Abstract
Objective
To evaluate the effect of coenzyme Q10 treatments in male infertility, specifically in these parameters: live birth and pregnancy rates, CoQ10 seminal concentration, sperm concentration, and sperm motility.
Materials and methods
Systematic review and meta-analysis in male infertility patients with CoQ10 oral treatments. Three trials were included: 149 males in CoQ10 group and 147 males in placebo group.
Results
None of the included trials provided any data regarding live births. The results of this meta-analysis show that supplementing infertile men with CoQ10 does not increase pregnancy rates. The analysis showed, among patients receiving CoQ10 treatment, a statistically significant increase in: CoQ10 seminal concentration (RR 49.55, 95 % CI 46.44 to 52.66, I2 = 17 %), sperm concentration (RR 5.33, 95 % CI 4.18 to 6.47, I2 = 58 %), and sperm motility (RR 4.50, 95 % CI 3.92 to 5.08, I2 = 0 %)
Conclusion
There is no evidence in the literature that CoQ10 increases either live birth or pregnancy rates, but there is a global improvement in sperm parameters. Adequately powered, robust trials of individual and combination antioxidant therapies are required to guide clinical practice.
Keywords: Coenzyme Q10, Male infertility, Randomized controlled trial, Live birth
Introduction
Male infertility is not always easy to assess because of multifocal origin of the seminal fluid. Taking into account the latest data released by the Society for Assisted Reproductive Technology (SART, www.sart.org), corresponding to IVF patients in 2011, the incidence of male factor was: male factor (17 %), mixed male and female factor (18 %), other factors (8 %) and unknown factors (12 %). The aetiology of diminished semen quality is poorly understood, although both environmental and occupational factors, as well as lifestyle, have been implicated [5,13].
The damaging effects of oxidative stress on sperm are considered to be responsible for 30 % to 80 % of male subfertility cases [38]. It is well known that spermatozoa are susceptible to oxidative damage and reactive oxygen species (ROS), and numerous studies support the hypothesis that ROS are responsible for sperm dysfunction and male infertility [3,34].
The lipid composition of the plasma membrane of spermatozoa is highly specific. These membranes contain high levels of polyunsaturated fatty acids, which are responsible for the marked flexibility of these cells. However, these membrane lipids are also highly susceptible to damage induced by excessive ROS levels because they are the primary substrate for lipid peroxidation [13,26,34,37]. The male gamete undergoes continuous morphological and biochemical modifications during sperm development in the testis, maturation in the epididymis, and capacitation in the female reproductive tract. These changes affect lipids, carbohydrates and proteins of the plasma membrane [40].
Lipid peroxidation in biological membranes causes an impairment of membrane function, including decreased membrane fluidity and sperm motility, which results in a diminished fertilization potential. Enhanced pathological ROS generation leads to DNA degradation, which has been associated with a decreased mitochondrial membrane potential [41]. This situation can occur for many reasons: inflammation, infection, leucocytosis, presence of immature cells, smoking, etc.
The production of ROS is a physiological process in normal cell metabolism, including mitochondrial respiration. To some extent, low levels of ROS provide certain positive effects such as gene regulation, intracellular signalling, and, specific to sperm cells, enhancement of the ability to bind to the zona pellucida. Superoxide anion radicals are necessary to boost the capacitation and the acrosome reaction within the spermatozoon. However, when the excessive generation of ROS exhausts the antioxidant capacity of the spermatozoa, oxidative stress occurs [1,13,35].
Coenzyme Q10 (CoQ10) is an antioxidant molecule, component of the respiratory chain. Recently there has been growing interest in identifying reversible causes of male infertility, and numerous studies have been performed to investigate whether supplementing infertile men with antioxidants can improve seminal parameters [38].
Among the various antioxidants tested, CoQ10 (as a component of the mitochondrial respiratory chain) appears to play an important role in energy metabolism, as well as functioning as a liposoluble chain-breaking antioxidant for cell membranes and lipoproteins [38].
However, the number of patients included in these published studies is low, and the inclusion criteria across the studies are relatively heterogeneous. Additionally, clinical outcomes such as live birth and clinical pregnancy rates were generally not considered to be the main outcomes and were excluded from the analysis.
In the present meta-analysis, we sought to investigate whether supplementing infertile men with CoQ10 increases the live birth and clinical pregnancy rates and improves seminal parameters.
Materials and methods
This study was exempt from Institutional Review Board approval because this was a systematic review and meta-analysis. We followed the preferred reporting items from the systematic reviews and meta-analysis (PRISMA statement) to report the results of this systematic review [32].
Search strategy
We performed an exhaustive electronic search in the following databases (from their inception until December 2012): MEDLINE, EMBASE, Science Citation Index and The Cochrane Central Register of Controlled Trials (CENTRAL). The search combined terms and descriptors related to male infertility, antioxidants, CoQ10 and reproductive outcomes. The search strategy was modified to comply with the requirements of each database consulted. We added validated filters to this strategy to retrieve clinical trials [22]. We also searched for ongoing trials in the primary clinical trials registries, including www.controlled-trials.com, www.clinicaltrials.gov and the WHO International Clinical Trials Registry Platform (www.who.int/trialsearch). No language limits were used. We screened the reference lists of all of the relevant articles and overviews. The authors can provide the complete search strategy upon request.
