Abstract
INTRODUCTION: The purpose of this in vitro study was to assess the apical microleakage of AH26 sealer when three different root canal irrigant regimens were used.
MATERIALS AND METHODS: Eighty single-rooted human teeth were randomly divided into three experimental (n=20) and two control groups (n=10). NaOCl was used as irrigant during instrumentation, and apical patency was ensured in all teeth. Final irrigation was implemented as follow: group A- 17% EDTA + 5.25% NaOCl, Group B- 7% citric acid + 5.25% NaOCl, and group C- 20% citric acid + 5.25% NaOCl. The experimental and negative control groups were obturated by laterally condensed gutta-percha with AH26 sealer. The positive control group was obturated without sealer. The teeth were stored in 100% humidity and 37ºC for 48 hours. In the experimental groups and positive control group, the root surfaces except for the apical 2 mm were covered with nail polish and sticky wax. In the negative control group, the roots were completely covered. The samples were then immersed in 2% methylene blue dye for 72 hours at 37ºC. After that the roots were sectioned longitudinally and the dye penetration was measured. The results were statistically analyzed by One-way Variance and Post Hoc Tukey tests.
RESULTS: Statistically significant difference was found between groups (P<0.05). Group C showed the least (1.072 mm) and group A showed the most (2.072 mm) amount of dye penetration.
CONCLUSION: When a resin-based sealer is used for the obturation of the root canal system, it is better to use a citric acid irrigant instead of EDTA to remove the smear layer and to improve the apical seal.
Key Words: AH26 Sealer, Citric Acid, Dental Leakage, EDTA, Irrigation, Smear Layer
Introduction
The ultimate aim of root canal treatment is to prepare a clean, bacteria and debris-free canal for obturation. A three-dimensional seal of root canal system to prevent coronal and apical microleakage of bacteria and their by-products is an important phase in achieving good prognosis for endodontic treatments. Ingle believes most unsuccessful cases of root canal treatment are caused by percolation of fluid from inflamed periapical tissue into improperly obturated canals (1). Kakehashi et al. states that microorganisms and their by-products are the main etiological factors of pulpal and periradicular pathosis, and a successful endodontic treatment depends on the elimination of these bacteria with the prevention of future recontamination (2). Allen (3) and Strindberg (4) have shown that incomplete seal of the root canal system is one of the most important causes of long term treatment failures and they propose a fluid-tight seal for the entire root canal system.
A number of studies have shown the formation of a layer of a sludge material (smear layer) over the surfaces of instrumented root canal walls (5-6). Smear layer is an amorphous, irregular entity containing organic (pulp tissue, bacteria) and inorganic (dentin) material. The smear layer is composed of two separate layers: (I) a superficial layer which is loosely attached to the underlying dentin and (II) a deeper layer of debris compacted into the dentinal tubules called the smear plug (6). Mc Comb and Smith (5) suggest that smear layer can create a space between the inner wall of root canal and the obturating materials, thus preventing the complete locking and adherence of the root canal filling materials into the dentinal tubules. Pashley et al. believes that smear layer contains bacteria and bacterial by-products and thus must be completely removed from the root canal system (7). Haapasalo et al. suggests that removal of the smear layer can allow intracanal medicaments to penetrate the dentinal tubules for better disinfection (8).
According to Torabinejad et al. (9) smear layer is one of the factors that can adversely affect the apical and coronal microleakage compromising the long term success of the treatment. These unwanted layers of organic and inorganic materials should be removed before obturation of the root canal system. Many investigations have demonstrated that removal of smear layer causes a better adherence and penetration of sealer into the dentinal tubules preventing apical/coronal microleakage (10-15).
