Abstract
Purpose
To describe the presentation and fertility sparing treatment of a young woman found to have a steroid cell tumor not otherwise specified (NOS) and her spontaneous pregnancy and delivery shortly after surgery.
Methods
A 20-year-old Hispanic female presented with hirsuitism, virilization, and elevated androgen levels (testosterone 328 ng/dL) and was wrongly diagnosed with polycystic ovarian syndrome. Four months later she sought a second opinion. Her androgens were as follows: testosterone level 485 ng/dL, androstenedione 1,738 ng/dL and DHEA 1,459 ng/dL. She had normal levels of progesterone, estradiol, and DHEA-SO4. On transvaginal ultrasound she had a solid-appearing right ovarian mass. She underwent fertility sparing surgery with a laparoscopic right oophorectomy.
Results
Gross and histological pathology confirmed a benign steroid cell tumor NOS. She had rapid normalization of all androgens 13 days after surgery. She had spontaneous resumption of menses 4 months later. She conceived despite using emergency contraception approximately 9 months following surgery and delivered a healthy boy at term without complication.
Conclusion
Prompt evaluation for an androgen producing tumor should be performed when testosterone levels are greater than 200 ng/dL. Pregnancy following removal of this rare tumor has not previously been reported.
Keywords: Virilization, Steroid cell tumor not otherwise specified, Pregnancy, Androgen tumor, Elevated testosterone, Fertility preservation
Introduction
The vast majority of women that present with hyperandrogenism and irregular menstruation will be diagnosed with polycystic ovarian syndrome (PCOS). In fact, 5–10 % of all reproductive-age women have PCOS (ACOG Practice Bulletin No. 108 [1]). In addition to PCOS, the differential diagnosis for women presenting with signs of androgen excess should include non-classical adrenal hyperplasia and androgen-producing tumors of the ovary or adrenal gland. Cushing’s syndrome and acromegaly are rare causes of hyperandrogenism that are ruled-out only if suggested by other clinical signs or symptoms (ACOG Practice Bulletin No. 108 [1]). We report here on a patient that was initially thought to have PCOS but later determined to have an androgen-producing ovarian tumor that was removed and the patient subsequently conceived and delivered without complication. This case report was submitted to the University of Oklahoma Health Sciences Center Institutional Review Board (IRB) and determined to be exempt. Patient approval was obtained for publication.
Case
A 20-year-old, gravida 0, para 0 Hispanic female presented to the university student health clinic with amenorrhea, hirsutism, and acne. She had menarche at age 10 followed by irregular periods for which she was prescribed birth control pills starting at age 15. During her first year of college, she discontinued the birth control pills followed by amenorrhea. Her serum testosterone level was 328 ng/dL. Her past medical history was unremarkable. She was given information on polycystic ovarian syndrome and told to follow up when she returned home for the summer 4 months later.
She presented to the reproductive endocrinology clinic at the University of Oklahoma Health Sciences Center. The patient weighed 71.7 kg and measured 1.57 m tall. She had a deep voice, acne on her face and back, and an enlarged clitoris. Coarse hair covered her entire lower cheeks, chin, neck, breasts, complete abdomen, buttocks, and inner thighs.
Her testosterone level was 485 ng/dL, androstenedione 1,738 ng/dL and DHEA 1,459 ng/dL. Her progesterone, estradiol, and DHEA-SO4 were within normal limits and a pregnancy test was negative. On transvaginal ultrasound, her right ovary measured 6.3 × 4.7 × 4.9 cm with no identifiable antral follicles. She had a right adnexal mass that was echogenic in appearance consistent with a solid mass. The left ovary measured 2.3 × 1.5 × 2.2 cm with nine antral follicles measuring less than 10 mm, which was consistent with the patient’s age and not distinctly polycystic in appearance.
