Abstract
Purpose
The aim of this prospective descriptive study was to evaluate the efficacy of reducing sexual abstinence as a strategy to decrease sperm DNA fragmentation.
Methods
Men with one or more of the following characteristics were included in the study: older than 44, smoking more than 10 cigarettes per day, with a body mass index over 25, diabetes mellitus, varicocele, a previous chemotherapy treatment, severe oligozoospermia, prostatitis, cryptorchidism, having a partner with recurrent miscarriage and/or implantation failure, poor embryo morphology and/or fertilization failure. Patients were asked to produce a semen sample after 3 to 7 abstinence days which was subjected to a sperm DNA fragmentation test. When DNA fragmentation was above or equal to 30 %, it was considered to be altered. Patients with increased DNA fragmentation were asked to produce another semen sample following a “one abstinence day protocol”. This protocol required producing up to three semen samples with 1 day of abstinence and measuring sperm DNA fragmentation.
Results
Four hundred and sixteen patients produced a first semen sample after a sexual abstinence of 3 to 7 days. Sperm DNA fragmentation was altered in 46 samples (11.1 %). Thirty five patients with increased DNA fragmentation samples completed the “one abstinence day protocol”. DNA fragmentation decreased to normal values in one of the three attempts in 91.4 % of the patients: 81.3 % in the first attempt, 12.5 % in the second try and 6.3 % in the third.
Conclusions
This approach could be a simple, low-cost and effective way to decrease sperm DNA damage to normal values.
Keywords: Sexual abstinence days, DNA fragmentation, IVF, SCD
Introduction
It has been suggested that sperm DNA fragmentation is an outstanding cause of male infertility, and it has been negatively correlated with motility, morphology, and concentration of the ejaculated sperm [1].
Four main hypotheses have been postulated where DNA damage in the sperm can take place: before spermiation (as a result of alterations in the regulation of spermatogenesis); after spermiation (as a result of cross-damage of sperm by leukocytes and/or immature sperm producing reactive oxygen species—ROS—during comigration from the tubules to the epididymus); a combination of both of the former; or after ejaculation (as a result of cross-damage of sperm by ROS-producing leukocytes and/or immature sperm in semen) [2].
Many factors that have been linked to a reduction in male fertility have now been associated with an increase in the sperm DNA fragmentation index (DFI): male aging [3], smoking [4, 5], varicocele [6–8], quemotherapy [9], insulin dependent diabetes mellitus [10], high body mass index [11], as well as any factors which may increase the temperature in the scrotum (fever, hot tubs, tight clothing, cryptorchidism,etc.) [12]. Genitourinary infections by Chlamydia Thracomatis and Mycoplasma Sp. also increase DFI in comparison with fertile subjects without infection [13]; additionally, leukocytospermia is associated with increased ROS production by human spermatozoa [14] and with an increase in immature germ cell concentration [15].
It is now accepted that infertile men have more sperm DNA damage than fertile men [16–19]. Zini (2001) found a difference of 14.3 points in sperm DNA fragmentation between fertile and infertile men (13.3 ± 2.5 vs 27.6 ± 2.5. P = 0.016). Increased sperm DNA fragmentation has been associated with increased time of natural conception [17, 20], lower results in intrauterine insemination [21, 22], and lower fertilization rates [23] and embryo quality [24] in in vitro fertilization cycles (IVF) [25]. Additionally, increased DNA fragmentation may be related to miscarriages or failure of implantation in IVF and ICSI [26, 27], which has led to the recommendation of its evaluation prior to every IVF/ICSI [27].
There is also some experimental evidence that high values of sperm DNA fragmentation can lead to increased risk of cancer development and reduction of the longevity of the offspring [28–30].
Despite the normal recommendation of producing sperm samples for analysis after 2 to 7 days of abstinence [31], some authors have suggested that 1 day of abstinence leads to the best possible semen samples (in terms of WHO guidelines). Levitas et al. (2005) [32] evaluated the relationship between the duration of sexual abstinence and various characteristics of normal and subnormal semen in 9,489 semen samples from 6,008 patients. They concluded that the best possible semen samples (when analysing motility and morphology) from patients with male factor infertility were obtained after 1 day of sexual abstinence.
It is therefore possible that reducing the days of abstinence to one might also improve sperm DNA fragmentation. If this occurred it would seem logical to freeze the sperm sample for its use in assisted reproductive techniques (ART) to ensure that the sample had a correct DFI.
The aim of this study was to determine if 1 day of sexual abstinence could reduce sperm DNA fragmentation to normal values in patients with a first measurement of DFI above the threshold of normality (30 %) in samples produced after 3 to 7 days of abstinence.
Material and methods
This prospective descriptive study was performed from April 2008 to December 2011, following institutional review board approval. All men attending our clinic (URH-García del Real) for infertility counselling, with one or more of the following characteristics, were offered a DNA fragmentation test in their semen sample (obtained after 3 to 7 days of abstinence): men older than 44, smoking more than 10 cigarettes per day, with a body mass index (BMI) above 25, diabetes mellitus, varicocele, previous chemotherapy treatment, severe oligozoospermia (<10 M/ml in two semen samples), prostatitis, cryptorchidism, and/or a partner with recurrent miscarriage (2 or more miscarriages), implantation failure (2 cycles with embryo transfer or a total 4 embryos transferred in different IVF cycles with no pregnancy), poor embryo morphology and/or fertilization failure.