Resin-based sealers not only can penetrate the dentinal tubules, but also can adhere to the exposed dentin surface. A better adherence of resin-based sealers might be obtained if a larger surface area of dentin is created by the irrigation regimen. Different irrigation protocols in removing the smear layer create different dentin surface structures microscopically. Thus, one can assume that a particular surface structure of dentin is more suitable for a specific sealer. The purpose of this study was to assess the apical microleakage of AH26 sealer when three different root canal irrigant regimens (17% EDTA, 7% citric acid, and 20% citric acid) were used to remove the smear layer.
MATERIALS AND METHODS
This experimental study selected 80 freshly extracted human teeth (maxillary centrals and canines, mandibular premolars). The teeth had the following characteristics: 1) all had single canals, 2) the roots did not have fractures, caries and/or open or resorbed apices and finally 3) all roots were straight or had slight curvature (5-10 degree). In order to clean the surface of the teeth, they were placed in 2.5% NaOCl for 48 hours. All teeth were radiographically investigated to ensure that they have single canals and that there are no calcifications. To standardize the samples, the crowns of the teeth were cut at CEJ using a diamond disk so that an average length of the remaining roots was approximately 13-15 mm. Before instrumentation, the samples were randomly divided into three experimental groups A, B, and C (n=20) and two control groups (n=10). All teeth were kept in normal saline solution during the experiment. A #10 K-file (Mani, Japan) and rubber stop was used to determine the working length for each root.
All roots were instrumented up to a #40 K-file, and the coronal part was flared up to #70 K-file using step back technique. A #10 K-file was used to ensure apical patency between each file. Full strength NaOCl (5.25%) was used as irrigant during instrumentation of all canals. To remove the smear layer, the following three different final irrigation protocols were used (16-17):
Group A: 5mL of 17% EDTA (Merck, Darmstadt, Germany) for 1 min + 5mL of 5.25% NaOCl + 5mL of distilled water.
Group B: 5mL of 7% citric acid (Carlo Erba, Milan, Italy) for 1 min + 5mL of 5.25% NaOCl + 5mL of distilled water.
Group C: 5mL of 20% citric acid (Carlo Erba, Milan, Italy) for 1 min + 5 mL of 5.25% NaOCl + 5mL of distilled water.
Distilled water was used at the end of irrigation procedure to terminate the action and eliminate any precipitates from the irrigants. After completion of the final irrigation phase, the canals in groups A, B, and C were dried with paper points and then obturated laterally using #40 gutta-percha as master cone and #25 gutta- percha as accessory cones (AriaDent, Asia Chemi Teb Co., Tehran, Iran). AH26 (Dentsply, DeTrey, Konstanz, Germany) was used as sealer in this experiment. In the positive control group, the canals were dried and obturated without sealer, and in the negative control group, the canals were dried and obturated with gutta- percha and sealer.
The coronal 3 mm of the canals in all groups were sealed with Cavisol (Golchai, Tehran, Iran) temporary filling. To ensure setting of the sealer in the experimental groups, the samples were kept in 100% humidity with 37ºC temperature for 48 hours. Then all surfaces of the teeth except for the 2-3 mm of apical root were sealed using two coats of nail polish and one coat of sticky wax (experimental and positive control groups). In negative control group, all surfaces of the teeth including the 2-3 mm of apical root were completely sealed with nail polish and sticky wax.
The teeth in the three experimental groups and two control groups were placed in 2% methylene blue dye and kept in incubator for 72 hours. After removing the samples from the incubator, they were thoroughly washed with water and the nail polish and sticky wax were removed from the surfaces. A diamond disk was used to make buccal and lingual grooves on the root surfaces; using a spatula the roots were separated into two parts and the gutta-percha and filling materials were removed from the canals. The linear dye penetration (maximum point) was measured in one-thousandth of millimeters using a stereomicroscope (MBC-10, St. Petersburg, Russia) and with the help of a computer software (Motic Images Plus 2.0 ML, Motic Instrument Inc., Canada). The data collected were statistically analyzed by One-way Variance and Post Hoc Tukey tests.