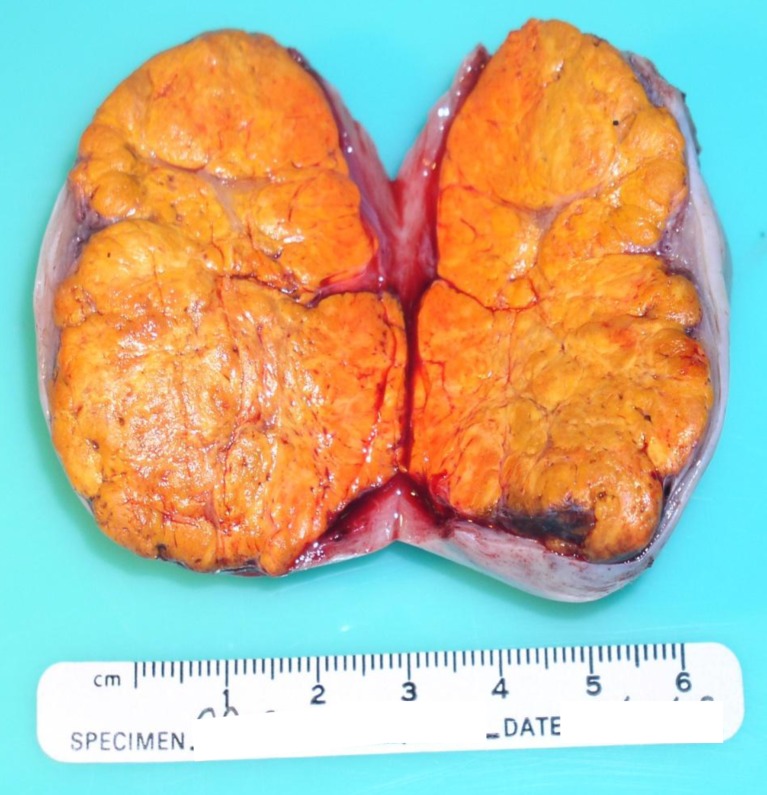
Because of the patient’s age and her desire to retain fertility, a laparoscopic right oophorectomy was performed 1 week after the initial visit (rather than a hysterectomy and bilateral oophorectomy). A well-circumscribed, solid tumor was found occupying 90 % of the ovarian tissue, measuring 5.5 × 4.5 × 4.2 cm. The outer surface of the ovary was smooth and intact without adhesions to surrounding structures. The cut surface of the tumor was firm, yellow-orange, and lobulated (Fig. 1). There was no necrosis or hemorrhage.
Fig. 1.
The ovarian tumor cut surfaces are well-circumscribed, yellow-orange, and lobulated
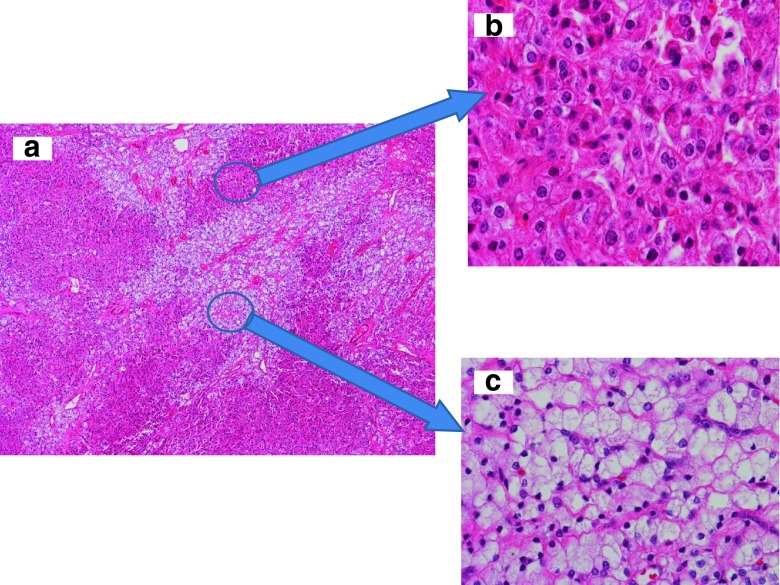
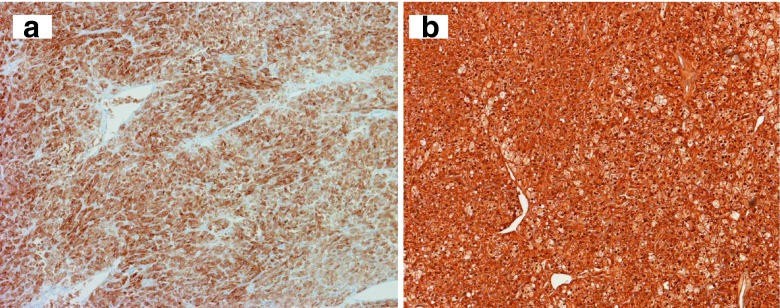
Microscopic examination of the tumor showed characteristic features of a steroid cell tumor not otherwise specified (NOS). There were two types of tumor cells arranged predominately in a diffuse pattern with delicate fibrovascular septa, along with some areas of small nests and cords (Fig. 2a). The tumor cells were polygonal and medium-to-large in size with distinct cell borders and contained abundant eosinophilic granular cytoplasm or vacuolated (lipid-rich) clear cytoplasm. The nuclei were small and uniform, and had inconspicuous nucleoli. The cells with abundant eosinophilic granular cytoplasm resembled the Leydig cells of the ovary (Fig. 2b). The clear cells had more cytoplasm as well as small and slightly irregular nuclei (Fig. 2c) that resemble adrenal cortical cells. Signs of mitosis were extremely rare. No hemorrhage or necrosis was present. Immunohistochemical staining resulted in diffuse positive staining for Inhibin-alpha and calretinin (Fig. 3a and b). There was focal positivity for pancytokeratin and Low molecular weight cytokeratin (LMW-CK). The tumor was negative for high molecular weight cytokeratin (HMW-CK), Epithelial Membrane Antigen (EMA), Carcinoembryonic Antigen (CEA), alpha-fetoprotein, S-100, chromogranin, synaptophysin, Thyroid Transcription Factor-1 (TTF-1), and CD-10. No Reinke crystals were identified, which is a feature of Leydig cell tumor. The histological features and immunohistochemical stain results were consistent with a steroid cell tumor NOS [2,5].