Eligibility criteria
The review included randomised controlled clinical trials (RCTs) that evaluated the effects of antioxidant therapy with CoQ10 on idiopathic male infertility. The type of intervention evaluated was the effect of administering CoQ10 compared to a placebo.
Outcome measures
The primary outcomes of interest for this review were the live birth and clinical pregnancy rates, as well as the levels of CoQ10 in the seminal plasma. The secondary outcomes were sperm motility and sperm morphology after the intervention. All of the outcomes of interest were considered per randomised male (Table 1).
Table 1.
Study eligibility criteria
| Target population | Men with a minimum of 1 year infertility and regular unprotected sexual activity |
|---|---|
| Intervention | • CoQ10 versus placebo |
| Outcome measure | • Primary |
| ○ Live birth | |
| ○ Pregnancy rate | |
| ○ Level of CoQ10 in seminal plasma | |
| • Secondary | |
| ○ Sperm concentration | |
| ○ Total sperm motility | |
| Design | • Randomised controlled trial |
The outcomes were defined according to the terminology provided by the ICMART (International Committee Monitoring Assisted Reproductive Technologies) glossary [44] and the updated and revised nomenclature for the description of early pregnancy events [14].
Data mining
The data were collected using standard forms in which the study design, participants, interventions, comparisons and the main results were recorded. Two independent authors (R.L. and M.G.C.) judged study eligibility, assessed the risk of bias and mined the data, solving discrepancies by agreement. If necessary, a consensus was achieved with a third author (M.A.C). The agreement between reviewers was analysed using the weighted kappa for each inclusion criterion [15].
Assessment of the risk of bias
We assessed the risk of bias in the included studies following the guidance suggested by the Cochrane Collaboration. We addressed six specific domains (explicit eligibility criteria, sequence generation, allocation concealment, patient blinding, outcome assessor blinding and patients lost to follow-up) and assessed the adequacy of the study report relative to each domain. A judgment of “yes” for all domains indicates a low risk of bias, and a judgement of “no” for one or more domains indicates a high risk of bias. We interpreted the risk of bias in the specific domains as unclear when the information was unavailable. Patients lost to follow-up were described as a percentage.
Analysis
For each study, the treatment effect was measured with risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, both of which were presented with their corresponding 95 % confidence intervals (CI). We extracted event data following the intention-to-treat principle. When possible, we pooled outcome data from each study using a Mantel-Haenszel model and applying the fixed effects model.
We quantified the statistical heterogeneity using the I2 statistic, which describes the percentage of the total variation across studies due to heterogeneity rather than sampling error [21]. We conducted all of the analyses using Review Manager Software (RevMan, version 5.1, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011).
Results
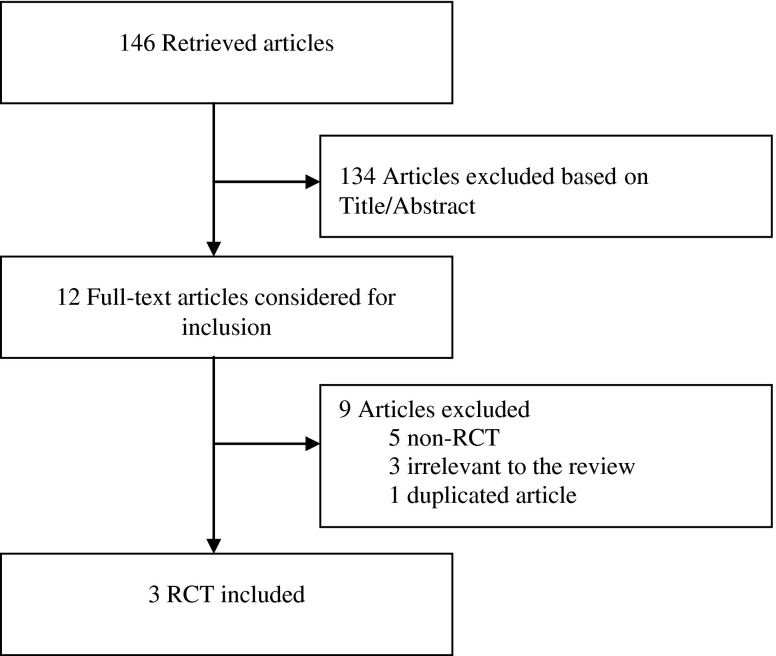
Out of a total of 146 documents identified in the initial electronic search, 12 studies were considered potentially eligible to be included in the meta-analysis by either one or both reviewers, whereas 134 were excluded based on their title and abstract because the results were irrelevant for the purpose of this meta-analysis. During the second phase of the inclusion process, 5 of the 12 studies were excluded because of non-randomised comparisons; three were excluded because of their irrelevance to the review; and one was a duplicate article. This process left three randomised controlled trials that met the inclusion criteria, and these were included in our meta-analysis: Balercia et al. [7], Nadjarzadeh et al. [33] and Safarinejad [36]. A flow chart of the inclusion and exclusion criteria for the search is shown in Fig. 1. The two reviewers achieved positive agreement on the selection of the trials [weighted kappa 0.87 (p < 0.0001), standard error 0.073].
