Abstract
Objective
To evaluate the effects of zinc oxide nanoparticles on mouse spermatogenesis.
Methods
Thirty two adult male NMRI mice were used. Experimental Groups (ZNP-1-ZNP-3) received one of the following treatments daily for 35 days: 5, 50 and 300 mg/kg zinc oxide nanoparticles respectively. Control group received only distilled water. Epididymal sperm parameters, testicular histopathology, morphometric analysis and spermatogenesis assessments were performed for evaluation of the zinc oxide nanoparticles effects on testis.
Results
Epididymal sperm parameters including sperm number, motility and percentage of abnormality were significantly changed in 50 and 300 mg/kg zinc oxide nanoparticles treated mice (p < 0.01). Histopathological criteria such as epithelial vacuolization, sloughing of germ and detachment were significantly increased in 50 and 300 mg/kg zinc oxide nanoparticles treated mice (p < 0.001). 300 mg/kg zinc oxide nanoparticles induced formation of multinucleated giant cells in the germinal epithelium. 50 and 300 mg/kg zinc oxide nanoparticles also caused a significant decrease in seminiferous tubule diameter, seminiferous epithelium height and maturation arrest (p < 0.001).
Conclusion
Zinc oxide nanoparticles act as testicular toxicant and further studies are needed to establish its mechanism of action upon spermatogenesis.
Keywords: Zinc oxide nanoparticles, Nanomaterials, Spermatogenesis, Mice
Introduction
Spermatogenesis is a complex process of germ cell proliferation and differentiation which leads to the production and release of spermatozoa from the testis. This elaborate process is dependent on hormonal and dynamic interactions between the Sertoli cells and the germ cells [1, 2]. Tight junctions between adjacent Sertoli cells create two separate compartments within the seminiferous epithelium: a basal compartment below the tight junction and an adluminal compartment above. Sertoli cells secrete hormonal and nutritive factors into the adluminal compartment which creates a specialized microenvironment for development and viability of germ cells. In addition, Sertoli cells provide efficient paracrine signaling mechanisms between these cells as well as physical support to developing germ cells [3]. The intricate regulation and cellular interactions that occur in the testis provide multiple distinct targets by which toxicants can disrupt spermatogenesis [2].
Many recent in vivo and in vitro studies demonstrate that most nanoparticles (NPs) show an adverse or toxic effect on male germ cells [4, 5]. NPs are materials with at least one dimension ≤100 nm, and this large surface-to-volume ratio results in unique characteristics compared to their corresponding bulk materials [6]. Recent studies have shown that administration of NPs to mice results in their accumulation in the various tissues including the brain and the testis. This indicates that they easily pass through the blood–brain and blood–testis barriers [7, 8]. Not all NPs will necessarily demonstrate an adverse effect leading to toxicity. For example, some NPs show a beneficial or nontoxic effect on spermatogenesis [9, 10]. It has been reported that nanoselenium diet supplementation produced positive effects on sperm quality in male goats [9].
Thus, NPs must be investigated on a case-by-case basis to determine whether a NP will have a positive or negative effect on spermatogenesis. Metal NPs and their oxides have a considerable number of present and future applications in the medical and industrial fields [11]. Among the various metal nanomaterials, zinc oxide nanoparticles (ZNP) are used in several products such as sunscreens, biosensors, food additives, pigments, rubber manufacture, and electronic materials [12]. Delouise reported that ZNPs are nontoxic to cultured human dermal fibroblasts [13]. Other reports suggest that these nanoparticles are toxic to neuroblastoma cells [14], vascular endothelial cells [15], liver and kidneys [16, 17]. In present study, toxic effects of ZNP on the mouse spermatogenesis were investigated.
Materials and methods
Animals
In this study, 32 healthy adult male NMRI (Naval Medical Research Institute) mice (6–8 weeks old, 25–30 g) were used. The animals were obtained from Ahvaz Jundishapur University of Medical Sciences, Experimental Animal Research Center, and this study was approved by the ethics committee of Jundishapur University and carried out in an ethically proper way by following the guidelines provided. The animals were kept under standard laboratory conditions (12 h dark and 12 h light cycle, relative humidity of 50 ± 5 % and 22 ± 3 °C) for at least 1 week before the experiment and those conditions were preserved until the end of the experiment. Animal cages were kept clean, and commercial food (pellet) and water were provided ad libitum.
