Abstract
Objective. To assess the prevalence, clinical and laboratory characteristics, and short-term outcomes of poststroke hip fracture (HF). Methods. A cross-sectional study of 761 consecutive patients aged ≥60 years (82.3 ± 8.8 years; 75% females) with osteoporotic HF. Results. The prevalence of poststroke HF was 13.1% occurring on average 2.4 years after the stroke. The poststroke group compared to the rest of the cohort had a higher proportion of women, subjects with dementia, history of TIA, hypertension, coronary artery disease, secondary hyperparathyroidism, higher serum vitamin B12 levels (>350 pmol/L), walking aid users, and living in residential care facilities. The majority of poststroke HF patients had vitamin D insufficiency (68%) and excess bone resorption (90%). This group had a 3-fold higher incidence of postoperative myocardial injury and need for institutionalisation. In multivariate analysis, independent indicators of poststroke HF were female sex (OR 3.6), history of TIA (OR 5.2), dementia (OR 4.1), hypertension (OR 3.2), use of walking aid (OR 2.5), and higher vitamin B12 level (OR 2.3). Only 15% of poststroke patients received antiosteoporotic therapy prior to HF. Conclusions. Approximately one in seven HFs occurs in older stroke survivors and are associated with poorer outcomes. Early implementation of fracture prevention strategies is needed.
1. Introduction
Stroke and osteoporotic hip fracture (HF) are two highly prevalent conditions among the elderly, both with poor clinical outcomes and high medical and social costs. Worldwide the prevalence of these events is expected to increase dramatically because of rapid population ageing. Osteoporosis and low-impact trauma are the main determinants of HF in the elderly. Stroke increases the risk of falls [1–5] as a result of impaired locomotor function and muscle weakness [6, 7], accelerates bone loss (especially in the hemiplegic leg) [8–26], and subsequently leads to fractures [4, 27–29]. Although accumulating evidence suggests bidirectional links between vascular diseases, including stroke, and osteoporotic fractures [9–11, 30–35], there is still considerable uncertainty regarding the contribution of stroke to osteoporotic HF.
The incidence of HF after a stroke was reported to be 1.5–4 times [27, 29, 36, 37] or even >7 times [28] higher than in the general population, but this was not observed in other studies [38–40]. Similarly, the reported prevalence of previous stroke among patients with HF ranges from 3% to 38.5% [4, 41–43].
Data concerning clinical characteristics, outcomes, prediction, and prevention of poststroke HF are also inconsistent [3, 35, 41, 44, 45]. Most previous studies have examined only a small number of the many clinical and pathogenic factors involved in stroke and HF. The exact aetiology and pathogenesis of poststroke osteoporosis remain not fully understood. Studies have generally focused on bone mineral density (BMD) and documented accelerated bone loss, especially in paretic limbs [10, 12–14, 22], with only few analysing mineral and bone metabolism in poststroke patients [15, 21, 46, 47]. Fracture prevention and osteoporosis care are not included in current guidelines for stroke management.
The aims of this study were (1) to investigate the prevalence of poststroke HF, (2) to compare in HF patients with and without a previous stroke the demographic, clinical, and laboratory characteristics and short-term outcomes, (3) to test in both groups the associations of parameters related to mineral and bone metabolism, and (4) to determine whether any clinical or laboratory characteristics are indicative/predictive for a poststroke HF.
2. Materials and Methods
2.1. Study Patients
A total of 847 consecutive patients aged 60 years and older who were admitted to the Canberra Hospital with low-trauma HF and underwent surgery were investigated. After the exclusion of 86 patients with pathological HF (metastatic bone cancer, multiple myeloma, and Paget's disease) or primary hyperparathyroidism, a total of 761 patients (570 women and 191 men) were included in the analysis. All patients or their guardians gave informed consent to undergo examination and surgical treatment. The study was conducted according to the Helsinki Declaration and approved by the regional Human Research Ethics Committee.
2.2. Clinical Data Collection
The data were collected prospectively: demographic, medical, lifestyle, and residential characteristics, and in-hospital management, peri-operative complications and short-term outcomes were recorded. In all patients a detailed medical history and full physical examination were performed. The presence of comorbidities was identified based on clinical manifestations, review of hospital medical records, general physician's progress notes/letters, and medication used. Data on a history of previous stroke and transient ischaemic attack (TIA) were initially abstracted from the medical records. A patient was included in the group with a history of stroke if he met the following criteria: (1) presence of neurological deficit consistent with a stroke and/or (2) medical documentation confirming a stroke before the HF.
Short-term outcome measures included (1) postoperative myocardial injury as defined by cardiac troponin I rise (cTnI > 0.06 μg/L), (2) prolonged length of stay (LOS ≥ 20 days), (3) discharge to a permanent residential care facility (RCF) for patients who were admitted from home, and (4) all-cause in-hospital death.