Fig. 1.
Flowchart of the identification of eligible trials involving coenzyme Q10
Description of the studies
A total of 332 infertile men were randomised to an intervention group (n = 166) and a control group (n = 166). A total of 17 patients in the CoQ10 group and 19 patients in the placebo group were excluded for various reasons, which left 149 patients in the CoQ10 group and 147 in the placebo group.
The inclusion criteria were relatively homogeneous among the three studies. Balercia et al. considered infertile men with idiopathic asthenozoospermia, whereas Safarinejad and Nadjarzadef et al. studied infertile men with idiopathic oligoasthenoteratozoospermia. All the included studies described seminal parameters as stated in the WHO criteria [42]. Exclusion criteria included men with leucocytosis of the semen or the presence of a systemic disease as well as men who were reported to be receiving medication for any indication, or have a history of drug, alcohol or substance abuse. Only Safarinejad considered a body mass index of 30 kg/m2 or greater as an exclusion criterion.
The mean age reported across the studies was comparable (Table 2), and each study reported no significant difference regarding this parameter among both the intervention and the control groups.
Table 2.
Description of the included trials
| [7] | [36] | [33] | |
|---|---|---|---|
| Methods | Placebo-controlled, double-blind randomised trial | Placebo-controlled, double-blind randomised trial | Placebo-controlled, double-blind randomised trial |
| Participants | N = 60 | N = 212 | N = 60 |
| Diagnosis: Infertile men with idiopathic asthenozoospermia | Diagnosis: Infertile men with idiopathic oligoasthenoteratozoospermia | Diagnosis: Infertile men with idiopathic oligoasthenoteratozoospermia | |
| Mean age: 32 (range 27–39) | Mean age: 28 (range 21–42) | Mean age: 34 (range 25–46) | |
| Inclusion criteria: | Inclusion criteria: | Inclusion criteria: | |
| (1) age 20–40 years, infertility >2 years, regular sexual intercourse with a potentially fertile female, | (1) minimum 2 years unprotected intercourse and 2 years of reluctant childlessness, | (1) minimum of 1 year of regular unprotected sexual activity without achieving pregnancy, | |
| (2) normal rheological characteristics, (appearance, consistency and liquefaction) of semen and volume and pH in normal range, | (2) male infertility diagnosed if 1 or more standard seminal parameters were below cutoff levels accepted by World Health Organization (WHO), | (2) sperm count less than 20 × 106/ml and progressive motility (grade a + b) <50 % and normal morphology <30 % (18), | |
| (3) sperm count >20 × 106/ml sperm motility <50 % (WHO [42]), normal morphology >30 %, | (3) fertile female partner, | (3) seminal white blood cells (WBC) less than one million per millilitre, | |
| (4) seminal white blood cells <1 × 106/ml, negative sperm culture and chlamydia and M. urealyticum detection, | (4) no known medical condition that could account for infertility, testicular volume 12 ml or greater, | ||
| (5) normal levels of gonadotropins, T, E2 and PRL, | (5) no medical therapy for at least 12 weeks before the study begins, | (4) absence of anatomical abnormalities of the genital tract, | |
| (6) absence of genital disease and anatomical abnormalities of the genital tract including varicocele and antibodies, | (6) only patients seeking medical attention for infertility were included. | (5) absence of infectious genital diseases or systemic diseases, | |
| (7) absence of systemic disease or treatment with other drugs within 3 months of being enrolled in the study. | Exclusion criteria: | (6) absence of treatment with other drugs and dietary supplement during the 3 months before enrolling in the study, | |
| (8) absence of smoking, alcohol and drug addiction as well as of exposure to occupational chemicals. | (1) azoospermia or severe oligospermia (sperm count less than 5 million/ ml), | (7) absence of either smoking, drug and alcohol use or occupational chemical exposure. | |
| Exclusion criteria: | (2) history of epypidymo-orchitis, prostatitis, genital trauma, testicular torsion, inguinal or genital surgery, | ||
| (1) transient decrease in semen quality during run in and | (3) any genital or central nervous system disease, endocrinopathy, or use of cytotoxic drugs, immunosuppressants, anticonvulsives, androgens, antiandrogens, | ||
| (2) sudden improvement in seminal parameters during run in. | (4) a recent history of a sexually transmitted disease, | ||
| (5) psychological or physiological abnormalities that would impair sexual functioning or ability to produce sperm samples | |||
| (6) drug, alcohol or substance abuse, | |||
| (7) liver disease, renal insufficiency or chromosome abnormalities, | |||
| (8) occupational and environmental exposures to reproductive toxins, | |||