Experimental design
The mice were randomly divided into four groups, all of which contained eight animals. The doses of ZNP (Sigma) were selected according to previous studies that demonstrated significant toxicity in rodents [15]. The stock solution of ZNP (2 mg/ml) was prepared in Milli-Q water and dispersed for 10 min by using a sonicator to prevent aggregation. The stock solution of ZNP was kept at 4 ◦C and used within 1 week for the experiments. Prior to each experiment, the stock solution was sonicated on ice for 10 min, then immediately diluted in Milli-Q water. Experimental groups (ZNP-1, ZNP-2 and ZNP-3) received 5, 50 and 300 mg/kg ZNP for 35 consecutive days, respectively. The duration time of treatment was selected according to the timing of mouse spermatogenesis [13]. Control group received distilled water orally for 35 consecutive days. One day after the last administration, the mice were sacrificed by cervical dislocation under ether anesthesia and testicles from each animal were dissected out and weighed. Right testis from each animal was fixed in Bouin’s solution. The samples were embedded in paraffin, sectioned (5 μm) and stained with haematoxylin and eosin (H&E) for histopathology, Johnsen’s scoring and morphometry. The left testis was homogenized for assessment of testicular sperm head counts.
Testicular sperm counts
Testicular sperm head numbers were assessed to evaluate the numbers of mature elongate spermatids in the testis. Briefly, mouse testes were homogenized in an 8 ml solution of 0.9 % NaCl and 0.05 % Triton X-100, and sperm heads were counted using a hemocytometer [18]. Each sample was counted four times and averaged. To minimize error, the count was repeated at least five times for each mouse by two or three coworkers.
Epididymal sperm parameters
Spermatozoa were taken from right epididymal cauda and placed into a petri dish containing 1 ml saline of the motility and frequency of morphologically abnormal spermatozoa. One drop of suspension was put onto concave object glass and observed under the microscope with magnification of 400×. The observation was carried out onto 100 spermatozoa with five times replication in each mouse. Sperm motility was grouped into four categories, type A, spermatozoa move rapid progressively; B. spermatozoa move slow progressively; C. Spermatozoa move in place, and D, spermatozoa is immotile. Motility is regarded as normal if A > 25 % or A + B > 50 %. A drop of suspension was put onto an object glass, stained with 1 % eosin and 10 % nigrosin, and smeared. Morphological observation was conducted in 100 spermatozoa with 5 replication in each mouse with magnification of 400×. The observation data were differentiated based on normal and abnormal morphology. The morphology was regarded as normal when the acrosome is curved like a hook, and the neck is straight with free-end single tail. Abnormal morphology was found when the head was smaller than normal, the neck was broken, the tail was branched or cut, etc. The left epididymis was macerated and minced in 0.8 ml of 1 % trisodium citrate solution for 7–8 min, then more solution was added (up to total amount 8 ml) and mixed for about 1 min. The sperm suspension was diluted 1:1 in 10 % buffered formalin. Spermatozoa were counted using improved Neubauer haemocytometer. The sperm suspension from right testes was diluted 1:1 in 10 % buffered formalin. Spermatozoa were counted using improved Neubauer haemocytometer [19].
Histopathology
Six microscopy slides per animal were examined for signs of germ cell degeneration including the following histopathological alterations: detachment (appearance of breaking off of cohorts of spermatocytes from the seminiferous epithelium), sloughing (release of clusters of germ cells into the lumen of the seminiferous tubule) and vacuolization (appearance of empty spaces in the seminiferous tubules). For each treatment, the average percentage of normal and regressed tubules was determined.
Average percentages were calculated for each sample by dividing the number of round tubules with a histopathology index (vacuolization, detachment, sloughing) or normal tubules in a randomly microscopic field by the total number of round tubules in the same field and the result multiplied by 100. For each slide the mean of three fields was considered [20, 21].
Assessment of spermatogenesis
Maturity of the germinal epithelium was graded by using the Johnsen’s scoring method [22], a simple way for assessment of spermatogenesis. By using a 40× magnification, 150 tubules per animal were evaluated and each tubule was given a score ranging from 1 to 10. The tubules having complete inactivity were scored as 1 and those with maximum activity (at least five or more spermatozoa in the lumen) scored as 10.
Morphometry
The diameters of the seminiferous tubules and the lumen diameter were measured by fitting a graticule of a calibrated linear scale in the 10× eyepiece of Leitz microscope at objective lens 40×. Only circular and near circular tubules were assessed. The height of the seminiferous epithelium was calculated by subtracting the lumen diameter from the tubule diameter. For each animal 150 tubules were analyzed [23, 24].
