Abstract
Purpose
To investigate the association of Progesterone Receptor (PR) gene variations and male infertility
Methods
DNA extraction, PCR and sequencing of PR gene, PROGINS insertion by PCR. Association of the variations with seminal parameters and outcomes of ICSI.
Results
Four known SNPs in the PR gene were identified in the study of which three (rs3740753, rs1042838, rs104283) were co-inherited and in complete linkage disequilibrium with the PROGINS Alu insertion. There were no differences in their frequencies between fertile and infertile males. The rs2020880 was found at a very low frequency only in the controls but not in the infertile subjects. The sperm counts, fertilization rate, embryo quality or pregnancy rates were not different in individuals with or without PROGINS allele.
Conclusion
PR gene alterations are not associated with male infertility or ICSI outcome.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-013-0074-2) contains supplementary material, which is available to authorized users.
Keywords: Progesterone receptor, Polymorphism, PROGINS, Male infertility, ICSI, Azoospermia, Sperm counts
Introduction
It is estimated that nearly 15 % of couples in the reproductive age experience involuntary childlessness. Amongst these, approximately 40 % are due to defects in the female partner, 40 % in the male partner and 10 % are hitherto unexplained. Clinically representing as azoospermia (no sperm in the semen) or oligozoospermia (<15 × 106 spermatozoa/mL of ejaculate); male factor subfertility is a frequently observed medical problem [13,42]. Several factors like; cryptorchidism, environmental toxins, infections, drugs, alcohol, radio/chemotherapy are known to reduce sperm counts; in about one third of the cases, the cause of impaired spermatogenesis is unknown and is often referred to as idiopathic infertility [13,22]. Identification of the etiology in these cases is necessary for accurate diagnosis and designing rational therapies for male infertility.
Studies in the last decennia have implicated genetic alterations in the Y chromosome and autosomes as a causative factor for male infertility. Microdeletions on the long arm of the Y chromosome (Yq), encompassing critical genes involved in spermatogenesis, has been observed in 5–10 % of infertile men [1,14,26,35]. In addition, mutations/polymorphisms in several autosomal genes have been identified in a subset of individuals with male factor infertility [15,26]. Genome-wide association studies have identified multiple autosomal loci across the genome that may be implicated in male factor infertility [10,18,21]. These observations suggest a strong genetic involvement of both sex chromosome and autosomal loci and male infertility appears to be polygenic in nature. This is not surprising as spermatogenesis is a complex developmental process involving multiple cell types and tightly regulated by endocrine, autocrine and paracrine mechanisms. Thus, it is likely that there could be more than one genetic defect in the same individual which may compromise spermatogenesis in concert through epistatic interactions [10]. Therefore, there is a need to identify more susceptibility loci in infertile men in order to determine the etiology of this yet enigmatic condition.
Amongst the several hormonal factors, progesterone has recently emerged as one of the factors that affect spermatogenesis. It has been observed that co-administration of progestins in androgen based contraceptive regimens augment the induction of gonadotropin induced spermatogenic suppression [8,27]. Based on assessment of the gonadotropin levels, testicular germ cell counts, intra-testicular hormone levels, expression of various genes and the time required to induce azoospermia in men treated with testosterone with and without progesterone has suggested that progesterone can act directly in the testis [7,24,28]. Indeed, we reported the presence of progesterone receptors (PR) in the germ cells of human testis and its differential expression during various stages of spermatogenesis [37,38]. PRs are also detected in testis of other mammals [17,20,25] suggesting that progesterone may regulate spermatogenesis. While PR knockout mice (PRKO) have larger testes, greater sperm production, increased numbers of Sertoli and Leydig cells [25]; in humans, PR mRNA and protein are reduced in testis of azoospermic men with maturation arrest and hypospermatogenesis [2,16] and also in spermatozoa of oligoasthenoteratozoospermic infertile men [12]. These observations are definitive evidence to suggest that PR has a role in regulation of spermatogenesis and reduced PR expression is associated with defective spermatogenesis. However, the reason for the loss of PR expression in the testis of infertile men is yet not clear.
Several genetic variants have been reported in the PR gene; of these the most common is the PROGINS insertion. Characterized by a 320 bp PV/HS-1 Alu insertion in intron G and two point mutations, V660L in exon 4 and H770H (silent substitution) in exon 5; the Alu element contains a half oestrogen-response element/Sp1-binding site (Alu-ERE/Sp1), which acts as an in-cis intronic enhancer leading to increased transcription of the PROGINS allele in response to 17beta-oestradiol. The Alu element reportedly reduces the stability of the PROGINS transcript compared with the wild allele; the amino acid substitution V600L leads to differences in PR phosphorylation and degradation upon ligand binding, most likely as a result of differences in the three-dimensional structures of the variant [33]. As a consequence, the PROGINS variant displays decreased transactivation activity, the resulting protein has poor bioactivity and affects cell cycle in cultured endometrial cells [11,33]. Intriguingly, studies have demonstrated that the presence of this polymorphism increases the risk of women towards gynecologic disorders including cancers [30,32,34,39]. Thus, we hypothesized that such genetic variation in the PR gene may reduce PR mRNA/protein expression in the testis leading to male factor infertility. However, to the best of our knowledge, genetic changes, if any, in the PR gene of men with azoospermia or oligozoopsermia has not been investigated. Thus in the present study we aimed to study the PR gene variations determine and its association with male infertility.