| (9) a body mass index (BMI) of 30 kg/m2 or over, | |||
| (10) participation in another investigational study and a likelihood of being unavailable for follow up. | |||
| Study design | Randomisation: unclear (“randomisation list was opened at the end of the study”) | Randomisation: yes, using permuted blocks | Randomisation: yes, though does not describe the sequence generation. |
| Allocation concealment: unclear, not described | Allocation concealment: People geographically and operationally independent of the study | Allocation concealment: unclear, not described | |
| Blinding: Double, soft tablets with the same composition apart from + Q10 in the intervention group, tablet’s appearance not specified | Blinding: Double, tablets identical in appearance | Blinding: Double, placebo were “identical tablets containing lactose” | |
| Follow up: 5 patients dropped out the study (2 from intervention group/3 from placebo group). | Follow up: 18 patients dropped the study: 8 from intervention group/10 from placebo group (withdrawal of consent 2/3, missing data 3/4, lost to follow up 3/3). None due to adverse events. | Follow up: 13 patients (21 %) dropped out the study for “personal reasons” (7 from intervention group/6 from placebo group). | |
| Global quality: moderate | Global quality: high | Global quality: moderate | |
| Intervention | Intervention: CoQ10 100 mg 2x/day (n = 30) | Intervention: CoQ10 300 mg once daily (n = 106) | Intervention: CoQ10 200 mg once daily (n = 30) |
| Control: Placebo 2x/day (n = 30) | Control: Placebo once daily (n = 106) | Control: Placebo once daily (n = 30) | |
| Duration of treatment: 6 months | Duration of treatment: 26 weeks | Duration of treatment: 12 weeks | |
| Duration of study: 9 months | Duration of study: 20 months (February 2005 – October 2006) | Duration of study: 19 months (June 2008 – December 2009) | |
| Outcomes | Primary: Seminal parameters | Primary outcomes: seminal parameters and testicular volume | Primary outcomes: seminal parameters, lipid peroxidation |
| Secondary: Pregnancy rate (was not in the original list of outcomes, but was reported in results – “although pregnancy was not an end point for this controlled study, because of many possible interferences, it is interesting to note that six pregnancies occurred with patients given CoQ10”) | Secondary outcomes: adverse effects and hormone levels | Secondary outcomes: antioxidant capacity of seminal plasma, adverse effects | |
| Pregnancy outcome not stated as an outcome initially but it was reported on the discussion | Pregnancy outcome not stated as an outcome initially but it was reported on the discussion |
Intervention
Treatment protocols were relatively heterogeneous among the studies with regard to the dose and duration of the treatment with CoQ10. Balercia et al. used 200 mg CoQ10 per day for 24 weeks (28 cases of 149 patients); Safarinejad used 300 mg per day for 26 weeks (98 cases of 149 patients); and Nadjarzadeh et al. used 200 mg per day for 12 weeks (23 cases of 149 patients)
Internal validity of the trials
The trials provided complete data regarding the methodology of the RCT included in this meta-analysis and did not show major biases in their design or execution. However, whereas Safarinejad provided a detailed description of the study design, the randomisation and allocation concealment in the other two trials (Nadjarzadeh et al. and Balercia et al.) remain unclear. The methodological data regarding the included trials are shown in Table 2.
All of the included trials applied a blinded patient intervention with an identical placebo in the control group, although Balercia et al. did not specify the appearance of the placebo tablet. Additionally, all of the trials reported the patients lost to follow-up and the patients who dropped out of the study (n = 36), resulting in 149 patients in the intervention group and 147 patients in the control group.
Outcomes of interest
Live birth
Although live birth was intended to be a primary outcome in the present meta-analysis, none of the included trials provided any data regarding this outcome.
Pregnancy rate
All of the trials provided information regarding the pregnancy rate. Balercia et al. reported nine pregnancies, six within the CoQ10 group (6/28) and three within the placebo group (3/27). The other two trials did not report any spontaneous pregnancy in either the intervention or the placebo groups.
Coenzyme Q10 seminal concentration
Two of the trials (Balercia et al. and Safarinejad, n = 249), reported the seminal concentration of CoQ10. The pooled analysis showed a higher CoQ10 seminal concentration in the treated group (n = 126) than the control group (n = 123). The analysis showed a statistically significant increase in the CoQ10 seminal concentration in men who received CoQ10 treatment (RR 49.55, 95 % CI 46.44 to 52.66, I2 = 17 %) (Fig. 2).
Fig. 2.