2.3. Sample Collection and Methods of Laboratory Analyses
In each patient venous blood and second morning urine samples were collected under standardised conditions after a 12-hour overnight fast usually within 24 hours after arrival at the emergency department. After centrifugation of blood, one serum sample as well as the urine sample was frozen and stored at −70°C until further analysis. Routine haematological and biochemical assessments were performed by standardized methods on autoanalyzers at the day of collection.
All patients had the following tests performed: complete blood count, urea, creatinine and electrolytes, liver function tests, fasting blood glucose (and HbA1C in diabetic patients), thyroid function tests (TSH, T4, and T3 if indicated), C-reactive protein (CRP), and cTnI (two-step chemiluminescent microparticle immunoassay, Chemiflex, Abbott Labs, Mississauga, Ontario, Canada). Glomerular filtration rate (eGFR) was calculated using a standardized serum creatinine-based formula normalized to a body surface area of 1.73 m² [48]. Full blood count, urea, creatinine and electrolytes, serum cTnI, and CRP were also assessed within 24 hours postoperatively and then after if clinically indicated.
In order to evaluate mineral and bone metabolism in all patients, the following biochemical parameters were determined: serum concentrations of 25(OH) vitamin D (25(OH)D), intact PTH, total calcium, phosphate, magnesium, osteocalcin (OC), and bone-specific alkaline phosphatise (BAP) as markers of bone formation and urinary concentrations of deoxypyridinoline (DPD/Cr), and cross-linked N-telopeptide of type 1 collagen (NTx/Cr) as markers of bone resorption (both corrected for urinary creatinine concentrations in the same sample). Serum 25(OH)D was measured by radioimmunoassay kit (Dia Sorin, Stillwater, MN, USA; sensitivity 0.7 nmol/L) and intact PTH by two-site chemiluminescent enzyme-linked immunoassay on DPC Immulite 2000 (Diagnostic Products Corp, Los Angeles, CA, USA; sensitivity 0.07 pmol/L). Serum OC was determined by electrochemiluminescent immunoassay (Elecsys 1010, Roche Diagnostics, Ltd. Corp., IN, USA), serum BAP by Metra BAP ELISA (Quidel Corp., San Diego, CA, USA), urinary NTx by ELISA (Wampole Labs, Princeton, NJ, USA), and urinary DPD by 2-site chemiluminescent enzyme-labelled immunoassay (DPC Immulite 2000, Diagnostic Products, Los Angeles, CA, USA). All samples were analysed with commercially available kits of the same lot number according to the manufacturer's protocol and blind to any clinical information. In these methods both the intra- and interassay coefficient of variations (CVs) ranged from 2.1% to 12.7%. Serum calcium concentrations were corrected for serum albumin.
For the analyses, insufficiency of vitamin D was defined as 25(OH)D < 50 nmol/L and deficiency as 25(OH)D < 25 nmol/L based on current recommendations. Secondary hyperparathyroidism (SHPT) was defined as elevated serum PTH (>6.8 pmol/L, the upper limit of the laboratory reference range). For levels of bone turnover markers, we used the standard laboratory reference ranges and data provided by the manufacturer. In the analyses chronic kidney disease (CKD) was defined as eGFR < 60 mL/min/1.73 m² (CKD stage ≥ 3). To express the balance between bone formation and resorption, the data of biochemical bone turnover markers were evaluated as Z-scores, and a bone balance status index (BSI) was calculated [49, 50]. The formula for Z-scores was Z = (x i − X)/SD, where, x i was the patient's value of a marker and X and SD were the normal mean and standard deviations for this parameter, respectively. For BSI the formula was BSI = (Z-scores of OC + BAP)/2 − (Z-scores of DPD/Cr + NTx/Cr)/2.
2.4. Statistical Analyses
Descriptive statistics included means and standard deviations (SD) for continuous variables or percentages for categorical variables. Comparisons between groups were performed using analysis of variance and Student's t-test (for continuous variables) and χ 2 test (for categorical variables). Pearson's correlation coefficient with log-transformed data (to achieve normal distribution) was used to study the correlation between variables; Bonferroni and Sidak adjustments for multiplicity have been performed. Univariate and multivariate linear regression analyses were performed to determine the associations between different parameters related to mineral and bone metabolism in patients with and without history of stroke; all potential confounding variables (demographic, clinical, and biochemical) with statistical significance ≤0.20 on univariate analyses were included in multivariate analysis. The relationships between presence of poststoke HF and clinical characteristics as well as different laboratory parameters as independent variables were investigated by linear regression analyses. To quantify the significance of multicollinearity phenomena in regression analysis, the variance inflation factor was calculated. Two-sided P < 0.05 values were considered statistically significant. The Stata software version 10 (StataCorp, College Station, TX, USA) was used for all statistical analyses.