Material and methods
5 mL of blood samples were collected from the men, who were attending the male infertility clinic at NIRRH or INKUS IVF Center for assisted reproduction. Infertile subjects were selected based on the sperm counts. Fertile controls were normozoospermic men, who had fathered a child in the last 1 year or whose partners had female factor infertility and had conceived after assisted reproduction. A third group of individuals were normozoospermic male partners of infertile couples, where the female partners also did not have any known causes of infertility and defined as infertile normozoospermic. The study protocol was approved by the Institutional Ethics Committee and the written informed consent has been obtained from all the participants.
Subjects with obstructive azoospermia, hypogonadism, hypoandrogenism, chronic diseases, history of pelvic/spinal injuries or those reported to be heavy smokers and/or alcohol intake were excluded from this study. Initial genetic testing included karyotyping and detection for Yq microdeletions by PCR. Karyotyping was done using a standard G-banding technique. At least 25 metaphases were counted and two were karyotyped to determine numerical or gross structural abnormalities. Testing for Yq microdeletions was done by PCR using six pairs of STS primers that span the azoospermic factor (AZFa, AZFb and AZFc) loci. The primers and the method for STS PCR has been detailed elsewhere [1,2,35]. Individuals with karyotype abnormalities or Yq microdeletions were excluded from the study.
A total of 225 infertile men that included 139 oligozoospermic men (sperm count <15 million/mL), 42 non-obstructive azoopsermic men and 44 normozoospermic infertile men were included in this study. As controls, 232 fertile men were also included.
Amplification and direct sequencing of PR gene
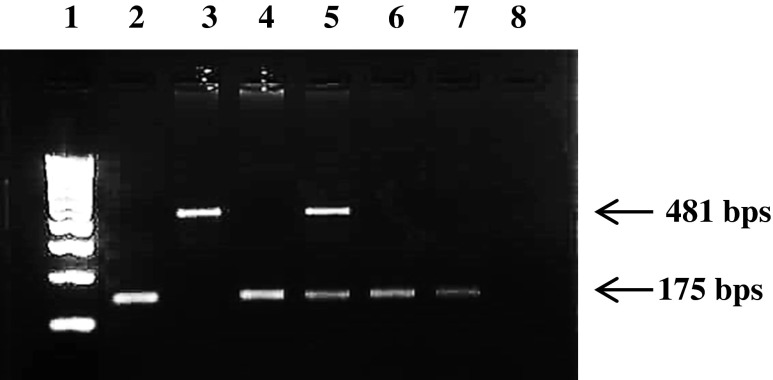
Genomic DNA was isolated from whole blood using a commercial kit (Sigma-Aldrich St. Louis USA). Primers were designed to amplify all the 7 exons of PR gene and encompassed the exon intron boundary. The sequences for the same are shown in supplementary Table 1. PCR was performed at an optimized annealing temperature and has been detailed elsewhere [1,5,40]. The amplicons were further subjected to direct sequencing [5,40] using BigDye Terminator cycle sequencing kit (Applied Biosystems, Foster City USA) and 3730 DNA analyzer (Applied Biosystems). The PROGINS amplified products were loaded in a 2 % agarose gel stained with ethidium bromide and observed under UV transillumination (Fig. 1).
Fig. 1.
Detection of PROGINS polymorphism. Representative image showing homozygote (T1) allele, heterozygote (T1/T2), and homozygote (T2) Lane 1 is 100 bp ladder, Lane 2, 4 6 and 7 are T1 allele, lane 5 is heterozygote, and lane 3 is heterozygote for the T2 allele
Assessment of sperm counts, fertilization rate, embryo quality and pregnancy rate
Data was available for 208 subjects, who approached for assisted reproduction for biological parenthood. All the subjects (irrespective of sperm counts) underwent ICSI as per the standard protocol at INKUS IVF Centre. The protocol for ovarian hyperstimulation, oocyte retrieval, ICSI and embryo culture has been detailed elsewhere [9,29].