When the first semen sample revealed a DFI above 30 %, men were asked to enter the “one abstinence day protocol”. This protocol consisted in producing up to three sperm samples, after 24 h of abstinence each, in which DNA fragmentation was measured. Once we obtained a sample with a DFI below 30 %, no more samples were requested, and the sperm was frozen for its use in ART.
DNA sperm fragmentation test
Semen samples were produced by masturbation in the clinic. A basic spermiogram and a DNA fragmentation test were performed in raw semen starting as soon as liquefaction was observed, and always in the first hour after production of the sample. Sperm DNA fragmentation was analyzed with the Sperm Chromatin Dispersion (SCD) test (Halotech DNA, Madrid, Spain).
The technique used was based on that described by Fernández et al. (2003) [33], with some modifications. Gelled low-melting-point agarose was fused in a water bath at 90–100 °C for 3 min and then set in a heat chamber at 37 °C for 5 min for temperature equilibration. 25 μL of sperm aliquots containing 5–10 million spermatozoa per mL were mixed with the agarose and pipetted onto a pretreated glass slide (provided in the Kit), covered with a coverslip and left to solidify at 4 °C for 5 min.
Covers were gently removed and slides were immersed horizontally in 10 mL of acid solution (80 μL of HCL + 10 mL of distilled water) for 7 min for DNA denaturation. Slides were then placed for 25 min in a tray with 10 mL of lysing solution. After washing them in distilled water for 5 min, the slides were dehydrated in increasing concentrations of ethanol (70 %–90 %–100 %) for 2 min in each and air-dried.
For bright field microscopy reading, slides had to be stained. For this purpose, dry slides were immersed in 10 mL of a xanthene buffered solution (quick panoptic Nr. 2, Química Clínica Apliacada S.A. Spain) for 7 min and then into 10 mL of a thiazine buffered solution (quick panoptic Nr. 3, Química Clínica Apliacada S.A. Spain) for 6 min. After removing the slide from the solution we added 6 μL of distilled water and covered the slide with a coverslip so it was ready for assessment.
It has been described that when nuclear proteins are removed, the compacted loops expand and form a central core and a peripheral halo of DNA loops. The acid treatment produces DNA unwinding just in the nuclei with a high rate of DNA breakage. After the lysing solution, sperm cells with DNA breakage produce very small or no halos of dispersed DNA. However, nuclei without DNA fragmentation release their DNA loops to form large halos [28].
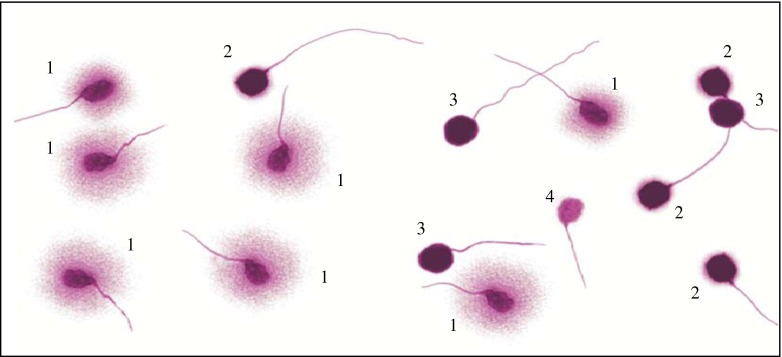
A minimum of 500 spermatozoa per sample were scored into four groups (Fig. 1):
Spermatozoa with large halos (halo width is similar or larger than the minor diameter of the core) + spermatozoa with medium size halos (halo size is between those with large and very small halo).
Spermatozoa with very small-size halo (halo width is similar or smaller than 1/3 of the minor diameter of the core).
Spermatozoa without a halo.
Degenerated spermatozoa (halo weakly or irregularly stained).
Fig. 1.
Group of different sperm halos: 1. Spermatozoa with large halos (halo width is similar or higher than the minor diameter of the core) + spermatozoa with medium size halos (halo size is between those with high and with very small halo). 2. Spermatozoa with very small-size halo (halo width is similar or smaller than 1/3 of the minor diameter of the core). 3. Spermatozoa without a halo. 4. Degenerated spermatozoa (halo weakly or irregularly stained)
Sperm cells with scores two, three and four were considered to have their DNA fragmented [33]. Following Halotech recommendations, sperm DNA fragmentation was considered increased when the percentage of fragmented spermatozoa was 30 % or above.
Sperm freezing
Gradient-prepared sperm was frozen in CBS High Security sperm straws (Cryo Bio System, France) using the Sperm CryoProtect II media (Nidacon International AB, Sweden) as described in the commercial protocol.
Statistical analysis
Continuous variables for analysis of groups “3 to 7 abstinence days” vs. “one abstinence day” were compared with the Student’s T test. Spearman non parametric correlation was used to determine the correlation between days of abstinence and sperm DNA fragmentation. A P value < 0.05 was regarded as being significant.