Results
In the positive control group, the dye leaked throughout the canal in all samples. In the negative control group, penetration of dye was not observed in any of the samples. The maximum mean dye penetration was in group A with 2.072 mm and the minimum mean dye penetration was for group C with 1.072 mm (Table 1). The difference in mean linear dye leakage between groups "A and B" and "A and C" was statistically significant (P=0.012 and P=0.002 respectively), but not significant between groups "B and C" (P=0.792).
Table 1.
Mean dye leakage (mm) in experimental groups
| Experimental Groups (n=20) | Mean (SD) |
|---|---|
| A-17% EDTA | 2.072 (0.9971) |
| B-7% citric acid | 1.252 (0.7205) |
| C-20% citric acid | 1.072 (0.8852) |
Discussion
A three dimensional obturation and complete coronal and apical seal is one of the important aims of root canal treatment. Since microorganisms may remain in the root canal system after instrumentation, a tight apical seal is desired to prevent bacteria and their by- products from invading the apex. A perfect apical seal is also desired to prevent apical percolation.
In this regard, smear layer is one of the factors that can affect coronal and apical microleakage and thus compromise the long term success of endodontic treatment.
Since the smear layer prevents the complete locking and adherence of the root canal filling materials to the dentinal wall, many studies recommend the removal of this layer before the obturation phase of the treatment (5,9-15). Farhad and Elahi showed that Roth's 801 sealer had a significantly better apical seal when the smear layer was removed (13). Economides et al. indicated that resin-based sealers have superior apical sealing ability with the removal of smear layer (14). In a coronal bacterial leakage study, Farhad et al. demonstrated that AH26, Roth's 801, and pure ZOE sealer have better coronal seal against bacterial penetration when the smear layer was removed, and the best results were obtained when AH26 sealer was coupled with the removal of smear layer (12). The advantage of resin-based sealers like AH26 over ZOE-based sealers is that they can not only lock into open dentinal tubules but also adhere to the exposed dentinal surfaces. This characteristic of resin-based sealers is similar to the adherence capacity of composites to the dentin and enamel of teeth.
Different irrigation protocols have been introduced to remove the smear layer during root canal treatment. These irrigation regimens can create dentinal surfaces which are very different structurally. The ideal purpose is to create a particular surface of dentin which is more suitable for the specific sealer used in the obturation of the root canal system. The hypothesis is that if a dentinal surface and a sealer can complete and complement each other characteristically, ultimately they can produce a better hermetic coronal and apical seal. Considering the qualities of AH26, if the surface area of dentin exposed to this sealer is increased, the adhering and penetrating capacity of AH26 is improved and better seal is expected. In this regard, irrigation solutions which cause more erosion of dentinal wall and create a porous etched surface would be a reasonable choice. Khademi et al. demonstrated that 20% citric acid produces more erosion of dentin compared to 7% citric acid and 17% EDTA (16). Reis et al. showed that the chelating effect of 1, 5, and 10% citric acid is significantly more than 17% EDTA (18). Machado-Silveiro et al. in their study indicated that the decalcifying effect of 10% citric acid on dentin is more than 17% EDTA (19). Dilenarda et al. concluded that 1mol/L citric acid is more effective than 15% EDTA in removing the smear layer (20). On the other hand, Khedmat and Shokohinejad showed that there was no statistical difference between 7% citric acid and 17% EDTA for removal of smear layer (21). Also, Yamada et al. indicated that 17% EDTA is more effective in removing the smear layer than 25% citric acid (22). The factors that may be considered in obtaining these conflicting results are: type of teeth used, the concentration of the irrigation solutions, the order the irrigation solutions were used, time span of irrigation, and the criteria considered by the investigators as being effective and successful. This study was designed to test the apical sealing ability of AH26 when three different irrigation protocols were used to remove the smear layer. The results of this investigation showed that AH26 sealer has significantly better apical seal when 20% or 7% citric acid was used to remove the smear layer compared to 17% EDTA. The reason for this difference is that 17% EDTA opens the dentinal tubules with minimal changes to surrounding dentin structure, but 20% and 7% citric acid cause more erosion of the dentinal wall creating more adhesion surface area for a resin-based sealer (Figure 1). The difference between 20% citric acid group and 7% citric acid group was not significant, but AH26 showed the best resistance against apical dye penetration when it was coupled with 20% citric acid irrigation regimen. The improvement in 20% citric acid group compared to 7% citric acid group could be attributed to the fact that 20% citric acid causes more etching of the dentin and thus creating a more favorable surface for AH26 to achieve a better apical seal.