In all these cases, the female partner was clinically normal with no apparent cause of infertility and only good quality oocytes were considered for microinjection. Embryos with two polar bodies and two distinct and opposing pronuclei (PN) with evenly distributed nucleoli were considered as fertilized. Fertilization rate was calculated as the number of oocytes fertilized and undergone first cleavage with respect to the number of oocytes microinjected.
Embryo cleavage and quality was evaluated 40–44 h after ICSI. Embryo quality was defined as Grade 1, Grade 2, Grade 3, Grade 4 and Grade 5 as described earlier [9]. Briefly Grade 1 embryos were defined as blastomeres of equal size without cytoplasmic fragments. Grade 2 embryos were defined as blastomeres of equal size with minor cytoplasmic fragments or blebs. Grade 3 embryos were defined as blastomeres of distinctly unequal size with few or none cytoplasmic fragments. Grade 4 embryos were defined as blastomeres of equal or unequal size with significant cytoplasmic fragmentation. Grade 5 embryos were defined as few blastomeres of any size with severe or complete fragmentation.
A maximum of three embryos were transferred per cycle. Pregnancy was confirmed by measuring serum β hCG concentration 2 weeks after embryo transfer and ultrasonography was done 2 weeks after measuring β hCG levels using commercial assays.
Statistical analysis
Allele and genotype distribution, embryo quality and pregnancy rates between groups were evaluated using Z test statistical significance at the 95 % confidence interval. Z > 2 is considered significant. Sperm counts and fertilization rates were analysed using Students-t test. A p-value of <0.05 was considered as significant. The 95 % Confidence limits and the Standard Error of Proportion were calculated as described previously [35].
Results
Progesterone receptor gene variations
Sequencing for all the exons of PR gene carried out in 100 infertile and 152 controls identified four known SNPs. These were rs3740753 (Exon 1: G 1774C, S344T), rs1042838 (Exon 4: G2721T, L660V), rs104283 (Exon 5: C3053T H770H), rs2020880 (Exon 7: C3337T, T865C). The nucleotide numbers are based on the mRNA sequence NM_000926.4 and the amino acid numbers are based on the protein sequence NP_000917.3. No other SNPs/variations were observed in any of the PR gene exons in the population studied herein.
Amongst the four SNPs, three (rs3740753, rs104283, rs104283) were found to co-inherit and in complete linkage disequilibrium. The major allele for all the three SNPs is referred to as *1 allele and the minor allele as *2. The genotypic and the allelic frequencies for wild type (*1), heterozygotes (*1/*2) and homozygotes (*2) in the infertile group are shown in Table 1. As evident, the genotypic or allelic frequencies were not significantly different between any of the groups (Z < 2). The 95 % CL and the standard error of proportion for the genotypic and allelic frequencies for the groups are shown in supplementary Tables 2 and 3.
Table 1.
Genotypic and allelic frequencies of progesterone receptor gene polymorphisms in fertile and infertile men
| Haplogroup S344T; L660V; H770H | ||||||
| Total screened | *1 | *1/*2 | *2 | *1 allele | *2 allele | |
| Fertile normozoospermia | 152 | 137 (90 %) | 15 (10 %) | 0 | 289 (95 %) | 15 (5 %) |
| Total infertile | 100 | 89 (89 %) | 10 (10 %) | 1 (1 %) | 188 (94 %) | 12 (6.0 %) |
| Oligozoospermia | 60 | 54 (90 %) | 5 (8 %) | 1 (2 %) | 113 (94 %) | 7 (6 %) |
| Azoospermia | 20 | 17 (85 %) | 3 (15 %) | 0 | 37 (92.5 %) | 3 (7.5 %) |
| Infertile normozoospermia | 20 | 18 (90 %) | 2 (10 %) | 0 | 38 (95 %) | 2 (5 %) |
| Total | 252 | 225 (89 %) | 26 (10.5 %) | 1 (0.5 %) | 477 (94.6 %) | 27 (5.4 %) |
| rs2020880 S865L | ||||||
| Total screened | SS | SL | LL | S allele | L allele | |
| Fertile normozoospermia | 152 | 145 (95 %) | 7 (5 %) | 0 | 297 (97.6 %) | 7 (2.4 %) |
| Total infertile | 100 | 100 (100 %) | 0 | 0 | 200 (100 %) | 0 |
| Oligozoospermia | 60 | 60 (100 %) | 0 | 0 | 120 (100 %) | 0 |
| Azoospermia | 20 | 20 (100 %) | 0 | 0 | 40 (100 %) | 0 |
| Infertile normozoospermia | 20 | 20 (100 %) | 0 | 0 | 40 (100 %) | 0 |
| Total | 252 | 245 (97 %) | 7 (3 %) | 0 | 497 (98.6 %) | 7 (1.4 %) |
The SNP rs2020880 was found at a very low frequency in our population (1.4 %) and observed only in the controls; this polymorphism was not detected in the infertile subjects. Sequencing of the exon 7 in additional 81 infertile subjects did not reveal the presence of this polymorphism (not shown). The differences in the frequency of this polymorphism between fertile and infertile subjects is statistically significant (Z > 2).