We considered a statistical precision of 20 %, which established that the sample size needed of “one abstinence day” cases was 24.
All analyses were performed with the commercial software SPSS version 13.0.
Results
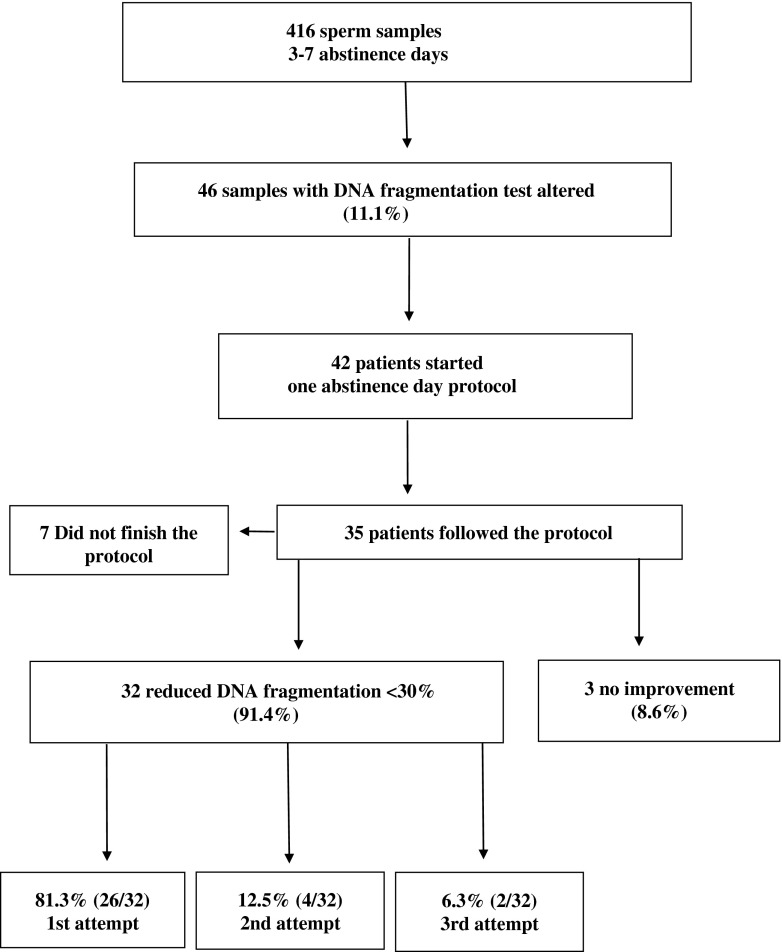
Four hundred and sixteen patients agreed to subject their semen sample to a DNA fragmentation test: 257 patients had one indication (61.8 %) and 159 patients had a combination of indications (38.2 %) for performing the test. Overall, 46 of them (11.1 %) had samples with increased DFI.
All men were caucasian, with an average age of 38 ± 5,5 years (95 % confidence interval, 37,5–38,5), mean BMI was 26,9 ± 3,4 (95 % confidence interval, 26,6–27,3), average smoking consumption was 3,9 ± 5,5 cigarettes per day (95 % confidence interval, 3,1–4,6) and the average number of alcohol units per week was 4,1 ± 7,9 (95 % confidence interval, 3,6–4,7). None of the men reported fever during 12 weeks prior to producing the sperm samples. Abstinence time was 4.8 ± 2.1 days (95 % confidence interval, 4.6–5 days). DNA fragmentation testing started 29.9 min ± 12.4 min (95 % confidence interval, 28–30.5 min) after production of the sample. The mean, range and 95 % confidence limits of the 416 semen samples with 3 to 7 abstinence days included in the study are given in Table 1.
Table 1.
Semen parameter descriptive statistics of the 416 semen samples with 3 to 7 abstinence days
| Semen parameter | Mean | Range | 95 % Confident limits |
|---|---|---|---|
| Volume (mL) | 3,37 | 0,5–11 | 3,2–3,5 |
| Concentration (106/mL) | 54,7 | 0,07–225 | 50,5–58,9 |
| Motility (%) | 52,9 | 0–91,9 | 51,2–54,7 |
| Grade A Motility (%) | 20,9 | 0–63,3 | 19,7–22,1 |
To determine the incidence of DNA fragmented sperm according to the indication, we only took into account cases with a single indication: 29.4 % of patients with severe oligozoospermia revealed an increase in sperm DFI, as did 25 % of the patients older than 44, 22.2 % of men with varicocele, 19.4 % of those with partners with implantation failure, 9.6 % of patients whose partners had suffered recurrent miscarriages and 5.6 % of patients with BMI above 25. Patients with other single indications (smoking, cryptorchidism, prostatitis, chemotheraphy, poor embryo morphology, diabetes mellitus, fertilization failure and others) did not present sperm samples with altered DFI (Table 2).
Table 2.