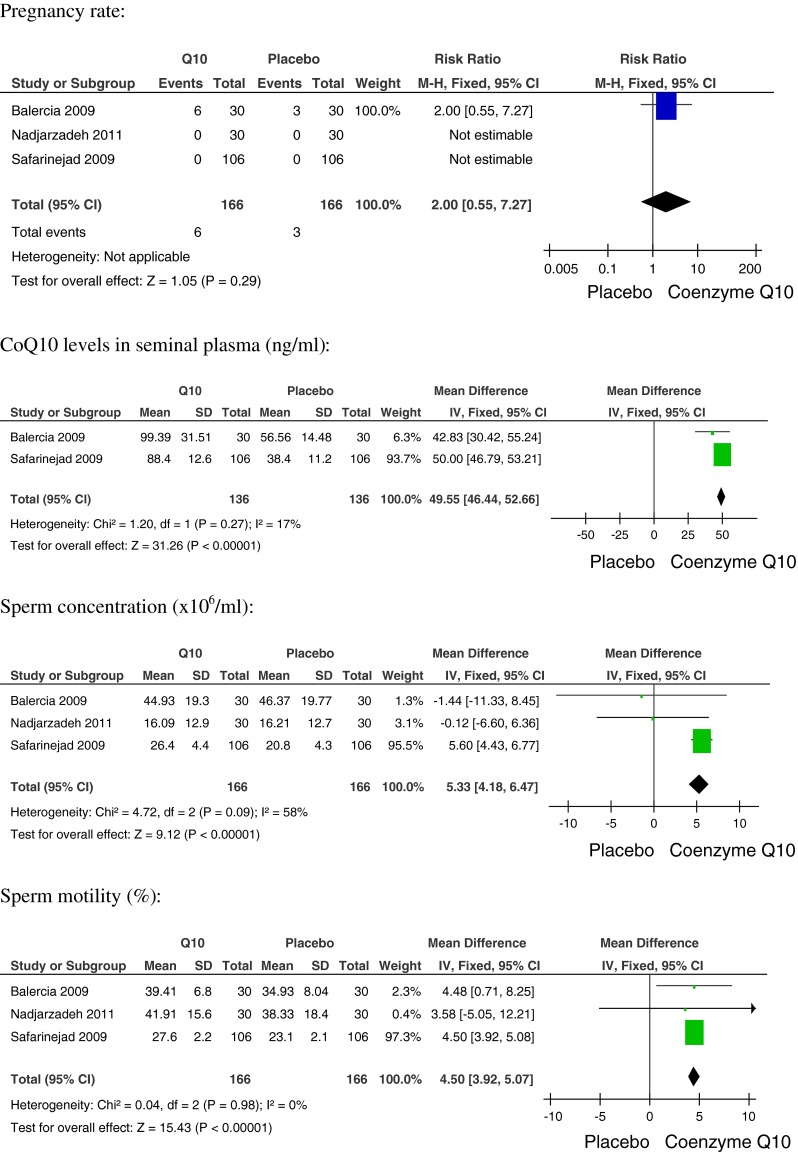
Effectiveness of CoQ10 treatments versus placebo
Sperm concentration
With regard to the sperm concentration, the analysis of the three trials showed a higher sperm concentration in the CoQ10 group (98/149) compared to the placebo group. Only one trial observed an increase in sperm concentration. The analysis showed a statistically significant higher sperm concentration among patients receiving CoQ10 treatment (RR 5.33, 95 % CI 4.18 to 6.47), though the results were moderately inconsistent (I2 = 58 %, Fig. 2).
Total sperm motility
Only two trials achieved a higher total sperm motility percentage in the CoQ10 treated group (126/149) compared to the control group. The analysis showed a statistically significant increase in sperm motility in men receiving CoQ10 treatment (RR 4.50, 95 % CI 3.92 to 5.08, I2 = 0 %). (Fig. 2)
Discussion
This systematic review gathered previously published evidence and data obtained from the original authors to provide pooled estimates regarding the use of CoQ10 among infertile men. The results of this meta-analysis show that supplementing infertile men with CoQ10 does not increase pregnancy rates; however, there are no available data with regard to the live birth rate, which was one of the main outcomes sought after in the present study. Interestingly, a significant improvement was observed regarding seminal parameters such as concentration and motility as well as concentration of CoQ10 in the seminal plasma. However, only one trial observed an increase in sperm concentration, confirming the relationship of CoQ10 especially with the improvement in sperm motility, to be involved in mitochondrial metabolism and cell antioxidant capacity.
The fundamental role of CoQ10 in mitochondrial function and its well-known antioxidant properties constitute the basis for its clinical application, although some of its effects may be related to a mechanism of gene induction involved in cell signalling, metabolism, and transport [20]. Presumably, some of the effects exerted by exogenous CoQ10 might be due to these functions.
Several authors have observed that spermatozoa from oligospermic patients remain within the epididymis for a longer time; hence, their exposure to ROS is increased [18,23,43]. Therefore, it is reasonable to assume that exogenous CoQ10 could improve seminal parameters.
A systematic review by Cochrane [38] was published to evaluate the effect of oral supplementation with different antioxidants for male partners of couples undergoing assisted reproduction techniques. Compared with the present study, Showell et al. [38], which included only two trials, obtained comparable results and concluded that although the seminal parameters appeared to improve after supplementation with CoQ10, there was no change in the pregnancy rate.
In vivo trials in humans have shown that administration of antioxidants improves sperm quality in heavy smokers [12] and in patients with male factor infertility [27,31] as well as increases the fertilisation potential of healthy men with a high concentration of seminal ROS [24] and of fertile normozoospermic men with low fertilisation rates (based on previous IVF cycles) [19].
Several studies have been conducted to evaluate the effects of supplementation with antioxidants on seminal parameters among infertile couples [30]. Although the review by Ross et al. [35] reported a higher pregnancy rate among men who were treated with a combination of various antioxidants, the study by Littarru and Tiano [30] observed an improvement in seminal parameters only in men who were supplemented with CoQ10.