PROGINS polymorphisms
The analyses of PROGINS Alu insertion was studied in fertile and infertile subjects. The homozygous wild types, heterozygous and homozygous cases with Alu insertion were detected by PCR and the results are shown in Fig. 1. The wild type (no insertion) is referred to as T1; the insertion was referred to as T2 allele. All the subjects that had *2 allele always had the T2 alleles indicating that these are in complete linkage disequilibrium.
The genotypic and the allelic frequencies of T1 and T2 alleles in the fertile and infertile subjects are shown in Table 2. As evident, the difference in the genotypic or allele frequencies between fertile and total infertile subjects was not significant (Z < 2). No differences were observed when the infertile group was further sub grouped into oligozoopsermic and azoopsermic cases. The 95 % CL and the standard error of proportion for the genotypic and allelic frequencies of T1 and T2 in the different groups are shown in supplementary Tables 4 and 5.
Table 2.
Genotypic and allelic frequencies of the PROGINS insertion. Wild type (T1) is without insertion and polymorphic allele (T2) is with insertion in progesterone receptor gene in fertile and infertile men
| Total screened | T1 | T1/T2 | T2 | T1 Allele | T2 Allele | |
|---|---|---|---|---|---|---|
| Fertile normozoospermia | 232 | 209 (90 %) | 23 (10 %) | 0 | 441 (95 %) | 23 (5 %) |
| Total infertile | 225 | 198 (88 %) | 25 (11 %) | 2 (1 %) | 421 (94 %) | 29 (6 %) |
| Oligozoospermia | 139 | 126 (91 %) | 11 (7 .5 %) | 2 (1.5 %) | 263 (95 %) | 15 (5 %) |
| Azoospermia | 42 | 33 (79 %) | 9 (21 %) | 0 | 75 (89 %) | 9 (10 %) |
| Infertile normozoospermia | 44 | 39 (89 %) | 5 (11 %) | 0 | 83 (94 %) | 5 (6 %) |
| Total | 457 | 407 (89 %) | 48 (10.5 %) | 2 (0.5 %) | 862 (94 %) | 52 (6 %) |
Association of PR gene polymorphism and PROGINS with sperm counts and ART outcome
Data of sperm counts, fertilization rate, embryo quality and pregnancy rate were available for 208 subjects. Of these 115 were normozoospermic fertile, 47 had oligozoospermia, 6 had azoospermia and 40 were normozoospermic infertile. Since the number of subjects with PROGINS insertion were low, we pooled the data of all these groups and compared the sperm counts and ART outcomes in individuals with (n = 32) and without (n = 176) PROGINS alleles.
The mean sperm counts were identical in men with and without PROGINS insertion irrespective of the fertility status (Table 3). No significant differences were noted in the fertilization rates (p = 0.3) or embryo quality (Z < 2) in the group with PROGINS allele as compared with the wild type. The pregnancy rates were half in the group with the T2 allele as compared to T1 allele. However, this difference was not statistically significant (Z = 0.67). The 95 % CL and the standard error of proportion for the data are shown in Table 3.
Table 3.
Effects of paternal PROGINS polymorphism on ART outcome; 95 % CL and Standard Error of Proportion (SEP) values for the sperm counts and ART outcome parameters in individuals with and without PROGINS insertion
| T1 | 95%CL | T1 + T2 | 95%CL | SEPa | |
|---|---|---|---|---|---|
| Mean sperm count (million/ml) | 44 (range 0.1-110) | – | 44 (0.1-89) | – | 0.21 |
| No. of embryos transferred | 548 (40.80 %) | 36.60–44.99 | 99 (45.4 %) | 33.39–55.40 | −0.85 |
| Grade I | 532 (97.08 %) | 95.64–98.51 | 98 (98.99 %) | 96.98–100.99 | −1.53 |
| Grade II | 16 (2.92 %) | 1.48–4.35 | 1 (1.01 %) | -0.99–3.01 | 0.18 |
| Pregnant | 43 (24.4 %) | 11.3–37.49 | 4 (12.5 %) | -20.57–45.57 | 0.67 |
acalculated in comparison to T1
Discussion
The results of the present study demonstrate that the PR gene variations are not associated with male infertility and the PROGINS polymorphism has no association with sperm counts. In addition, we also demonstrate that the presence of these polymorphisms in the paternal genome does not affect fertilization, embryonic growth or pregnancy rates in an assisted reproductive technology (ART) program.