Percentage of patients with an altered DNA fragmentation test according to their characteristics
| Indication | Mean %DFI ± SD | % Of patients with altered fragmentation |
|---|---|---|
| Severe oligospermia (<10 M/ml) | 24,3 ± 13,3 | 29,4 % (5/17) |
| Age (>44 years old) | 27 ± 18,9 | 25 % (3/12) |
| Varicocele | 18,8 ± 17,7 | 22,2 % (2/9) |
| Implantation failure | 20,1 ± 14,2 | 19,4 % (6/31) |
| Recurrent miscarriage (≥2) | 15,7 ± 9,4 | 9,6 % (3/31) |
| Body mass index (>25) | 14,1 ± 9,5 | 5.6 % (7/124) |
| Smokers (>10 cigarretes/day) | 12,6 ± 5,6 | 0 % (0/13) |
| Cryptorchidism | 10,7 ± 1,8 | 0 %(0/3) |
| Prostatitis | 15,2 ± 7,2 | 0 % (0/3) |
| Chemoteraphy | 7,3 | 0 % (0/1) |
| Poor embryo morphology | 14,4 ± 7,2 | 0 % (0/2) |
| Diabetes Mellitus | 12,3 ± 10,3 | 0 % (0/2) |
| Fertilization failure | 6,7 ± 2,6 | 0 % (0/6) |
| Others | 14,3 ± 3,1 | 0 % (0/3) |
| Multiple indications | 17,9 ± 12.7 | 12.6 % (20/159) |
Multiple indications = more than one characteristic
DNA fragmentation increased in 12.6 % of patients who presented more than one indication. In this group, there were five patients with four indications (40 % of them with increased DFI), 38 cases with three indications (18.4 % with increased DFI) and 116 cases with two indications (10,3 % with increased DFI).
Of the 46 patients with sperm DNA fragmentation equal or above 30 %, 42 (91.3 %) agreed to enter our “one abstinence day protocol”. The mean, range and 95 % confidence limits of semen parameters of these “one abstinence day” samples are given in Table 3. Seven patients (16.7 %) failed to complete the entire protocol. Of the 35 patients who followed the protocol, 32 (91.4 %) produced sperm samples with a DFI below 30 % in one of the three attempts: 81.3 % (26) in the first one, 12.5 % (4) in the second and 6.3 % (2) in the third (Fig. 2). The indications and DNA fragmentation reduction obtained per patient with the “one abstinence day protocol” is reflected in Table 4. Sperm DNA fragmentation reduction was 17.8 percentual points overall. Volume (2.7 vs 3.37 mL), concentration (34.2 vs 54.7 106/mL), motility (19.4 % vs 52.9 %) and grade A motility (7.8 % vs 20.9 %) were significantly lower in the “one abstinence day” group compared with the “3 to 7 abstinence days” group (P < 0.05).
Table 3.
Semen parameter descriptive statistics of the one abstinence day semen samples
| Semen parameter | Mean | Range | 95 % Confident limits |
|---|---|---|---|
| Volume (mL) | 2,7 | 0,5–9 | 2,3–3 |
| Concentration (106/mL) | 34,2 | 0–162 | 25,8–42,8 |
| Motility (%) | 19,4 | 0,01–122 | 13,7–25,1 |
| Grade A motility (%) | 7,8 | 0–45 | 5,3–10,3 |
Fig. 2.
Flow chart: Patients included in the study and results
Table 4.
Indication and sperm DNA fragmentation decrease per patient SOZ = Severe oligozoospermia
| DNA fragmentation (%) | |||||
|---|---|---|---|---|---|
| Patient | Indication | 3–7 Abstinence days | 1 Abstinence day | Decrease | Attempt |
| 1 | SOZ | 38 | 7.5 | 30.5 | 1st |
| 2 | BMI | 30 | 16 | 14 | 1st |
| 3 | AGE + IF | 38.2 | 10.7 | 27.5 | 1st |
| 4 | BMI + IF | 32 | 14.9 | 17.1 | 1st |
| 5 | BMI | 39.9 | 21 | 18.9 | 1st |
| 6 | AGE + IF | 77.5 | 18 | 59.5 | 1st |
| 7 | RM | 35.3 | 20.5 | 14.8 | 1st |
| 8 | IF | 30 | 17.6 | 12.4 | 1st |
| 9 | BMI | 49.1 | 28 | 21.1 | 1st |
| 10 | BMI + SMOKING | 41.8 | 19.4 | 22.4 | 1st |
| 11 | BMI + SMOKING | 31.2 | 19.7 | 11.5 | 1st |
| 12 | SOZ | 37.5 | 26 | 11.5 | 1st |
| 13 | RM | 34.7 | 21.7 | 13 | 1st |
| 14 | BMI | 43.6 | 29.2 | 14.4 | 1st |
| 15 | BMI + SMOKING | 44.9 | 29.3 | 15.6 | 1st |
| 16 | IF | 47 | 18.7 | 28.3 | 1st |
| 17 | BMI + CRYP | 30.6 | 15.6 | 15 | 1st |
| 18 | IF | 37.7 | 15.7 | 22 | 1st |
| 19 | AGE + BMI + SOZ | 30.1 | 28.2 | 1.9 | 1st |
| 20 | AGE + SMOKING | 53.3 | 10.9 | 42.4 | 1st |
| 21 | BMI | 33.6 | 23 | 10.6 | 1st |
| 22 | BMI + SOZ | 65.1 | 15.4 | 49.7 | 1st |
| 23 | AGE + SMOKING | 32.4 | 24.3 | 8.1 | 1st |
| 24 | BMI + SMOKING | 30 | 25.5 | 4.5 | 1st |
| 25 | BMI | 43.3 | 10 | 33.3 | 1st |
| 26 | AGE + IF | 44.4 | 22.2 | 22.2 | 1st |
| 27 | BMI | 46.5 | 25.9 | 20.6 | 2nd |
| 28 | AGE + BMI | 39 | 27.8 | 11.2 | 2nd |
| 29 | BMI | 31.6 | 28.9 | 2.7 | 2nd |
| 30 | VARICOCELE | 42 | 29.7 | 12.3 | 2nd |
| 31 | SOZ | 34 | 9 | 25 | 3rd |
| 32 | BMI | 55.2 | 22.9 | 32.3 | 3rd |
| 33 | RM | 39,3 | ND | – | – |
| 34 | SOZ | 62,5 | ND | – | – |
| 35 | IF | 51,8 | ND | – | – |
BMI Body Mass Index, IF Implantation Failure, RM Recurrent miscarriage, CRYP Cryptorchidism, ND No decrease below 30 %
When applying the Spearman test to determine the correlation between days of abstinence and sperm DNA fragmentation we obtained a P value = 0.055. With the 35 cases included in our study, we reached a statistical precision of 16.5 %.
