for "failed" fontan: A single-institution experience. J Thorac Cardiovasc Surg. 2012;143:1183–1192. e1184. doi: 10.1016/j.jtcvs.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Rodefeld MD, Boyd JH, Myers CD, LaLone BJ, Bezruczko AJ, Potter AW, Brown JW. Cavopulmonary assist: Circulatory support for the univentricular fontan circulation. Ann Thorac Surg. 2003;76:1911–1916. doi: 10.1016/s0003-4975(03)01014-2. discussion 1916. [DOI] [PubMed] [Google Scholar]
- 20.Russo P, Wheeler A, Russo J, Tobias JD. Use of a ventricular assist device as a bridge to transplantation in a patient with single ventricle physiology and total cavopulmonary anastomosis. Paediatric anaesthesia. 2008;18:320–324. doi: 10.1111/j.1460-9592.2008.02435.x. [DOI] [PubMed] [Google Scholar]
- 21.Sharma MS, Forbess JM, Guleserian KJ. Ventricular assist device support in children and adolescents with heart failure: The children's medical center of dallas experience. Artificial organs. 2012 doi: 10.1111/j.1525-1594.2012.01443.x. [DOI] [PubMed] [Google Scholar]
- 22.Mackling T, Shah T, Dimas V, Guleserian K, Sharma M, Forbess J, Ardura M, Gross-Toalson J, Lee Y, Journeycake J, Barnes A. Management of single-ventricle patients with berlin heart excor ventricular assist device: Single-center experience. Artificial organs. 2012;36:555–559. doi: 10.1111/j.1525-1594.2011.01403.x. [DOI] [PubMed] [Google Scholar]
- 23.Rodefeld MD, Frankel SH, Giridharan GA. Cavopulmonary assist: (em)powering the univentricular fontan circulation. Seminars in thoracic and cardiovascular surgery. Pediatric cardiac surgery annual. 2011;14:45–54. doi: 10.1053/j.pcsu.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt B, Steendijk P, Ovroutski S, Lunze K, Rahmanzadeh P, Maarouf N, Ewert P, Berger F, Kuehne T. Pulmonary vascular resistance, collateral flow, and ventricular function in patients with a fontan circulation at rest and during dobutamine stress. Circ Cardiovasc Imaging. 2010;3:623–631. doi: 10.1161/CIRCIMAGING.109.931592. [DOI] [PubMed] [Google Scholar]
- 25.Senzaki H, Masutani S, Ishido H, Taketazu M, Kobayashi T, Sasaki N, Asano H, Katogi T, Kyo S, Yokote Y. Cardiac rest and reserve function in patients with fontan circulation. J Am Coll Cardiol. 2006;47:2528–2535. doi: 10.1016/j.jacc.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Robbers-Visser D, Jan Ten Harkel D, Kapusta L, Strengers JL, Dalinghaus M, Meijboom FJ, Pattynama PM, Bogers AJ, Helbing WA. Usefulness of cardiac magnetic resonance imaging combined with low-dose dobutamine stress to detect an abnormal ventricular stress response in children and young adults after fontan operation at young age. Am J Cardiol. 2008;101:1657–1662. doi: 10.1016/j.amjcard.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 27.Oosterhof T, Tulevski II, Roest AA, Steendijk P, Vliegen HW, van der Wall EE, de Roos A, Tijssen JG, Mulder BJ. Disparity between dobutamine stress and physical exercise magnetic resonance imaging in patients with an intra-atrial correction for transposition of the great arteries. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2005;7:383–389. doi: 10.1081/jcmr-200053454. [DOI] [PubMed] [Google Scholar]
- 28.Tulevski II, van der Wall EE, Groenink M, Dodge-Khatami A, Hirsch A, Stoker J, Mulder BJ. Usefulness of magnetic resonance imaging dobutamine stress in asymptomatic and minimally symptomatic patients with decreased cardiac reserve from congenital heart disease (complete and corrected transposition of the great arteries and subpulmonic obstruction) Am J Cardiol. 2002;89:1077–1081. doi: 10.1016/s0002-9149(02)02279-8. [DOI] [PubMed] [Google Scholar]
- 29.Karamlou T, Diggs BS, Welke K, Tibayan F, Gelow J, Guyton SW, Slater MS, Broberg C, Song HK. Impact of single-ventricle physiology on death after heart transplantation in adults with congenital heart disease. The Annals of thoracic surgery. 2012;94:1281–1287. doi: 10.1016/j.athoracsur.2012.05.075. discussion 1287–1288. [DOI] [PubMed] [Google Scholar]
- 30.Bhama JK, Shulman J, Bermudez CA, Bansal A, Ramani R, Teuteberg JJ, Shullo M, McNamara DM, Kormos RL, Toyoda Y. Heart transplantation for adults with congenital heart disease: Results in the modern era. J Heart Lung Transplant. 2013;32:499–504. doi: 10.1016/j.healun.2013.01.1047. [DOI] [PubMed] [Google Scholar]
- 31.Lamour JM, Kanter KR, Naftel DC, Chrisant MR, Morrow WR, Clemson BS, Kirklin JK Cardiac Transplant Registry D, Pediatric Heart Transplant S. The effect of age, diagnosis, and previous surgery in children and adults undergoing heart transplantation for congenital heart disease. J Am Coll Cardiol. 2009;54:160–165. doi: 10.1016/j.jacc.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Gamba A, Merlo M, Fiocchi R, Terzi A, Mammana C, Sebastiani R, Ferrazzi P. Heart transplantation in patients with previous fontan operations. J Thorac Cardiovasc Surg. 2004;127:555–562. doi: 10.1016/j.jtcvs.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Jayakumar KA, Addonizio LJ, Kichuk-Chrisant MR, Galantowicz ME, Lamour JM, Quaegebeur JM, Hsu DT. Cardiac transplantation after the fontan or glenn procedure. J Am Coll Cardiol. 2004;44:2065–2072. doi: 10.1016/j.jacc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 34.Dardas T, Mokadam NA, Pagani F, Aaronson K, Levy WC. Transplant registrants with implanted left ventricular assist devices have insufficient risk to justify elective organ procurement and transplantation network status 1a time. J Am Coll Cardiol. 2012;60:36–43. doi: 10.1016/j.jacc.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 35.Blume ED, Naftel DC, Bastardi HJ, Duncan BW, Kirklin JK, Webber SA Pediatric Heart Transplant Study I. Outcomes of children bridged to heart transplantation with ventricular assist devices: A multi-institutional study. Circulation. 2006;113:2313–2319. doi: 10.1161/CIRCULATIONAHA.105.577601. [DOI] [PubMed] [Google Scholar]