We and others have previously demonstrated that PR is expressed in the mammalian testis and the expression is downregulated in testis of infertile men [2,17,20,25,37,38]. In the present study, we tested if loss of PR was due to any sequence alterations in the PR gene and may be a cause of oligo or azoospermia. To the best of our knowledge, PR gene has not been studied with regards to male infertility in any of the populations. Bidirectional sequencing of the PR coding region identified four SNPs of which rs3740753, rs104283 and rs104283 are in complete linkage disequilibrium. Referred to as *2 allele, these SNPs have been previously reported and occur at frequencies nearly identical to that reported in the Indian population [5]. However, the genotypic or allelic frequencies of *2 were not significantly different between normozospermic controls vs oligozoopsermic or azoopsermic or infertile normozoospermc. In addition, we also identified the rare polymorphism rs2020880 (S865L) in our population that had an allele frequency of 1.4 %, which is higher when compared to the Asian or other population (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?type=rs&rs=2020880). While the rs2020880 polymorphism was only found in the normozoospermic controls, all the infertile patients were monomorphic and had the wild type allele. This polymorphism was not detected in the DNA of additional 81 infertile individuals sequenced for only exon 7 (not shown). Although the differences observed in the frequency of this polymorphism in fertile and infertile subjects is statistically significant, considering the low prevalence of the polymorphic allele, a larger sample size will be required to determine if the SNP has any association with the fertility status. Beyond these, no other SNPs or mutations were identified in any of the sequenced individuals. These observations suggest that PR gene SNPs or mutations are not a cause of compromised spermatogenesis atleast in the Indian population.
PROGINS is a polymorphic variant in the PR gene and has been shown to act as a risk-modulating factor in several gynaecological disorders including cancers [30,32,34,41]. This polymorphic variant is also functionally relevant as presence of PROGINS in the PR affects the stability of its mRNA and the resulting protein has reduced response to progesterone [33]. To test if the PROGINS insertion may be a cause of reduced PR expression and or its activity in the infertile men, we screened for its presence in the DNA of fertile and infertile individuals. PROGINS insertion was found to be in complete linkage disequilibrium with the *2 allele in our population, as reported by others [34,41]. However, no significant differences were observed in the frequency of the PROGINS insertion in both fertile and infertile subjects irrespective of whether the subjects were azoopsermic or oligozoospermic or infertile normozoospermic. These results suggest that PROGINS insertion is not a risk factor for predisposition to infertility.
While the laboratory classification of oligozoospermia and normozoospermia is based on the sperm counts with a cutoff of 15 million/ml [42], the correlation of these counts to fertility is often poor and an individual can father a child even at sperm concentrations lower then this cutoff [13,40]. Thus, the fertility status is often a misleading parameter for association based studies and it is recommended to compare the data with sperm counts. In the present study, we compared the total sperm counts of individuals with or without PROGINS and observed no significant differences between the two groups suggesting that PROGINS insertions is not associated with low sperm counts in the Indian population.
Previous studies have demonstrated that the presence of *2 allele in the mother is associated with recurrent abortions, implantation failure and pre-term births [34,39]. While there are reports that have refuted these findings [5,23], the influence of maternal genome on embryonic growth cannot be underestimated. Beyond the maternal genome, evidences are now available where the paternal genome has also been suggested to influence embryogenesis. Growing body of evidences suggest that mature sperm provide appropriate epigenetic marks that drive specific genes toward activation and contribute to the pluripotent state of the embryonic cells [19]; in addition it also provides several classes of non-coding RNAs including miRNAs, lncRNAs, novel elements and mRNAs that are likely to be essential for early development [6,36]. It is possible that defects in the paternal genome and/or abnormal RNA profiles in the spermatozoa may lead to failure of embryonic growth, pregnancy loss, and/or developmental abnormalities. Recent clinical and experimental evidences have indeed suggested that epigenetic defects in the sperm DNA is associated with recurrent spontaneous abortions [4,31]. Since the PR mRNA is expressed in the mature spermatozoa [37,38] and the PROGINS polymorphism is associated with defective PR transcription [11,33], we tested if the PROGINS insertion in the paternal genome has any effect on fertilization, embryo quality and pregnancy rates. However, no significant differences were noted when these parameters were compared in the group, where the male partner had PROGINS allele vs the wild type allele. At this conjuncture, it is important to note that the fertilization rates and embryo quality observed in the two groups are comparable to that routinely observed in our clinic [9,29]. Interestingly, the pregnancy rates were half in the group with PROGINS insertion as compared to the wild type controls; however the differences were not statistically significant. These results suggest that although the paternal PROGINS allele does not affect early embryo quality, the pregnancy rates may be lower. However, it is important to note that the PROGINS allele in this group was in the heterozygous state and the status of maternal PR polymorphisms is also unknown; thus, it is difficult to estimate how many transferred/implanted embryos would have a PROGINS allele. It has been shown that the status of PROGINS allele both in the mother and the fetus alter the risk of development of transient tachypnea [3]. Therefore, it will be essential to determine the status of both maternal and paternal PR polymorphisms and correlate with ART outcome mainly pregnancy rates.