Discussion
Our results suggest that sperm DNA fragmentation can be decreased by reducing the days of sexual abstinence. This is in accordance with previous publications. Spano et al. (1998) [34] published an epidemiological study of 277 healthy Danish men finding that the sperm chromatin structure assay (SCSA) can be influenced by age, smoking habits, the presence of leukocytes and immature germ forms and the duration of sexual abstinence. Richthoff et al. (2002) [35] studied the impact of testicular and post testicular function on SCSA parameters in 278 military conscripts. They found a negative correlation between sperm concentration and DFI and a positive correlation with abstinence time. Gosalvez et al. (2011) [36] studied two cohorts of normozoospermic individuals: 21 males attending a clinic with clearly adverse female factors and a group of 12 selected donors. They assessed sperm DNA fragmentation after 24 h of abstinence with recurrent ejaculations (one every 24 h) and also before and after sperm selection with abstinence of 3 h. They observed lower baseline levels of DNA fragmentation after shorter periods of abstinence between ejaculations (24 h and 3 h). Although these three studies were based on fertile men and our study dealt with infertile men, the results are similar.
However, other authors comparing samples after 1, 3, 5 and 8 days of abstinence in 11 men undergoing infertility investigation did not find that abstinence days influenced sperm DNA fragmentation [37]. It is suggested that results in DNA fragmentation depend on the time that the mature gamete stays in the epididymis. The longer the stay, the greater the probability of the sperm undergoing a deterioration process, eventually leading to increased susceptibility of chromatin to acid denaturation in situ [34]. A possible explanation for this phenomenon might be attributable to changes in the thiol disulphide status of protamines during epididymal storage leading to a higher instability of the sperm chromatin [38].
Other factors such as the presence of ROS, leucocytes or immature sperm have also been related to an increase in DNA fragmentation. Immature sperm produce ROS due to the cytoplasmatic content in the proximal droplets that can damage the DNA of mature sperm [39]. Therefore, a higher concentration of immature sperm found in samples with 1 day compared with 5 days of abstinence [37] might explain why the 1 day abstinence protocol does not work in all cases.
It is also possible that the presence of infertility affects the results. While studies showing an improvement in DFI by reducing the abstinence days were performed in a healthy population [34–36], De Jonge ’s study (2004) [37] was done on an infertile population. Reducing the abstinence time to 1 day might reduce the deterioration process of the sperm in the epididymis, but it most probably cannot improve the regulation of spermiogenesis in cases where it was damaged. In our series, performed on an infertile population, most cases responded to the “one abstinence day” protocol, irrespective of the diagnosis, and we did not find any common indicator in cases where DFI did not improve after three attempts (8.6 %).
Differences observed in the studies might also depend on the processing of sperm samples. We chose to study DNA fragmentation on neat sperm samples because many publications refer to this data when relating DNA fragmentation to fertility problems [17, 20, 24, 25]. However, some authors have described an improvement of DFI after 3 h of abstinence versus 96 h only when the samples were centrifuged in density gradients, but not in neat samples [36].
In our study DNA fragmentation was altered in a high proportion of patients older than 44, with severe oligozoospermia, a history of varicocele, or implantation failure in their partners. In other clinical conditions, however, the percentage of males affected was similar to the general population [18]. An increase in the number of cases of the indications studied is needed to obtain significant conclusions.
Given the within-subject variation in sperm parameters, and the described intraindividual variation in sperm chromatin structure assay [40], we requested up to three semen samples with “one abstinence day” to observe a possible benefit of the protocol. To our knowledge this has not been described before. Most cases (81.3 %) improved after one sample, while 18.3 % needed a second or third sample to decrease the DFI. We cannot exclude that requesting several “3 to 7 abstinence day” samples may have allowed a reduction of the DFI below 30 %, due to the same predicted within-subject variation. As other authors have published [1], we observe that when the abstinence time is reduced, volume, concentration, motility and grade A motility also diminish. This has to be taken into account when selecting the ART procedure, though in most cases it did not modify the technique previously chosen (unpublished data).