Figure 1.
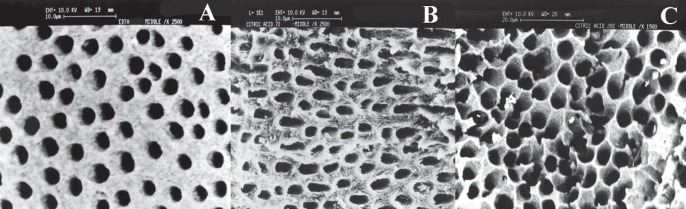
Scanning electrone microscope appearance of dentin surface after irrigation with A) 17% EDTA, B) 7% citric acid, and C) 20% citric acid
The results obtained in this study are in complete agreement with our previous investigation. In our study (17), the coronal sealing ability of two sealers (AH26 and TubliSeal) against microleakage of Entrococcus faecalis was compared using the same irrigation protocols mentioned above for removing the smear layer; it was shown that:
a) 20% and 7% citric acid groups in association with AH26 had the best resistance against coronal bacterial leakage,
b) 20% citric acid coupled with AH26 had the least coronal microleakage, and
c) 20% citric acid in association with TubliSeal had the greatest coronal microleakage.
Different methods such as electrochemical, radioisotope spectrometry, radiolabeled isotopes, bacterial leakage, and dye leakage techniques have been introduced to evaluate the apical seal. Because of its simplicity and low cost, dye leakage studies are popular tests. If the unwanted variables are eliminated and the experimental conditions are standardized, dye leakage studies can prove valid (23). Since dye molecules are much smaller than bacteria, investigations using dye penetration method may be less applicable to in vivo conditions compared to bacterial leakage techniques.
CONCLUSION
In summary, the results of this in vitro study indicate that when a resin-based sealer is used for obturation of the root canal system, it may be a reasonable and smart choice to use citric acid to remove the smear layer. Furthermore, it can be concluded that the main purpose of removing the smear layer is not only to clean the dentinal tubules from organic and inorganic debris, but also to create a dentinal surface structure that best fits the characteristics of the sealer used.
ACKNOWLEDGEMENT
This research was supported by a grant (386236) from Isfahan University of Medical Sciences. The authors wish to thank the staff of Torabinajad Research Center for their assistance with data collection. The authors also wish to thank Dr. Bahram Soleimani for his help with statistical analysis.
Conflict of Interest: ‘None declared’.