Fig. 2.
Histology of the steroid cell tumor not otherwise specified (NOS). a Diffuse growth pattern with two cell types, eosinophilic cells and clear cells (40× magnification). b The eosinophilic cells with abundant eosinophilic granular cytoplasm and small to intermediate nuclei with small nucleoli and distinct cell borders (200× magnification). c The clear cells vacuolated clear cytoplasm and small nuclei (200× magnification)
Fig. 3.
Tumor cells (a) stain positive for alpha-inhibin (100× magnification) and (b) stain positive for calretinin (100× magnification)
At the post operative visit 13 days after surgery, the patient’s plasma testosterone was 32 ng/dL. At four months following surgery regular menstrual cycles resumed. However, her virilization symptoms persisted with extensive hirsuitism, mild clitoromegaly, and lower voice. Her acne had cleared and she stated that the terminal hair growth was softer and grew more slowly than previously. Nine months following surgery, the patient had intercourse in which the condom broke. She took levonorgestrel 1.5 mg orally within 24 h of the incident; however, the over-the-counter emergency contraception was unsuccessful. She conceived and had an uncomplicated pregnancy and delivered a female infant weighing 3,579 g at 40 weeks gestation.
Comment
Steroid cell tumors of the ovary are classified as sex-cord stromal tumors and constitute less than 0.1 % of all ovarian tumors. Scully and Young first used the term “steroid cell tumor” in 1987 [4]. Before 1987, these tumors were described as lipoid cell tumors, lipid cell tumors, adrenal-like tumors, masculinovoblastomas, luteomas, hypernephroid tumors and adrenal rest tumors [4,7,9,11]. Steroid cell tumors are classified into three subtypes: steroid cell tumor not otherwise specified (NOS), stromal luteoma and Leydig cell tumor.
Steroid cell tumor NOS is the most common of all the subtypes, accounting for 60 % of these tumors, and can occur at any age between 3 and 80 years with average age of presentation being 43 years [4]. Most tumors are unilateral; only 6 % of patients have bilateral tumors. About 25 % of the tumors are non-secretory with patients experiencing abdominal distension and pain. The remaining 75 % of steroid cell tumors NOS can secrete a variety of substances including estrogens, androgens, cortisol, and rarely rennin. The majority of these tumors secretes androgenic steroids and can cause hirsutism or virilization in 56–77 % of cases [4]. Up to 23 % secrete estrogens, which are associated with menorrhagia, postmenopausal bleeding, or even endometrial adenocarcinoma [4]. Less than 10 % secrete cortisol, in which case the patient presents with Cushing’s syndrome [3]. A rare renin-secreting steroid cell tumor associated with polycythemia has been reported [10].
The biologic behavior of the ovarian steroid cell tumors NOS is varied; 25–43 % of tumors are found to be malignant. Hayes and Scully determined the most accurate predictor of malignant behavior in these tumors is ≥2 mitotic figures per 10 high-power fields. They also found that the majority of malignant tumors demonstrated grade 2–3 nuclear atypia, necrosis, hemorrhage, and a diameter of ≥7 cm [4]. Fortunately, our patient’s tumor was benign and did not exhibit these characteristics upon histological examination.