Additionally, the freezing of sperm samples with a reduced DFI after 1 day of abstinence gives the patient the chance to use a good semen sample regarding DNA fragmentation for ART. In keeping with a previous publication that showed that the freezing-thawing process does not affect DNA structure [41], in our post freeze-thawed sperm samples the DFI did not increase (data not shown).
Reducing the time of abstinence is a simple, low-cost and non-invasive technique to reduce DFI in comparison with other strategies like antioxidants, testicular biopsy, MACS, antibiotics or hyaluronic acid binding. Oral antioxidants appear to reduce sperm DNA damage, but more information is required about which antioxidants are better and which patients will benefit from its use. At the moment, some authors find clear benefits, while others detect only partial or no success [42–46]. Testicular biopsy has been described to improve ongoing pregnancy rates in males with increased DFI [47]. However, it is an invasive technique and it provides immature spermatozoa for ICSI, which have been suggested to have an inferior ability to produce normal zygotes [48]. The use of magnetic-activated cell sorting (MACS) allows for a reduction of apoptotic sperm [49] and a reduction of DFI [50]. However, MACS requires expensive equipment and it is not known yet if the magnetic separation has any secondary effects in the sperm. Additionally, MACS separates apoptotic sperm, but there can be fragmented sperm that have not yet started apoptosis and would not be retained by the column.
Antibiotics are effective in reducing the DFI when it rises due to an infection [13]. Reducing the abstinence time may be an effective co-treatment; leucocytes will not be eliminated but their time of contact with the sperm will be reduced. Finally, the use of hyaluronic acid to bind spermatozoa with low DNA fragmentation and normal nucleus appears to be another way of treatment [51], but it can only be applied in ICSI cases.
Although in 2013 the Practice Committee of the American Society for Reproductive Medicine [52] published that the current methods for assessing sperm DNA integrity do not reliably predict ART outcomes and cannot be recommended routinely for clinical use, the many strategies recently developed to reduce DNA fragmentation reflect the increasing interest in controlling a possible contributing factor to sterility. Patients in whom a reduction of DNA fragmentation is not achieved by reducing the abstinence days to one may require other techniques to be applied.
There are some limitations and shortcomings to this study. Firstly, the research was conducted without a control group. Secondly, we cannot exclude that requesting several “3 to 7 abstinence day” samples might have the same results. In addition, the population of the experimental group was small.
The Spearman test to determine the correlation between days of abstinence and sperm DNA fragmentation was near to statistical significance (P = 0.055). We continue with our study to reach a higher number of cases with a view to reaching statistical significance that will support the results of our study, where in 90 % of the cases we obtained a decrease in the sperm DNA fragmentation under the threshold of normality following the “one abstinence day protocol”.
We have observed that severe oligozoospermia, age over 44, varicocele and having a partner with implantation failure were the situations most frequently linked to an increase in sperm DNA fragmentation in our series. Producing a sperm sample after one abstinence day can be a simple, low cost and effective way of reducing DNA fragmentation. More studies are needed to determine if reducing the DNA fragmentation index below 30 % can improve ART results and therefore, if this should be recommended to every male that is going through an ART cycle.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule One abstinence day may be a simple, low cost and effective way to lower sperm DNA fragmentation to normal values.
References
- 1.Sun J-G, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod. 1997;56:602–7. doi: 10.1095/biolreprod56.3.602. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez JG, Sharma Rakesh K, Ollero M, Saleh Ramadan A, Lopez MC, Thomas AJ, Jr, et al. Increased DNA damage in sperm from leukocytospermic semen samples as determined by the sperm chromatin structure assay. Fertil Steril. 2002;78:319–28. doi: 10.1016/S0015-0282(02)03201-6. [DOI] [PubMed] [Google Scholar]
- 3.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci. 2006;103:9601–6. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potts RJ, Newbury CJ, Smith G, Notarianni LJ, Jefferies TM. Sperm chromatin damage associated with male smoking. Mutat Res. 1999;423:103–4. doi: 10.1016/S0027-5107(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 5.Viloria T, Garrido N, Fernández JL, Remohí J, Pellicer A, Meseguer M. Sperm selection by swim-up in terms of deoxyribonucleic acid fragmentation as measured by the sperm chromatin dispersion test is altered in heavy smokers. Fertil Steril. 2007;88:523–5. doi: 10.1016/j.fertnstert.2006.11.135. [DOI] [PubMed] [Google Scholar]