References
- 1.Ingle JI, Bakland LK. Endodontics. 5th Edition. Hamilton: BC Decker Inc; 2002. p. 470. [Google Scholar]
- 2.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germfree and conventional laboratory rats. J South Calif Dent Assoc. 1966;34:449–51. [PubMed] [Google Scholar]
- 3.Allen DE. Hermetic sealing of root canals, value in successful endodontics. Dent Radiogr photogr. 1964;37:85–7. [Google Scholar]
- 4.Strindberg LZ. The dependence of the results of pulp therapy on certain factors. Acta Odontol Scand. 1956;14:Suppl 21. [Google Scholar]
- 5.McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod. 1975;1:238–42. doi: 10.1016/S0099-2399(75)80226-3. [DOI] [PubMed] [Google Scholar]
- 6.Cameron JA. The use of ultrasonics in the removal of the smear layer: a scanning electron microscope study. J Endod. 1983;9:289–92. doi: 10.1016/S0099-2399(83)80119-8. [DOI] [PubMed] [Google Scholar]
- 7.Pashley DH, Michelich V, Kehl T. Dentin permeability: effects of smear layer removal. J Prosthet Dent. 1981;46:531–7. doi: 10.1016/0022-3913(81)90243-2. [DOI] [PubMed] [Google Scholar]
- 8.Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66:1375–9. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 9.Torabinejad M, Cho Y, Khademi AA, Bakland LK, Shabahang S. The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. J Endod. 2003;29:233–9. doi: 10.1097/00004770-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy WA, Walker WA 3rd, Gough RW. Smear layer removal effects on apical leakage. J Endod. 1986;12:21–7. doi: 10.1016/S0099-2399(86)80277-1. [DOI] [PubMed] [Google Scholar]
- 11.Oksan T, Aktener BO, Sen BH, Tezel H. The penetration of root canal sealers into dentinal tubules. A scanning electron microscopic study. Int Endod J. 1993;26:301–5. doi: 10.1111/j.1365-2591.1993.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 12.Farhad AR, Havaei A, Khajali N, Karimi F, Poorsina F. Assesment of sealing ability of three sealers (AH26, Roth801, Pure ZOE paste) in presence or absence of smear layer. Res Med J. 2002;7:233–7. [Google Scholar]
- 13.Farhad AR, Elahi T. The effect of smear layer on apical seal of endodontically treated teeth. J of Research in Medical Scinces. 2004;3:28–31. [Google Scholar]
- 14.Economides N, Kokorikos I, Kolokouris I, Panagiotis B, Gogos C. Comparative study of apical sealing ability of a new resin-based root canal sealer. J Endod. 2004;30:403–5. doi: 10.1097/00004770-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Cobankara FK, Adanr N, Belli S. Evaluation of the influence of smear layer on the apical and coronal sealing ability of two sealers. J Endod. 2004;30:406–9. doi: 10.1097/00004770-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Khademi AA, Feizianfard M. The effect of EDTA and citric acid on smear layer removal in mesial canals of first mandibular molars: A scanning electron microscopic study. J of Research in Medical Sciences. 2004;9:27–35. [Google Scholar]
- 17.Farhad AR, Havaei A, Barekatain B, Narimani T. Comparing the bacterial leakage in endodontic therapy following using EDTA and citric acid as irrigation and AH26 or Tubliseal as sealers. J of Mashhad Dental School. 2007;31:83–92. [Google Scholar]
- 18.Reis C, De-Deus G, Leal F, Azevedo E, Coutinho- Filho T, Paciornik S. Strong effect on dentin after the use of high concentrations of citric acid: An assessment with co-site optical microscopy and ESEM. Dent Mater. 2008;24:1608–15. doi: 10.1016/j.dental.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Machado-Silveiro LF, Gonzalez-Lopez S, Gonzalez-Rodriguez MP. Decalcification of root canal dentine by citric acid, EDTA and sodium citrate. Int Endod J. 2004;37:365–9. doi: 10.1111/j.1365-2591.2004.00813.x. [DOI] [PubMed] [Google Scholar]
- 20.Di Lenarda R, Cadenaro M, Sbaizero O. Effectiveness of 1 mol L-1 citric acid and 15% EDTA irrigation on smear layer removal. Int Endod J. 2000;33:46–52. doi: 10.1046/j.1365-2591.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- 21.Khedmat S, Shokouhinejad N. Comparison of the efficacy of three chelating agents in smear layer removal. J Endod. 2008;34:599–602. doi: 10.1016/j.joen.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: Part 3. J Endod. 1983;9:137–42. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- 23.Roig M, Ribot J, Jane L, Canalda C. Estudio de la filtracion apical de cuatro cementos de obturacion. Endodoncia. 1996;14:21–7. [Google Scholar]