There is little evidence to support the impact of supplemental CoQ10 among infertile men on live birth and pregnancy rates. Firstly, most of these studies were not designed to evaluate either live birth or pregnancy rates because they were not included as primary outcomes. Additionally, these studies present some limitations, such as the small number of included patients and the heterogeneity of the published results. The trials conducted on animal models indicate that antioxidant therapies could be successful in humans. Controversial data often result from the many uncontrolled studies carried out to support such a treatment, and the efficacy of this treatment has not yet been proven [2]. It is important to consider the lifestyle habits of patients in these studies, as some included only non-smokers [13] and others included both smokers and non-smokers [10]. Smokers require two to three doses of antioxidants to maintain levels comparable to the levels found in the blood plasma of non-smokers [17].
There have been several trials that aimed to investigate the mechanisms by which antioxidants, specifically CoQ10, exert their effects. It is well-known that CoQ10 is a highly lipophilic molecule, which led to Balercia et al. [8] hypothesising that CoQ10 diffuses through the phospholipid bilayer of cellular membranes in their transport from the peripheral blood to testicular and accessory male genital glands. However, whether this transport takes place either passively or through an active mechanism remains unknown. The distribution of CoQ10 between intracellular and extracellular compartments appears to be an active process. In this study, Balercia et al. [8] observed a positive correlation between the CoQ10 levels in the seminal plasma and sperm motility. Hence, it could be hypothesised that, in certain circumstances, an increased oxidative stress in sperm cells could somehow overwhelm the CoQ10 levels, which could lead to the detrimental effect of its bioenergetic role on spermatogenesis. However, a deeper insight into the molecular mechanisms of CoQ10 might lead to a greater knowledge of unexplained infertility [8].
Conversely, Littarru and Tiano [29] observed that supplementation with CoQ10 resulted in increased levels of ubiquinol-10 within the circulating lipoproteins and increased resistance of human low-density lipoproteins to the initiation of lipid peroxidation, which could reduce oxidative stress.
Alleva et al. [4] measured the content of both the reduced and oxidised forms of CoQ10 (ubiquinol/ubiquinone) as well as the organic peroxide concentrations in seminal plasma and seminal fluid in 32 infertile patients. A significant positive correlation was observed between ubiquinol content, motility and sperm count; an inverse correlation between ubiquinol content and organic peroxide concentrations was also observed. These findings suggest that ubiquinol-10 inhibits organic peroxide formation in both the seminal fluid and seminal plasma, which reduces the oxidative stress to which sperm cells may be subjected.
Antioxidant therapies have been tested in human model systems. Dawson et al. [12] and Lanzafame et al. [25] observed an improvement in the seminal parameters of heavy smokers. Increased fertilisation potential was described among normozoospermic men in couples who had low fertilisation rates and were undergoing IVF cycles [19]. In this sense, it seems logical to administer these antioxidant treatments to patients with such indications and not to use these treatments indiscriminately.
Eskenazi et al. [13] demonstrated that higher antioxidant intake over the normal dietary and supplement daily amounts was associated with higher sperm counts and increased motility. The study also reported that antioxidant intake, to some extent, may attenuate the impact of age on sperm motility. Some studies that implemented an oral antioxidant therapy with different antioxidants were associated with a significant improvement in both spontaneous and assisted conception pregnancy rates [9,11,28,39].
Safarinejad [36] also detected a significant decrease in the presence of serum follicle stimulating hormone and luteinising hormone after treatment with CoQ10. Low levels of serum follicle stimulating hormone are associated with better spermatogenesis. Additionally, the levels of inhibin B, which reflects the functionality of Sertoli cells, were increased in the group treated with CoQ10.
The overall study quality is moderate, which requires a moderate level of confidence in the results. Further studies with more cases could change the direction of this meta-analysis. The current study has several potential limitations. First, the number of events included in the meta-analysis is relatively small, and neither live births nor pregnancy rates are the primary outcome of the included trials.
It is necessary to highlight the heterogeneity of the included trials regarding the inclusion criteria. Balercia et al. considered only asthenozoospermia, whereas the other two trials considered oligoasthenoteratozoospermia as defined by the WHO criteria from 2009. In the future, it would be interesting to unify the inclusion criteria to include patients with abnormalities in concentration, motility and morphology, which is based on the WHO criteria from 2010.
The marked heterogeneity with regard to the mean age of patients in each study is also relevant because Safarinejad included patients with a significantly lower mean age compared to the other two studies. The relevance is because advanced age is correlated with higher cellular oxidation, which in turn affects spermatogenesis.
However, we believe that there is considerable homogeneity regarding the administration and dosage of CoQ10 across the three trials (between 200 and 300 mg/day). The results from other studies have shown a significant improvement in fertilisation rates after supplementing with only 60 mg/day of CoQ10 for a mean of 103 days [6]. Thus, differences in the CoQ10 doses provided in the three studies considered in this meta-analysis would not be a limiting factor.