- 6.Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ., Jr Evaluation of nuclear DNA damage in spermaozoa from infertile men with varicocele. Fertil Steril. 2003;80:1431–6. doi: 10.1016/S0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]
- 7.Chi-huang C, Shang-sen l, Da-chang C, Hsin-hsuan C, I-ching C, Yung-ning C, et al. Apoptosis and Kinematics of ejaculated spermatozoa in patients with varicocele. J Androl. 2004;25:348–53. doi: 10.1002/j.1939-4640.2004.tb02799.x. [DOI] [PubMed] [Google Scholar]
- 8.Enciso M, Muriel l, Fernández JL, Goyanes V, Segrelles E, Marcos M, et al. Infertile men with varicocele show a high relative proportion of sperm cells with intense nuclear damage level, evidenced by the sperm chromatin dispersion test. J Androl. 2006;27:106–11. doi: 10.2164/jandrol.05115. [DOI] [PubMed] [Google Scholar]
- 9.Robaire B, Hales BF. Mechanisms of action of cyclophosphamide as a male-mediated developmental toxicant. Adv Exp Med Biol. 2003;518:169–80. doi: 10.1007/978-1-4419-9190-4_14. [DOI] [PubMed] [Google Scholar]
- 10.Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;7:1871–7. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- 11.Kort HI, Massey JB, Carlene WE, Mitchell-Leef D, Shapiro DB, Witt MA, et al. Impact os body mass index values on sperm quantity and quality. J Androl. 2006;27:450–2. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 12.Banks S, King SA, Irvine SD, Saunders TKP. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction. 2005;129:505–14. doi: 10.1530/rep.1.00531. [DOI] [PubMed] [Google Scholar]
- 13.Gallegos G, Ramos B, Santiso R, Goyanes V, Gosalvez J. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Micoplasma. Fertil Steril. 2008;90:328–34. doi: 10.1016/j.fertnstert.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 14.Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Jr, Nada EA, et al. Leukocytospermia is associated with increased ROS production by human spermatozoa. Fertil Steril. 2002;78:1215–24. doi: 10.1016/S0015-0282(02)04237-1. [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson MJ, White A, Barratt CLR, Bolton AE, Cooke ID. The removal of morphologically abnormal sperm forms by phagocytes: a positive role for seminal leukocites. Hum Reprod. 1992;4:517–22. doi: 10.1093/oxfordjournals.humrep.a137682. [DOI] [PubMed] [Google Scholar]
- 16.Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationship with semen quality. J Androl. 2000;21:33–44. [PubMed] [Google Scholar]
- 17.Spanò M, Bonde JP, Hjøllund HI, Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human fertility. Fertil Steril. 2000;73:43–50. doi: 10.1016/S0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 18.Zini A, Bielecki R, Phang D, Zenzes MT. Correlation between two marked sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril. 2001;4:674–7. doi: 10.1016/S0015-0282(00)01796-9. [DOI] [PubMed] [Google Scholar]
- 19.Zini A, Fischer MA, Sharir S, Shayegan B, Phang D, Jarvi K. Prevalence of abnormal sperm DNA denaturation in fertile and infertile men. Urology. 2002;60:1069–72. doi: 10.1016/S0090-4295(02)01975-1. [DOI] [PubMed] [Google Scholar]
- 20.Loft S, Kold-Jensen T, Hjollund NH, Giwercman A, Jesper Gyllemborg J, Ernst E, et al. Oxidative DNA damage in human sperm influences time to pregnancy. Hum Reprod. 2003;6:1265–72. doi: 10.1093/humrep/deg202. [DOI] [PubMed] [Google Scholar]
- 21.Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002;12:3122–8. doi: 10.1093/humrep/17.12.3122. [DOI] [PubMed] [Google Scholar]
- 22.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;1:174–9. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 23.Muriel L, Garrido N, Fernández JL, Remohí J, Pellicer A, De los Santos MJ, et al. Value of the sperm deoxyribonucleic acid fragmentation level, as measured by the sperm chromatin dispersion test, in the outcome of in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2006;85:371–83. doi: 10.1016/j.fertnstert.2005.07.1327. [DOI] [PubMed] [Google Scholar]
- 24.Velez de la Calle JP, Muller A, Walschaerts M, Clavere JL, Jiménez C, Wittemer C, et al. Sperm deoxyribonucleic acid fragmentation as assessed by sperm chromatin dispersion test in assisted reproductive technology programs: results of a large prospective multicenter study. Fertil Steril. 2008;90:1792–9. doi: 10.1016/j.fertnstert.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;6:1401–8. doi: 10.1093/humrep/deh280. [DOI] [PubMed] [Google Scholar]
- 26.Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum Reprod. 2000;8:1717–22. doi: 10.1093/humrep/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 27.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an inceased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;12:2663–8. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-González R, Nuno Moreira P, Pérez-Crespo M, Sánchez-Martín M, Ramirez MA, Pericuesta E, et al. Long-term effects of mouse intracytiplasmatic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008;78:761–72. doi: 10.1095/biolreprod.107.065623. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Crespo M, Moreira P, Pintado B, Gutiérrez-Adán A. Factors from damaged sperm affect its DNA integrity and its ability to promote embryo implantation in mice. J Androl. 2008;29:47–54. doi: 10.2164/jandrol.107.003194. [DOI] [PubMed] [Google Scholar]