Statistical analysis
The data were analyzed using one-way ANOVA followed by post hoc LSD test and were presented as the mean ± SD. p < 0.05 was considered significant.
Results
Organ weight
Weight of testicles in the ZNP-1 and ZNP-2 groups was slightly less than control group (p > 0.05). A significant reduction in testicular weight was observed in the ZNP-3 (p < 0.05) group (Table 1).
Table 1.
The number of testicular sperm heads per gram of testis and testicular weight in control and experimental groups. ZNP-1: 5 mg/kg ZNP, ZNP-2: 50 mg/kg ZNP, ZNP-3: 300 mg/kg ZNP
| Groups | Sperm head/testis (×106) | Weight(Mg) |
|---|---|---|
| Control | 22.3 ± 1.2 | 140.8 ± 8.5 |
| ZNP-1 | 21.7 ± 1 | 138.7 ± 14.2 |
| ZNP-2 | 11 ± 0.7* | 133.3 ± 10.8 |
| ZNP-3 | 4.8 ± 0.3** | 115.5 ± 7.2* |
Values expressed as mean ± SD for 8 mice. *p < 0.05, **p < 0.01
Testicular sperm counts
No significant decrease in the number of sperm heads was evidenced in ZNP-1 group (P > 0.05). ZNP-2 and ZNP-3 groups showed significant decrease in the number of sperm heads (Table 1).
Epididymal sperm parameters
There were no significant changes in the number of epididymal sperms in the ZNP-1 groups compared to the control. Exposure of the mice to the ZNP at the doses of 50 or 300 mg/kg exhibited a significant reduction in sperm number (p < 0.01).
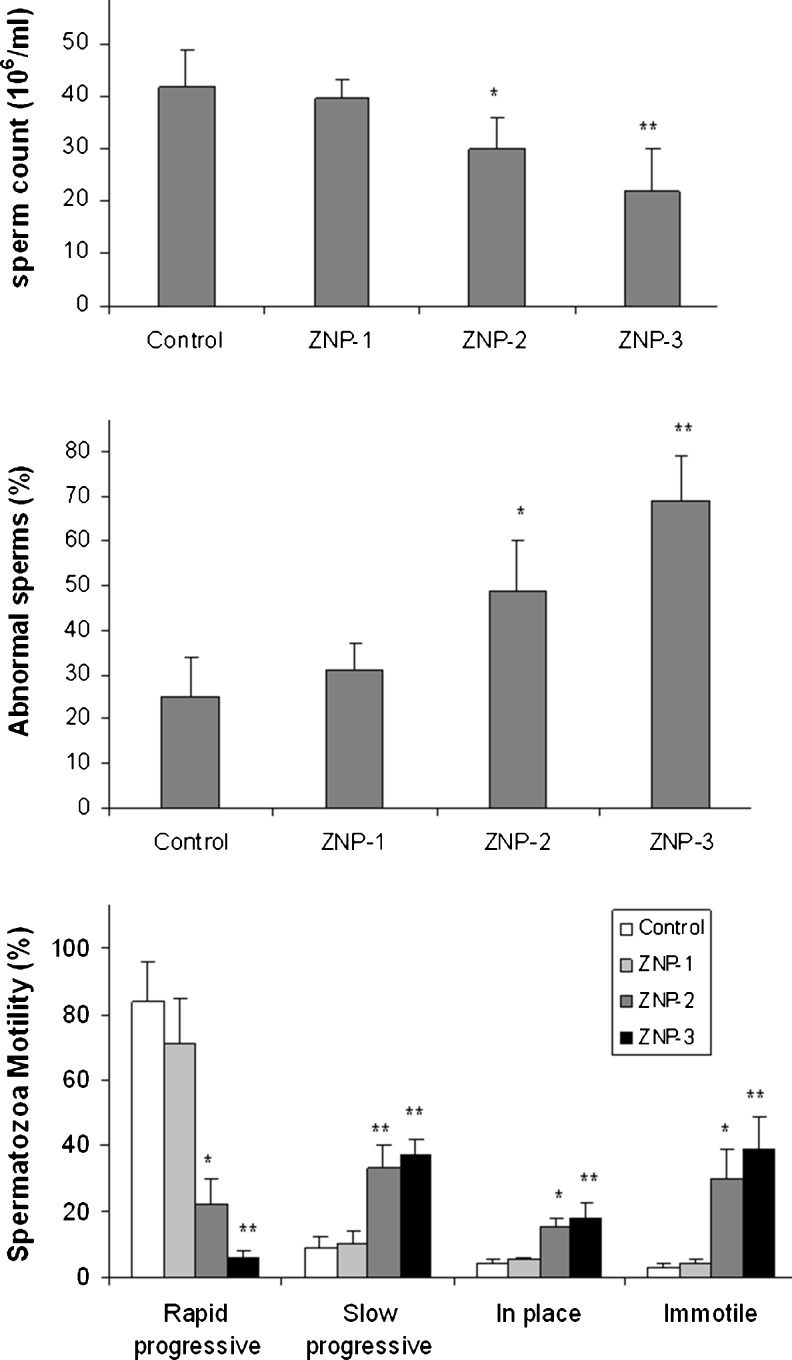
Motility of the sperms from the control was about 90 %. Treatment of the animals with ZNP at the doses of 50 or 300 mg/kg significantly reduced the sperm motility compared to the control group (p < 0.05). There were no significant changes in sperm abnormality in the ZNP-1 groups compared to the control (p > 0.05). Treatment of the animals with 50 and 300 mg/kg ZNP significantly decreased the abnormality of sperms (p < 0.05). The results of epididymal sperm parameters are depicted in Fig. 1.