In conclusion, within the limited cohort of Indian individuals, genetic alterations in the PR gene do not seem to contribute towards the aetiology of male infertility or ART outcome. Since this study is the first of its kind, one should await results from more populations of diverse ethnic and geographic backgrounds before making any conclusive statement on the role of PR in male fertility.
Electronic supplementary material
(DOC 42.5 KB )
(DOC 33.5 KB)
(DOC 31.0 KB)
(DOC 33.0 KB)
(DOC 31.5 KB)
Acknowledgments
We express our thanks to Director NIRRH and Director, CCMB for the support and encouragement. SS is thankful to the Lady Tata Memorial Trust for providing Junior Research Fellowship. AD is grateful to ICMR for Senior Research fellowship. The work included in this publication (NIRRH/M/100/13) has been supported financially by grants from the Indian Council of Medical Research (ICMR), New Delhi, India.
Authors contributions
Sanjukta Sen: Sample collection, Data collection, Data analysis, Manuscript preparation
Abhijit Dixit: Data collection, Data analysis, Manuscript preparation
Jyotsna Gokral: Patient recruitment, sample collection and screening, Manuscript preparation
Chitra Thakur: Sample collection, Data collection, Manuscript preparation
Indira Hinduja: Recruitment of ICSI candidates, Manuscript preparation
Kusum Zaveri: Recruitment of ICSI candidates, Manuscript preparation
Kumarasamy Thangaraj: Sequencing, Manuscript preparation
D. Modi: Conceptualization of the project, Data analysis, Manuscript preparation, Overall coordination
Footnotes
Capsule Genetic variations in progesterone receptor gene do not associate with male infertility or ICSI outcome.
References
- 1.Abid S, Maitra A, Meherji P, Patel Z, Kadam S, Shah J, et al. Clinical and laboratory evaluation of idiopathic male infertility in a secondary referral center in India. J Clin Lab Anal. 2008;22:29–38. doi: 10.1002/jcla.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abid S, Gokral J, Maitra A, Meherji P, Kadam S, Pires E, et al. Altered expression of progesterone receptors in testis of infertile men. Reprod Biomed Online. 2008;17:175–184. doi: 10.1016/S1472-6483(10)60192-7. [DOI] [PubMed] [Google Scholar]
- 3.Alter M, Pfab T, Guthmann F, Burdack A, Kempiners N, Kalk P, et al. Maternal and fetal PROGINS progesterone receptor polymorphism reduces the risk for transient tachypnea of the newborn. Clin Lab. 2010;56:559–567. [PubMed] [Google Scholar]
- 4.Ankolkar M, Patil A, Warke H, Salvi V, Kedia Mokashi N, Pathak S, et al. Methylation analysis of idiopathic recurrent spontaneous miscarriage cases reveals aberrant imprinting at H19 ICR in normozoospermic individuals. Fertil Steril. 2012;98:1186–1192. doi: 10.1016/j.fertnstert.2012.07.1143. [DOI] [PubMed] [Google Scholar]
- 5.Aruna M, Nagaraja T, Andal S, Tarakeswari S, Sirisha PV, Reddy AG, et al. Role of progesterone receptor polymorphisms in the recurrent spontaneous abortions: Indian case. PLoS One. 2010;5:e8712. doi: 10.1371/journal.pone.0008712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barroso G, Valdespin C, Vega E, Kershenovich R, Avila R, Avendaño C, et al. Developmental sperm contributions: fertilization and beyond. Fertil Steril. 2009;92:835–848. doi: 10.1016/j.fertnstert.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Bayne RA, Forster T, Burgess ST, Craigon M, Walton MJ, Baird DT, et al. Molecular profiling of the human testis reveals stringent pathway-specific regulation of RNA expression following gonadotropin suppression and progestogen treatment. J Androl. 2008;29:389–403. doi: 10.2164/jandrol.107.004168. [DOI] [PubMed] [Google Scholar]
- 8.Bebb RA, Anawalt BD, Christensen RB, Paulsen CA, Bremner WJ, Matsumoto AM. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. J Clin Endocrinol Metab. 1996;81:757–762. doi: 10.1210/jc.81.2.757. [DOI] [PubMed] [Google Scholar]
- 9.Bhilawadikar R, Zaveri K, Mukadam L, Naik S, Kamble K, Modi D et al. Levels of Tektin 2 and CatSper 2 in normozoospermic and oligoasthenozoospermic men and its association with motility, fertilization rate, embryo quality and pregnancy rate. J Assist Reprod Genet. 2013;30:513–23. [DOI] [PMC free article] [PubMed]