Plasma testosterone levels in these patients have been found to range from 130 to 3,546 ng/dL [4]. The upper limit of normal for testosterone in women varies from 60 to 80 ng/dL in most laboratories. The American College of Obstetricians and Gynecologists Practice Bulletin on polycystic ovarian syndrome states that there is no testosterone level that is considered pathognomonic for tumor (ACOG Practice Bulletin No. 108 [1]). Although there is no clear cut-off, an androgen-secreting tumor should be considered when testosterone levels are above 200 ng/dL (ACOG Practice Bulletin No. 108 [1,6]).
Ovarian steroid cell tumors NOS should be managed surgically. For women who do not desire future fertility, a total abdominal hysterectomy with bilateral salpingo-oophorectomy and complete surgical staging is indicated. For women desiring future fertility, a unilateral oophorectomy with appropriate staging should be performed. Surgical management requires follow-up evaluation, which includes tracking the patient’s sex hormone levels, especially if levels were elevated preoperatively. Adjuvant chemotherapy should be considered if the tumor is malignant based on histology and surgical staging [4,8].
Our patient would have benefited from earlier detection of her tumor. Testosterone levels above 150–200 ng/dL should raise suspicion for malignancy. Failure to fully investigate the cause of her testosterone level of 328 ng/dL led to delayed excision and continued irreversible virilization. Our patient underwent surgical treatment and testosterone levels decreased to 32 ng/dL within 13 days of surgical intervention. More significant were the rapid resumption of menses and retention of her fertility. This case is significant because of the rarity of ovarian steroid cell tumors NOS and the age of the patient when the tumor was detected. The patient became pregnant 9 months after surgical intervention. The pregnancy developed normally and the patient delivered 9 months later. A review of the literature revealed no reports of other cases in which a full-term pregnancy followed removal of this rare tumor.
Footnotes
Capsule Testosterone levels above 150–200 ng/dL should raise suspicion for malignancy. Following laparoscopic right oopherectomy for a steroid cell tumor NOS, this patient had quick resumption of menses, conceived nine months following surgery, and delivered without complication.
References
- 1.ACOG Practice Bulletin No. 108: polycystic ovary syndrome. Obstet Gynecol. 2009;114:936–49. [DOI] [PubMed]
- 2.Deavers MT, Malpica A, Ordonez NG, Silva EG. Ovarian steroid cell tumors: an immunohistochemical study including a comparison of calretinin with inhibin. Int J Gynecol Pathol. 2003;22:162–167. doi: 10.1097/00004347-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Elhadd TA, Connolly V, Cruickshank D, Kelly WF. An ovarian lipid cell tumour causing virilization and Cushing’s syndrome. Clin Endocrinol. 1996;44:723–725. doi: 10.1046/j.1365-2265.1996.693515.x. [DOI] [PubMed] [Google Scholar]
- 4.Hayes MC, Scully RE. Ovarian steroid cell tumors (not otherwise specified). A clinicopathological analysis of 63 cases. Am J Surg Pathol. 1987;11:835–845. doi: 10.1097/00000478-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Kommoss F, Oliva E, Bhan AK, Young RH, Scully RE. Inhibin expression in ovarian tumors and tumor-like lesions: an immunohistochemical study. Mod Pathol. 1998;11:656–666. [PubMed] [Google Scholar]
- 6.Meldrum DR, Abraham GE. Peripheral and ovarian venous concentrations of various steroid hormones in virilizing ovarian tumors. Obstet Gynecol. 1979;53:36–43. [PubMed] [Google Scholar]
- 7.Powell JL, Dulaney DP, Shiro BC. Androgen-secreting steroid cell tumor of the ovary. South Med J. 2000;93:1201–1204. [PubMed] [Google Scholar]
- 8.Reedy MB, Richards WE, Ueland F, Uy K, Lee EY, Bryant C, et al. Ovarian steroid cell tumors, not otherwise specified: a case report and literature review. Gynecol Oncol. 1999;75:293–297. doi: 10.1006/gyno.1999.5549. [DOI] [PubMed] [Google Scholar]
- 9.Scully RE, Young RH, Clement PB. Steroid cell tumors. In: Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament: Afip atlas of tumor pathology No. 23, 1st edn. American RRRegistry of Pathology; 1996. pp 227–38.
- 10.Stephen MR, Lindop GB. A renin secreting ovarian steroid cell tumour associated with secondary polycythaemia. J Clin Pathol. 1998;51:75–77. doi: 10.1136/jcp.51.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young R, Scully R. Steroid cell tumors of the ovary. In: Fox H (ed) Haines and Taylor obstetrical and gynecological pathology, 3rd edn. Churchill Livingstone; 2003. pp. 845–56.