Fig. 1.
Epididymal sperm parameters in control and experimental groups. Values are expressed as means ± SD for 8 mice. *p < 0.01, **p < 0.001, ZNP-1: 5 mg/kg ZNP, ZNP-2: 50 mg/kg ZNP, ZNP-3: 300 mg/kg ZNP
Histopathology
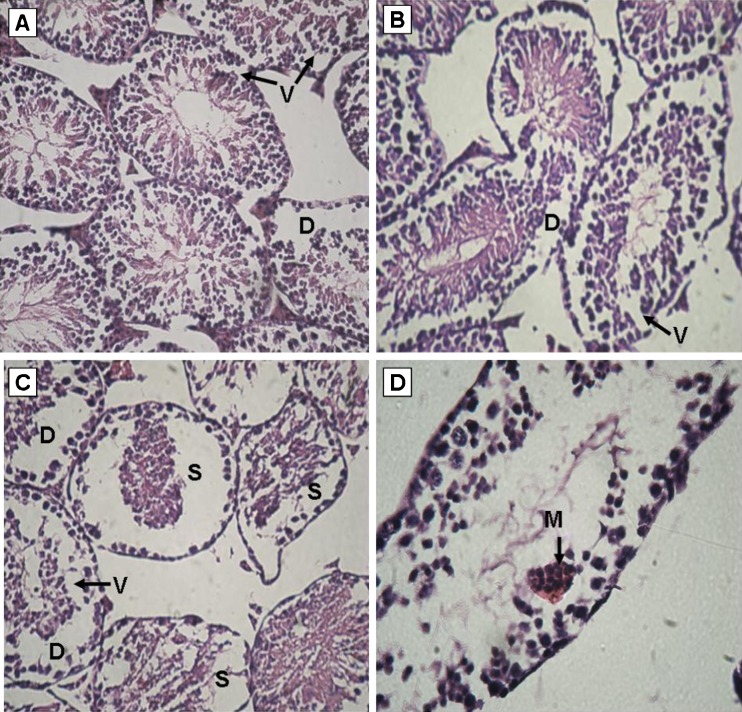
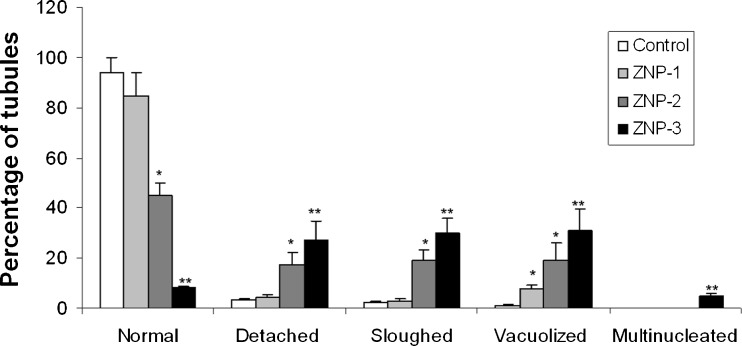
Testicular sections from control animals showed a low incidence of detached, sloughed or vacuolised seminiferous tubules. In ZNP-1 group, the majority of seminiferous tubules exhibited active spermatogenesis, but the percentage of vacuolised seminiferous tubules was significantly increased (Fig. 2a). No significant increase in detached or sloughed seminiferous tubules was evidenced in ZNP-1 group. In ZNP-2 group, varying degrees of germ cell degenerative changes occurred, ranging from loss of elongated spermatids, disorganization of germ cell layers, detachment and sloughing to vacuolization of the seminiferous tubules was observed (Fig. 2b). In the ZNP-3 group, detached, sloughed or vacuolised seminiferous tubules, contributing to eventual atrophy were markedly increased (Fig. 2c). In addition to these alterations, characteristic multinucleate giant cells (Fig. 2d) were also detected. Percentages of normal tubules were significantly decreased in ZNP-2 and ZNP-3 groups. Figure 3 shows the results obtained from the histopathological evaluations.