Antioxidants may play a critical role in protecting male germ cells against oxidative damage [16]. The production of ROS has been associated with loss of motility and decreased capacity for sperm-oocyte fusion [1]. The balance between the production of ROS and antioxidant activity is an important process that needs further study to better understand the effect of oral antioxidants on male infertility [35].
The wide variation in the duration of CoQ10 treatment and the heterogeneity in the characteristics of the study populations included could explain the discrepancies observed in this meta-analysis. Furthermore, there is no evidence in the literature that CoQ10 increases either live birth or pregnancy rates, but there is a global improvement in sperm parameters. A limitation of this study is that the statistical analysis of the results from limited number of patients and limited number of studies, may lead a misinterpretation and redundant expectations of the patients. So the definitive effect of replacement on semen parameters should be revealed with further randomized controlled trial on larger patient populations for standardization of doses and duration of supplementation.
Acknowledgments
Conflict of interest
The authors declare no conflict of interest.
Financial support
None
Footnotes
Capsule Oral treatment with Coenzyme Q10 improves sperm concentration and sperm motility, but there is no evidence that increases either live birth or pregnancy rates.
References
- 1.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–43. doi: 10.1016/S0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Said TM. Carnitines and male infertility. Reprod Biomed Online. 2004;8(4):376–84. doi: 10.1016/S1472-6483(10)60920-0. [DOI] [PubMed] [Google Scholar]
- 3.Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation and human sperm function. Biol Reprod. 1989;40:183–97. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- 4.Alleva R, Scararmucci A, Mantero F, Bompadre S, Leoni L, Littarru GP. The protective role of ubiquinol-10 against formation of lipid hydroperoxides in human seminal fluid. Mol Aspects Med. 1997;18(Suppl):S221–8. doi: 10.1016/S0098-2997(97)00040-X. [DOI] [PubMed] [Google Scholar]
- 5.Auger J, Eustache F, Andersen AG, Irvine DS, Jorgensen N, Skakkebaek NE, et al. Sperm morphological defects related to environment, lifestyle and medical history of 1001 male partners of pregnant women from four European cities. Hum Reprod. 2001;16:2710–7. doi: 10.1093/humrep/16.12.2710. [DOI] [PubMed] [Google Scholar]
- 6.Balercia G, Armeni T, Mantero F, Principato G, Regoli F. Total oxyradical scavenging capacity toward different reactive oxygen species in seminal plasma and sperm cells. Clin Chem Lab Med. 2003;41(1):13–9. doi: 10.1515/CCLM.2003.003. [DOI] [PubMed] [Google Scholar]
- 7.Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F, Amoroso S, Ricciardo-Lamonica G, Boscaro M, Lenzi A, Littarru G. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril. 2009;91(5):1785–92. doi: 10.1016/j.fertnstert.2008.02.119. [DOI] [PubMed] [Google Scholar]
- 8.Balercia G, Mancini A, Paggi F, Tiano L, Pontecorvi A, Boscaro M, et al. Coenzyme Q10 and male infertility. J Endocrinol Invest. 2009;32(7):626–32. doi: 10.1007/BF03346521. [DOI] [PubMed] [Google Scholar]
- 9.Cavallini G, Ferraretti AP, Gianaroli L, Biagiotti G, Vitali G. Cinnoxicam and L-carnitine/acetyl-L-carnitine treatment for idiopathic and varicocele-associated oligoasthenospermia. J Androl. 2004;25(5):761–70. doi: 10.1002/j.1939-4640.2004.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 10.Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, Depuydt CE. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukot Essent Fat Acids. 2000;63(3):159–65. doi: 10.1054/plef.2000.0174. [DOI] [PubMed] [Google Scholar]
- 11.Comhaire FH, El Garem Y, Mahmoud A, Eertmans F, Schoonjans F. Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: a double blind, randomized trial. Asian J Androl. 2005;7(3):257–62. doi: 10.1111/j.1745-7262.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- 12.Dawson EB, Harris WA, Teter MC, Powell LC. Effect of ascorbic acid supplementation on the sperm quality of smokers. Fertil Steril. 1992;58(5):1034–9. [PubMed] [Google Scholar]
- 13.Eskenazi B, Kidd SA, Marks AR, Sloter E, Block G, Wyrobek AJ. Antioxidant intake is associated with semen quality in healthy men. Hum Reprod. 2005;20(4):1006–12. doi: 10.1093/humrep/deh725. [DOI] [PubMed] [Google Scholar]
- 14.Farquharson RG, Jauniaux E, Exalto N, ESHRE Special Interest Group for Early Pregnancy (SIGEP) Updated and revised nomenclature for description of early pregnancy events. Hum Reprod. 2005;20(11):3008–11. doi: 10.1093/humrep/dei167. [DOI] [PubMed] [Google Scholar]
- 15.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2(2):121–45. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 16.Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A. 1991;88(24):11003–6. doi: 10.1073/pnas.88.24.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraga CG, Motchnik PA, Wyrobek AJ, Rempel DM, Ames BN. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat Res. 1996;351(2):199–203. doi: 10.1016/0027-5107(95)00251-0. [DOI] [PubMed] [Google Scholar]