- 10.Carrell DT, Aston KI. The search for SNPs, CNVs, and epigenetic variants associated with the complex disease of male infertility. Syst Biol Reprod Med. 2011;57:17–26. doi: 10.3109/19396368.2010.521615. [DOI] [PubMed] [Google Scholar]
- 11.D’Amora P, Maciel TT, Tambellini R, Mori MA, Pesquero JB, Sato H, et al. Disrupted cell cycle control in cultured endometrial cells from patients with endometriosis harboring the progesterone receptor polymorphism PROGINS. Am J Pathol. 2009;175:215–224. doi: 10.2353/ajpath.2009.080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Amicis F, Guido C, Perrotta I, Avena P, Panza S, Andò S, et al. Conventional progesterone receptors (PR) B and PRA are expressed in human spermatozoa and may be involved in the pathophysiology of varicocoele: a role for progesterone in metabolism. Int J Androl. 2011;34:430–445. doi: 10.1111/j.1365-2605.2010.01111.x. [DOI] [PubMed] [Google Scholar]
- 13.Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. [corrected] Clinics (Sao Paulo) 2011;66:691–700. doi: 10.1590/S1807-59322011000400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foresta C, Moro E, Ferlin A. Y chromosome microdeletions and alterations of spermatogenesis. Endocr Rev. 2001;22:226–239. doi: 10.1210/er.22.2.226. [DOI] [PubMed] [Google Scholar]
- 15.Hamada AJ, Esteves SC, Agarwal A. A comprehensive review of genetics and genetic testing in azoospermia. Clinics (Sao Paulo) 2013;68:39–60. doi: 10.6061/clinics/2013(Sup01)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Y, Feng HL, Sandlow JI, Haines CJ. Comparing expression of progesterone and estrogen receptors in testicular tissue from men with obstructive and nonobstructive azoospermia. J Androl. 2009;30:127–133. doi: 10.2164/jandrol.108.005157. [DOI] [PubMed] [Google Scholar]
- 17.Heikinheimo O, Mahony MC, Gordon K, Hsiu JG, Hodgen GD, Gibbons WE. Estrogen and progesterone receptor mRNA are expressed in distinct pattern in male primate reproductive organs. J Assist Reprod Genet. 1995;12:198–204. doi: 10.1007/BF02211799. [DOI] [PubMed] [Google Scholar]
- 18.Hu Z, Xia Y, Guo X, Dai J, Li H, Hu H, et al. A genome-wide association study in Chinese men identifies three risk loci for non-obstructive azoospermia. Nat Genet. 2011;44:183–186. doi: 10.1038/ng.1040. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins TG, Carrell DT. The sperm epigenome and potential implications for the developing embryo. Reproduction. 2012;143:727–734. doi: 10.1530/REP-11-0450. [DOI] [PubMed] [Google Scholar]
- 20.Kohler C, Riesenbeck A, Hoffmann B. Age-dependent expression and localization of the progesterone receptor in the boar testis. Reprod Domest Anim. 2007;42:1–5. doi: 10.1111/j.1439-0531.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- 21.Kosova G, Scott NM, Niederberger C, Prins GS, Ober C. Genome-wide association study identifies candidate genes for male fertility traits in humans. Am J Hum Genet. 2012;90:950–961. doi: 10.1016/j.ajhg.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab. 2011;25:271–285. doi: 10.1016/j.beem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Kurz C, Tempfer CB, Boecskoer S, Unfried G, Nagele F, Hefler LA. The PROGINS progesterone receptor gene polymorphism and idiopathic recurrent miscarriage. J Soc Gynecol Invest. 2001;8:295–298. doi: 10.1016/S1071-5576(01)00123-X. [DOI] [PubMed] [Google Scholar]
- 24.Lue Y, Wang C, Cui Y, Wang X, Sha J, Zhou Z, et al. Levonorgestrel enhances spermatogenesis suppression by testosterone with greater alteration in testicular gene expression in men. Biol Reprod. 2009;80:484–492. doi: 10.1095/biolreprod.108.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lue Y, Wang C, Lydon JP, Leung A, Li J, Swerdloff RS. Functional role of progestin and the progesterone receptor in the suppression of spermatogenesis in rodents. Andrology. 2013;1:308–317. doi: 10.1111/j.2047-2927.2012.00047.x. [DOI] [PubMed] [Google Scholar]
- 26.Massart A, Lissens W, Tournaye H, Stouffs K. Genetic causes of spermatogenic failure. Asian J Androl. 2012;14:40–48. doi: 10.1038/aja.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthiesson KL, McLachlan RI. Male hormonal contraception: concept proven, product in sight? Hum Reprod Update. 2006;12:463–482. doi: 10.1093/humupd/dml010. [DOI] [PubMed] [Google Scholar]