Fig. 2.
Light microscopy of cross sections of H&E stained testis from ZNP treated mice. a ZNP-1 (5 mg/kg ZNP) group: vacuoles (V) and detachment (D) are observed in some tubules. b ZNP-2 (50 mg/kg ZNP) group: histopathological changes including detachment and vacuolization are observed. c ZNP-3 (300 mg/kg ZNP) group: disorganization of germ cell layers includind sloughing (S), detachment and vacuolization are markedly increased. d multinucleated giant cells (M) in the seminiferous tubules are observed in ZNP-3 group. (a, b and c: ×250; d: ×400)
Fig. 3.
Testis histopathology assessments for control and experimental groups. Values are expressed as means ± SD for 8 mice. *p < 0.05, **p < 0.01, ZNP-1: 5 mg/kg ZNP, ZNP-2: 50 mg/kg ZNP, ZNP-3: 300 mg/kg ZNP
Assessment of spermatogenesis
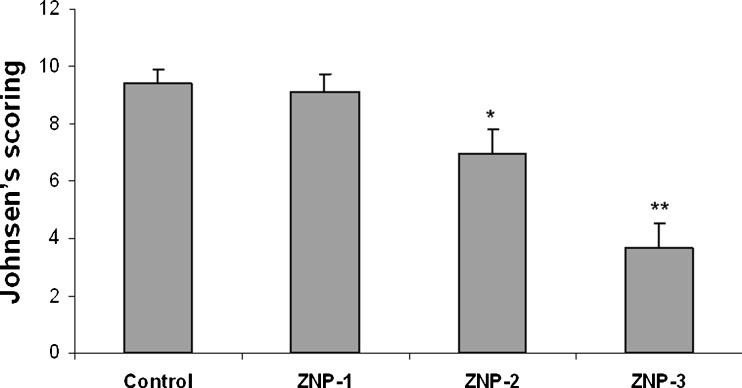
In the control group, normal spermatogenesis was observed and the mean Johnsen’s score was 9.4 ± 0.8. In ZNP-1 group, some sections of testes contained a few tubules with poor spermatogenesis and the mean Johnsen’s score was slightly lower than in the control group (P > 0.05). In ZNP-2 group, all sections of testes contained several tubules in which spermatogenesis were abnormal and the mean Johnsen’s score was significantly lower than in the control group (P < 0.05). ZNP-3 group showed a more significant decrease in the mean Johnsen’s score (P < 0.01). The results of Johnsen’s scoreing are depicted in Fig. 4.
Fig. 4.
Johnsen’s score in control and experimental groups. Values are expressed as means ± SD for 8 mice. *p < 0.05, **p < 0.01, ZNP-1: 5 mg/kg ZNP, ZNP-2: 50 mg/kg ZNP, ZNP-3: 300 mg/kg ZNP
Morphometry
ZNP-1group showed no significant alteration in testicular parameters compared with the control group. Diameters of the seminiferous tubules and height of the seminiferous epithelium were significantly decreased in both ZNP-2 and ZNP-3 groups. The results of morphometric studies are shown in Table 2.
Table 2.
Morphometric parameters in control and experimental groups. ZNP-1: 5 mg/kg ZNP, ZNP-2: 50 mg/kg ZNP, ZNP-3: 300 mg/kg ZNP
| Parameter (μm) | ||
|---|---|---|
| Group | STD | SEH |
| Control | 212.4 ± 18 | 164.3 ± 9.2 |
| ZNP-1 | 218 ± 19.2 | 163.3 ± 10.95 |
| ZNP-2 | 178 ± 14.6* | 109.4 ± 12* |
| ZNP-3 | 107 ± 10.9** | 81.7 ± 13.5** |
STD Seminiferous tubule diameter, SEH Seminiferous epithelium height
Values expressed as mean ± SD for 8 mice. *p < 0.01, **p < 0.001
Discussion
The present study demonstrated that ZNPs induce testicular damage in a dose dependent manner in mice. The presence of vacuoles in the cytoplasm of Sertoli cells shows direct damage to these cells. These lesions are early morphological signs of testicular injury and are considered as the main Sertoli cell response to many xenobiotics [2, 25, 26].