- 18.Ford WC, Whittington K. Antioxidant treatment for male subfertility: a promise that remains unfulfilled. Hum Reprod. 1998;13(6):1416–9. doi: 10.1093/oxfordjournals.humrep.a019707. [DOI] [PubMed] [Google Scholar]
- 19.Geva E, Bartoov B, Zabludovsky N, Lessing JB, Lerner-Geva L, Amit A. The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program. Fertil Steril. 1996;66(3):430–4. doi: 10.1016/s0015-0282(16)58514-8. [DOI] [PubMed] [Google Scholar]
- 20.Groneberg DA, Kindermann B, Althammer M, Klapper M, Vormann J, Littarru GP, Döring F. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human CaCo-2 cells. Int J Biochem Cell Biol. 2005;37(6):1208–18. doi: 10.1016/j.biocel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Versio 5.1.0. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org
- 23.Johnson L, Varner DD. Effect of daily spermatozoan production but not age on transit time of spermatozoa through the human epididymis. Biol Reprod. 1988;39(4):812–7. doi: 10.1095/biolreprod39.4.812. [DOI] [PubMed] [Google Scholar]
- 24.Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID, Barratt CL. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil Steril. 1995;64(4):825–31. doi: 10.1016/s0015-0282(16)57861-3. [DOI] [PubMed] [Google Scholar]
- 25.Lanzafame FM, La Vignera S, Vicari E, Calogero AE. Oxidative stress and medical antioxidant treatment in male infertility. Reprod Biomed Online. 2009;19(5):638–59. doi: 10.1016/j.rbmo.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Lenzi A, Picardo M, Gandini L, Dondero F. Lipids of the sperm plasma membrane: from polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum Reprod Update. 1996;2(3):246–56. doi: 10.1093/humupd/2.3.246. [DOI] [PubMed] [Google Scholar]
- 27.Lenzi A, Lombardo F, Sgrò P, Salacone P, Caponecchia L, Dondero F, Gandini L. Use of carnitine therapy in selected cases of male factor infertility: a double-blind crossover trial. Fertil Steril. 2003;79(2):292–300. doi: 10.1016/S0015-0282(02)04679-4. [DOI] [PubMed] [Google Scholar]
- 28.Lenzi A, Sgrò P, Salacone P, Paoli D, Gilio B, Lombardo F, Santulli M, Agarwal A, Gandini L. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil Steril. 2004;81(6):1578–84. doi: 10.1016/j.fertnstert.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 29.Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37(1):31–7. doi: 10.1007/s12033-007-0052-y. [DOI] [PubMed] [Google Scholar]
- 30.Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: an update review. Nutrition. 2010;26(3):250–4. doi: 10.1016/j.nut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 31.López G, Lafuente R, Checa MA, Monqaut A, Brassesco M. Effect of a treatment with vitamins, L-carnitine and coenzyme Q10 on sperm head vacuolization and DNA fragmentation on in vitro fertilization patients. Rev Int Androl. 2011;09(4):154–9. [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadjarzadeh A, Sadeghi MR, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in Idiopathic Oligoasthenoteratozoospermia: a Randomized Double-blind, placebo Controlled Trial. J Endocrinol Invest 2011; e224-e228. [DOI] [PubMed]
- 34.Rao B, Soufir JC, Martin M, David G. Lipid peroxidation in human spermatozoa as related to midpiece abnormalities and motility. Gamete Res. 1989;24:127–34. doi: 10.1002/mrd.1120240202. [DOI] [PubMed] [Google Scholar]
- 35.Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, Coomarasamy A, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online. 2010;20(6):711–23. doi: 10.1016/j.rbmo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 2009;182:237–48. doi: 10.1016/j.juro.2009.02.121. [DOI] [PubMed] [Google Scholar]
- 37.Sheweita S, Tilmisany A, Al-Sawaf H. Mechanisms of male infertility: role of antioxidants. Curr Drug Metab. 2005;6(5):495–501. doi: 10.2174/138920005774330594. [DOI] [PubMed] [Google Scholar]
- 38.Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev 2011, Issue 1. Art. No.: CD007411. DOI: 10.1002/14651858.CD007411.pub2 [DOI] [PubMed]
- 39.Tremellen K, Miari G, Froiland D, Thompson J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust N Z J Obstet Gynaecol. 2007;47(3):216–21. doi: 10.1111/j.1479-828X.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 40.Tulsiani DR. Carbohydrates mediate sperm-ovum adhesion and triggering of the acrosome reaction. Asian J Androl. 2000;2:87–97. [PubMed] [Google Scholar]
- 41.Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 2007;77(2):190–201. doi: 10.1095/biolreprod.107.060558. [DOI] [PubMed] [Google Scholar]
- 42.WHO laboratory manual for the examination and processing of human semen. Fourth edition. 1999.
- 43.Wolff H, Politch JA, Martinez A, Haimovici F, Hill JA, Anderson DJ. Leukocytospermia is associated with poor semen quality. Fertil Steril. 1990;53(3):528–36. [PubMed] [Google Scholar]
- 44.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. [DOI] [PubMed]