- 28.McLachlan RI, O’Donnell L, Stanton PG, Balourdos G, Frydenberg M, de Kretser DM, et al. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab. 2002;87:546–556. doi: 10.1210/jc.87.2.546. [DOI] [PubMed] [Google Scholar]
- 29.Nagvenkar P, Zaveri K, Hinduja I. Comparison of the sperm aneuploidy rate in severe oligozoospermic and oligozoospermic men and its relation to intracytoplasmic sperm injection outcome. Fertil Steril. 2005;84:925–931. doi: 10.1016/j.fertnstert.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 30.Near AM, Wu AH, Templeman C, Van Den Berg DJ, Doherty JA, Rossing MA, et al. Ovarian Cancer Association Consortium; Australian Cancer Study (Ovarian Cancer) (ACS); Australian Ovarian Cancer Study Group (AOCS). Progesterone receptor gene polymorphisms and risk of endometriosis: results from an international collaborative effort. Fertil Steril. 2011;95:40–45. doi: 10.1016/j.fertnstert.2010.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathak S, Kedia-Mokashi N, Saxena M, D’Souza R, Maitra A, Parte P, et al. Effect of tamoxifen treatment on global and insulin-like growth factor 2-H19 locus-specific DNA methylation in rat spermatozoa and its association with embryo loss. Fertil Steril. 2009;91:2253–2263. doi: 10.1016/j.fertnstert.2008.07.1709. [DOI] [PubMed] [Google Scholar]
- 32.Rockwell LC, Rowe EJ, Arnson K, Jackson F, Froment A, Ndumbe P, et al. Worldwide distribution of allelic variation at the progesterone receptor locus and the incidence of female reproductive cancers. Am J Hum Biol. 2012;24:42–51. doi: 10.1002/ajhb.21233. [DOI] [PubMed] [Google Scholar]
- 33.Romano A, Delvoux B, Fischer DC, Groothuis P. The PROGINS polymorphism of the human progesterone receptor diminishes the response to progesterone. J Mol Endocrinol. 2007;38:331–350. doi: 10.1677/jme.1.02170. [DOI] [PubMed] [Google Scholar]
- 34.Schweikert A, Rau T, Berkholz A, Allera A, Daufeldt S, Wildt L. Association of progesterone receptor polymorphism with recurrent abortions. Eur J Obstet Gynecol Reprod Biol. 2004;113:67–72. doi: 10.1016/j.ejogrb.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Sen S, Pasi AR, Dada R, Shamsi MB, Modi D. Y chromosome microdeletions in infertile men: prevalence, phenotypes and screening markers for the Indian population. J Assist Reprod Genet. 2013;30:413–422. doi: 10.1007/s10815-013-9933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sendler E, Johnson GD, Mao S, Goodrich RJ, Diamond MP, Hauser R et al. Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic Acids Res. 2013;41:4104–17. [DOI] [PMC free article] [PubMed]
- 37.Shah CA, Modi D, Sachdeva G, Gadkar-Sable S, D’Souza S, Puri CP. N-terminal region of progesterone receptor B isoform in human spermatozoa. Int J Androl. 2005;28:360–371. doi: 10.1111/j.1365-2605.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- 38.Shah C, Modi D, Gadkar G, Sachdeva G, Puri C. Coexistence of intracellular and membrane bound progesterone receptor in human testis. J Clin Endocrinol Metab. 2005;90:474–483. doi: 10.1210/jc.2004-0793. [DOI] [PubMed] [Google Scholar]
- 39.Su MT, Lee IW, Chen YC, Kuo PL. Association of progesterone receptor polymorphism with idiopathic recurrent pregnancy loss in Taiwanese Han population. J Assist Reprod Genet. 2011;28:239–243. doi: 10.1007/s10815-010-9510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thangaraj K, Joshi MB, Reddy AG, Rasalkar AA, Singh L. Sperm mitochondrial mutations as a cause of low sperm motility. J Androl. 2003;24:388–392. doi: 10.1002/j.1939-4640.2003.tb02687.x. [DOI] [PubMed] [Google Scholar]
- 41.Tong D, Fabjani G, Heinze G, Obermair A, Leodolter S, Zeillinger R. Analysis of the human progesterone receptor gene polymorphism progins in Austrian ovarian carcinoma patients. Int J Cancer. 2001;95:394–397. doi: 10.1002/1097-0215(20011120)95:6<394::AID-IJC1070>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. World Health Organization, Geneva. 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 42.5 KB )
(DOC 33.5 KB)
(DOC 31.0 KB)
(DOC 33.0 KB)
(DOC 31.5 KB)