Multinucleate giant cell formation was characteristic of the ZNP-3 group. The multinucleate giant cells derive mainly from clumped spermatogenic cells that have lost their contact to Sertoli cells [27, 28]. The number of testicular sperm heads, which is known as a clear index of testicular cytotoxicity, correlated well with histopathological findings. It was previously reported that evaluation of testicular sperm head counts seems to be a good indicator of spermatogenic damages and that the number of testicular sperm heads corresponds to the number of elongate spermatids in the testis [29].
In this study, epididymal sperm parameters including sperm number, motility and percentage of abnormality were also significantly changed in ZNP-2 and ZNP-3 groups. Yoshida et al. reported that exposure to diesel exhaust induce Leydig cell degeneration, increase the number of damaged seminiferous tubules, and reduce daily sperm production [30]. Gromadzka-Ostrowska et al. also showed that even small amounts of silver NPs have a toxic impact on the germ cells and reduced sperm quality [19].
Previous studies demonstrate that NPs have the capacity to penetrate the blood–testis barrier and some of them have toxic action on male germ cells [30–33]. Braydich-Stolle et al. showed that mammalian spermatogonial stem cells are sensitive to Ag-NPs [5].
The exact mechanism of ZNP effects on mouse testis is not obtained from this study. However, the multinucleated giant cell formation, sloughing of immature germ cells from the seminiferous tubules, and vacuolization of Sertoli cells by ZNP indicates that this agent affect Sertoli cell functions. Another possible is that ZNP may affect Leydig cells and inhibit testosterone synthesis. The Leydig cell numbers were significantly decreased in ZNP-1 and ZNP-2 groups (results not shown). Komatsu et al., have demonstrated that titanium oxide and carbon black nanoparticles were taken up by mouse Leydig TM3 cells, and affected the viability, proliferation and gene expression [34]. Additionally, the reduction in the morphometrical parameters may have been a consequence of the apoptotic effects of ZNP on spermatogenic cells.
It is well known that increase in seminiferous tubule diameter is indicative of fluid retention resulting from impaired emptying through the efferent ducts, whereas decrease in seminiferous diameter may indicate germ cell loss [35]. The results of Johnsen’s scoring also revealed poor spermatogenesis in ZNP treated animals. Alterations in the Johnsen’s scoring relate to germ cells degenerations.
Sharma et al. (2012) reported that ZNP exposure in HepG2 cells increases apoptotic cell death [36]. Recently, some NPs types have been identified as a novel class of autophagy activators and inducers of autophagic cell death [37, 38]. Also, Yu et al. (2009) showed that rare element NPs including samar- ium/europium and gadolinium/terbium, induce serious autophagy in human liver cells [39]. Yu et al. (2013) showed that ZNP could induce autophagy in normal skin cells [40].
In summary, this study has established that ZNP has cytotoxic actions on testicular germ cells in a dose dependent manner. The multinucleated giant cell formation and sloughing of immature germ cells from the seminiferous tubules indicates that these NPs might also affect Sertoli cell functions. Alterations in the Johnsen’s scoring and morphometric studies may relate to induction of apoptosis or autophagy in testicular germ cells. However, to state the mechanism by which ZNP exerts its effects needs more investigations.
Footnotes
Capsule Zinc oxide nanoparticles exert significant toxic effects on the mouse spermatogenesis.