- 30.Lewis SEM, Aitken RJ. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005;322:33–41. doi: 10.1007/s00441-005-1097-5. [DOI] [PubMed] [Google Scholar]
- 31.Laboratory manual for the examination and processing of human semen and sperm-cervical mucus interaction. 4. Cambridge: University Press; 1999. [Google Scholar]
- 32.Levitas E, Lunenfeld E, Weiss N, Friger M, Har-Vardi I, Koifman A, et al. Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples. Fertil Steril. 2005;83:1680–6. doi: 10.1016/j.fertnstert.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 33.Fernández JL, Muriel M, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 34.Spanò M, Kolstad AH, Larsen SB, Cordelli E, Leter G, Giwercman A, et al. The applicability of the flow cytometric sperm chromatin structute assay in epidemiological studies. Hum Reprod. 1998;9:2495–505. doi: 10.1093/humrep/13.9.2495. [DOI] [PubMed] [Google Scholar]
- 35.Richthoff J, Spano M, Giwercman YL, Frohm B, Jepson K, Malm J, et al. The impact of testicular and accessory sex gland function on sperm chromatin integrity as assessed by the sperm chromatin structure assay (SCSA) Hum Reprod. 2002;12:3162–9. doi: 10.1093/humrep/17.12.3162. [DOI] [PubMed] [Google Scholar]
- 36.Gosálvez J, González-Martinez M, López-Fernández C, Fernández JL, Sánchez-Martin P. Shorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculate. Fertil Steril. 2011;96:1083–6. doi: 10.1016/j.fertnstert.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 37.De Jonge C, LaFromboise M, Bosmans E, Pharm D, Ombelet W, Cox A, et al. Influence of the abstinence period on human sperm quality. Fertil Steril. 2004;82:57–65. doi: 10.1016/j.fertnstert.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Seligman J, Kosower NS, Weissenberg R, Shalgi R. Thioldisulfide status of human sperm proteins. J Reprod Fertil. 1994;101:435–43. doi: 10.1530/jrf.0.1010435. [DOI] [PubMed] [Google Scholar]
- 39.Ollero M, Gil-Guzman E, Lopez MC, Sharma RK, Agarwal A, Larson K, et al. Characterizations of subsets of human spermatozoa at different stages of maturation: implications in the diagnosis and treatment of male infertility. Hum Reprod. 2001;9:1912–21. doi: 10.1093/humrep/16.9.1912. [DOI] [PubMed] [Google Scholar]
- 40.Erenpreiss J, Bungum M, Spano M, Elzanaty S, Orbidans J, Giwercman A. Intra-individual variation in sperm chromatin structure assay parameters in men from infertile couples: clinical implications. Hum Reprod. 2006;8:2061–4. doi: 10.1093/humrep/del134. [DOI] [PubMed] [Google Scholar]
- 41.Duru NK, Morshedi MS, Schuffner A, Oehninger S. Cryopreservation-thawing of fractionated human spermatozoa is associated with membrane phosphatidylserine externalization and Not DNA fragmentation. J Androl. 2001;22:646–51. [PubMed] [Google Scholar]
- 42.Agarwal A, Nallella KP, Allamaneni SR, Said TM. Role of antioxidants in treatment of male infertility: an overview of literature. Reprod Biomed Online. 2004;8:616–27. doi: 10.1016/S1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 43.Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005;26:349–53. doi: 10.2164/jandrol.04146. [DOI] [PubMed] [Google Scholar]
- 44.Greco E, Romano S, Iacobelli M, Ferrero S, Elena Baroni E, Minasi MG, et al. ICSI in cases of sperm DNA damage: beneficial effect of oral antioxidant treatment. Hum Reprod. 2005;9:2590–4. doi: 10.1093/humrep/dei091. [DOI] [PubMed] [Google Scholar]
- 45.Tremellen K, Miari G, Froiland D, Thompson J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. ANZJOG. 2007;47:216–21. doi: 10.1111/j.1479-828X.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 46.Tunc O, Thompson J, Tremellen K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online. 2010;18:761–8. doi: 10.1016/S1472-6483(10)60024-7. [DOI] [PubMed] [Google Scholar]
- 47.Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2004;1:226–30. doi: 10.1093/humrep/deh590. [DOI] [PubMed] [Google Scholar]
- 48.Palermo GD, Cohen J, Alikani M, Adler A, Rosenwaks Z. Intracytoplasmic sperm injection: a novel treatment for all forms of male factor infertility. Fertil Steril. 1995;63:1231–40. doi: 10.1016/s0015-0282(16)57603-1. [DOI] [PubMed] [Google Scholar]
- 49.Said TM, Agarwal A, Zborowski M, Grunewald S, Glander H-J, Paasch U. Utility of magnetic cell separation as a molecular sperm preparation technique. J Androl. 2008;29:134–42. doi: 10.2164/jandrol.107.003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rawe VY, Boudria HU, Alvarez Sedóa C, Carroa M, Papiera S, Nodara F. Healthy baby born after reduction of sperm DNA fragmentation using cell sorting before ICSI. Reprod Biomed Online. 2010;20:320–3. doi: 10.1016/j.rbmo.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Parmegiani L, Cognigni GE, Bernardi S, Troilo E, Ciampaglia W, Filicori M. “Physiologic ICSI”: Hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil Steril. 2010;93:598–604. doi: 10.1016/j.fertnstert.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 52.Practice Committee of the American Society for Reproductive Medicine The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013;99:673–7. doi: 10.1016/j.fertnstert.2012.12.049. [DOI] [PubMed] [Google Scholar]