References
- 1.Franca LR, Ghosh S, Ye SJ, Russell LD. Surface and surface to volume relationships of the Sertoli cells during the cycle of the seminiferous epithelium in the rat. Biol Reprod. 1993;49(6):1215–1228. doi: 10.1095/biolreprod49.6.1215. [DOI] [PubMed] [Google Scholar]
- 2.Boekelheide K, Fleming SL, Johnson KJ, Patel SR, Schoenfeld HA. Role of Sertoli cells in injury-associated testicular germ cell apoptosis. Exp Biol Med. 2000;225(2):105–115. doi: 10.1046/j.1525-1373.2000.22513.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheng CY, Mruk DD. Cell junction dynamics in the testis: sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82(4):825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 4.Braydich-Stolle LK, Lucas B, Schrand A, Murdock RC, Lee T, Schlager JJ, et al. Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicol Sci. 2010;116(2):577–589. doi: 10.1093/toxsci/kfq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. In Vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci. 2005;88(2):412–419. doi: 10.1093/toxsci/kfi256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roduner E. Size matters: why nanomaterials are different. Chem Soc Rev. 2006;35(7):583–592. doi: 10.1039/b502142c. [DOI] [PubMed] [Google Scholar]
- 7.Borm PJ, Kreyling W. Toxicological hazards of inhaled nanoparticles potential implications for drug delivery. J Nanosci Nanotechnol. 2004;4(5):521–531. doi: 10.1166/jnn.2004.081. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Xue Z, Zheng D, Xia K, Zhao Y, Liu T, et al. Sodium chloride modified silica nanoparticles as a non-viral vector with a high efficiency of DNA transfer into cells. Curr Gene Ther. 2003;3(3):273–279. doi: 10.2174/1566523034578339. [DOI] [PubMed] [Google Scholar]
- 9.Shi L, Xun W, Yue W, et al. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Ruminant Res. 2011;96(1):49–52. doi: 10.1016/j.smallrumres.2010.11.005. [DOI] [Google Scholar]
- 10.Shi L, Yang R, Yue W, Xun WJ, Zhang CX, Ren YS, et al. Effect of elemental nano-selenium quality, glutathione peroxidase activity, and testis ultrastructure in male Boer goats. Anim Reprod Sci. 2010;118(2–4):248–254. doi: 10.1016/j.anireprosci.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Tyner KM, Schiffman SR, Giannelis EP. Nanobiohybrids as delivery vehicles for camptothecin. J Control Release. 2004;95(3):501–514. doi: 10.1016/j.jconrel.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Lan Z, Yang WX. Nanoparticles and spermatogenesis: how do nanoparticles affect spermatogenesis and penetrate the blood–testis barrier. Nanomedicine. 2012;7(4):579–596. doi: 10.2217/nnm.12.20. [DOI] [PubMed] [Google Scholar]
- 13.Delouise LA. Applications of nanotechnology in dermatology. J Investig Dermatol. 2012;132(3 Pt 2):964–975. doi: 10.1038/jid.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeng HA, Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health A Toxicol Hazard Subst Environ Eng. 2006;41(12):2699–2711. doi: 10.1080/10934520600966177. [DOI] [PubMed] [Google Scholar]
- 15.Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: Effect of particle composition. Environ Health Perspect. 2007;115(3):403–409. doi: 10.1289/ehp.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma V, Singh P, Pandey AK, Dhawan A. Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutat Res. 2012;745(1–2):84–91. doi: 10.1016/j.mrgentox.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Wang L, Ding W, Zhang F. Acute toxicity of ferric oxide and zinc oxide nanoparticles in rats. J Nanosci Nanotechnol. 2010;10(12):8617–8624. doi: 10.1166/jnn.2010.2483. [DOI] [PubMed] [Google Scholar]
- 18.Blazak WF, Treinen KA, Juniewicz PE. Application of testicular sperm head counts in the assessment of male reproductive toxicity. In: Chapin RE, Heindel JJ, editors. Methods in toxicology. California: Academic; 1993. pp. 86–94. [Google Scholar]
- 19.Gromadzka-Ostrowskaa J, Dziendzikowskaa K, Lankoffb A, et al. Silver nanoparticles effects on epididymal sperm in rats. Toxicol Lett. 2012;214(3):251–258. doi: 10.1016/j.toxlet.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 20.D’Cruz OJ, Uckun FM. Vanadocene-mediated in vivo male germ cell apoptosis. Toxicol Appl Pharmacol. 2000;166(3):186–195. doi: 10.1006/taap.2000.8965. [DOI] [PubMed] [Google Scholar]
- 21.Oatley JM, Tibary A, de-Avila DM, Wheaton JE, McLean DJ, Reeves JJ. Changes in spermatogenesis and endocrine function in the ram testis due to irradiation and active immunization against luteinizing hormone-releasing hormone. J Anim Sci. 2005;83(3):604–612. doi: 10.2527/2005.833604x. [DOI] [PubMed] [Google Scholar]
- 22.Johnsen SG. Testicular biopsy score count: a method for registration of spermatogenesis in human testis. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 23.Ma YH, Hu JH, Zhou XG, Mei ZT, Fei J, Guo LH. Gammaaminobutyric acid transporter (GAT1) overexpression in mouse affects the testicular morphology. Cell Res. 2000;10(1):59–69. doi: 10.1038/sj.cr.7290036. [DOI] [PubMed] [Google Scholar]
- 24.Orazizadeh M, Khorsandi LS, Hashemitabar M. Toxic effects of dexamethasone on mouse testicular germ cells. Andrologia. 2010;42(4):247–253. doi: 10.1111/j.1439-0272.2009.00985.x. [DOI] [PubMed] [Google Scholar]
- 25.Zeinick H, Clegg ED. Assessment of male reproductive toxicity: a risk assessment approach. In: Hayes AW, editor. Principles and Methods of Toxicology. 2. New York: Raven; 1989. pp. 275–309. [Google Scholar]
- 26.Nolte T, Harleman JH, Jahn W. Histopathology of chemically induced testicular atrophy in rats. Exp Toxicol Pathol. 1995;47(4):267–286. doi: 10.1016/S0940-2993(11)80260-5. [DOI] [PubMed] [Google Scholar]
- 27.Neumann F, Schenck B. Formal genesis of giant cells in the germinal epithelium in the rat thioglucose model. Andrologia. 1977;9(4):323–328. doi: 10.1111/j.1439-0272.1977.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 28.Holstein AF, Eckmann C. Multinucleated spermatocytes and spermatids in human seminiferous tubules. Andrologia. 1986;18(1):5–16. doi: 10.1111/j.1439-0272.1986.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim JC, Kim KH, Chung MK. Testicular cytotoxicity of DA-125, a new anthracycline anticancer agent, in rats. Reprod Toxicol. 1999;13:391–397. doi: 10.1016/S0890-6238(99)00028-3. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida S, Sagai M, Oshio S, Umeda T, Ihara T, Sugamata M, et al. Exposure to diesel exhaust affects the male reproductive system of mice. Int J Androl. 1999;22(5):307–315. doi: 10.1046/j.1365-2605.1999.00185.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida M, Yoshida S, Sugawara I, Takeda K. Maternal exposure to diesel exhaust decreases expression of Steroidogenic Factor-1 and mullerian inhibiting substance in the murine fetus. J Heal Sci. 2002;48:317–324. doi: 10.1248/jhs.48.317. [DOI] [Google Scholar]
- 32.Yoshida S, Ono N, Tsukue N, Oshio S, Umeda T, Takano H, et al. In utero exposure to diesel exhaust increased accessory reproductive gland weight and serum testosterone concentration in male mice. Environ Sci. 2006;13(3):139–147. [PubMed] [Google Scholar]
- 33.Tsukue N, Yoshida S, Sugawara I, Takeda K. Effect of diesel exhaust on development of fetal reproductive function in ICR female mice. J Heal Sci. 2004;50:174–180. doi: 10.1248/jhs.50.174. [DOI] [Google Scholar]
- 34.Komatsu T, Tabata M, Kubo-Irie M, Shimizu T, Suzuki K, Nihei Y, et al. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol in Vitro. 2008;22(8):1825–1831. doi: 10.1016/j.tiv.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Moffit JS, Bryant BH, Hall SJ, Hall SJ. Dose-dependent effects of Sertoli cell toxicants 2, 5-hexanedione, carbendazim, and mono- (2-ethylhexyl) phthalate in adult rat testis. Toxicol Pathol. 2007;35(5):719–727. doi: 10.1080/01926230701481931. [DOI] [PubMed] [Google Scholar]
- 36.Sharma V, Anderson D, Dhawan A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2) Apoptosis. 2012;17(8):852–870. doi: 10.1007/s10495-012-0705-6. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Yang L, Feng C. Nano neodymium oxide induces massive vacuolization and autophagic cell death in non-small cell lung cancer NCI- H460 cells. Biochem Biophys Res Commun. 2005;337(1):52–60. doi: 10.1016/j.bbrc.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Zabirnyk O, Yezhelyev M, Seleverstov O. Nanoparticles as a novel class of autophagy activators. Autophagy. 2007;3(3):278–281. doi: 10.4161/auto.3916. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, Lu Y, Na M, Yu SH, Wen LP. Rare earth oxide nanocrystals induce autophagy in HeLa cells. Small. 2009;5(24):2784–2787. doi: 10.1002/smll.200901714. [DOI] [PubMed] [Google Scholar]
- 40.Yu KN, Yoon TJ, Minai-Tehrani A, Kim JE, Park SJ, Jeong MS, et al. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol in Vitro. 2013;27(4):1187–1195. doi: 10.1016/j.tiv.2013.02.010. [DOI] [PubMed] [Google Scholar]