3. Results
3.1. Prevalence and General Clinical Characteristics of Patients with Poststroke Hip Fracture
Of 761 older HF patients, 100 (13.1%) had a history of stroke, of whom 83.0% were women. The clinical characteristics of the patients according to stroke history are summarized in Table 1. HF occurred on average 2.4 ± 2.6 years after the stroke, in 68.0% on the hemiplegic side. Among poststroke HF patients, the proportion of females and individuals admitted from long-term residential care facilities (RCF) was significantly higher compared to the rest of the cohort. The predominance of women was not age related, as the age of women and men with poststroke HF did not differ, whereas in the nonstroke group females were significantly older (+2.1 year, P = 0.002). As would be expected, the group of poststroke HF patients has a higher prevalence of previous TIAs, dementia, hypertension, coronary artery disease (CAD), and myocardial infarction, American Society of Anaesthesiologists score ≥3 on admission, and using a walking device; atrial fibrillation was also more often among the poststroke patients, but the difference did not reach statistical significance. History of stroke was inversely related with male gender (Pearson correlation coefficient r = −0.151; P = 0.011) and positively associated with hypertension (r = 0.186, P = 0.002) and CAD (r = 0.128, P = 0.033). The two groups did not differ with regard to mean age, HF type, prevalence of diabetes mellitus, chronic obstructive pulmonary disease, Parkinson's disease, chronic kidney disease, alcohol consumption, and smoking habits.
Table 1.
Sociodemographic and clinical characteristics in older hip fracture patients with and without history of stroke.
| Characteristics | HF poststroke (n = 100) | HF without history of stroke (n = 661) | P value |
|---|---|---|---|
| Age, years (mean ± SD) | 84.1 ± 7.72 | 81.9 ± 7.96 | 0.120 |
| Age, females, years (mean ± SD) | 84.1 ± 8.03 | 82.5 ± 7.59 | 0.264 |
| Age, males, years (mean ± SD) | 83.3 ± 4.92 | 80.4 ± 8.62 | 0.521 |
| Females, % | 83.0 | 70.0 | 0.011 |
| Admitted from long-term RCF, % | 50.0 | 26.9 | 0.004 |
| Cervical/trochanteric HF, n | 55/45 | 346/315 | 0.698 |
| Coronary artery disease, % | 35.0 | 20.9 | 0.002 |
| Previous myocardial infarction, % | 10.0 | 4.6 | 0.024 |
| Hypertension, % | 60.0 | 42.1 | 0.001 |
| Atrial fibrillation, % | 18.0 | 12.5 | 0.092 |
| TIA, % | 16.0 | 6.1 | 0.001 |
| Dementia, % | 47.0 | 26.4 | 0.001 |
| Diabetes mellitus, % | 19.0 | 16.8 | 0.479 |
| COPD, % | 13.0 | 11.1 | 0.583 |
| Parkinson's disease, % | 3.0 | 4.6 | 0.473 |
| eGFR < 60 mL/min/1.73 m², % | 47.0 | 43.4 | 0.650 |
| eGFR < 30 mL/min/1.73 m², % | 7.9 | 4.5 | 0.371 |
| ASA score ≥3, % | 96.9 | 68.2 | 0.001 |
| Current Smoker, % | 6.0 | 5.3 | 0.785 |
| Ex-smoker, % | 12.0 | 9.1 | 0.365 |
| *Alcohol overuser, % | 5.0 | 5.6 | 0.795 |
| User of walking device, % | 50.0 | 32.2 | 0.001 |
HF: hip fracture; RCF: residential care facility; TIA: transient ischaemic attack; eGFR: estimated glomerular filtration rate; ASA: American Society of Anaesthesiologists; COPD: chronic obstructive pulmonary disease. *Three or more times per week.
Among 24 haematological and biochemical variables, including parameters of iron metabolism, vitamin B12, folic acid, liver, renal and thyroid functions, only mean values of leukocyte count and serum concentration of vitamin B12 were higher, and the mean corpuscular volume was lower in poststroke HF patients compared to the rest of the cohort (Table 2).
Table 2.
Haematologic, renal, liver, and thyroid parameters in older hip fracture patients with and without history of stroke.
| Characteristics | HF poststroke | HF without history of stroke | P value |
|---|---|---|---|
| Erythrocyte count, ×1012/L | 4.21 ± 0.61 | 4.08 ± 0.61 | 0.217 |
| Haemoglobin, g/L | 126.0 ± 18.9 | 124.7 ± 16.9 | 0.601 |
| Leukocyte count, ×109/L | 12.0 ± 4.22 | 10.5 ± 4.24 | 0.034 |
| Lymphocyte count, ×109/L | 1.24 ± 1.39 | 1.31 ± 1.22 | 0.742 |
| MCV, fl | 87.4 ± 16.5 | 90.8 ± 5.98 | 0.019 |
| MCH, pg/cell | 30.3 ± 3.35 | 30.7 ± 2.29 | 0.290 |
| MCHC, g/L | 338.0 ± 19.4 | 340.7 ± 37.1 | 0.660 |
| RDW, % | 15.1 ± 2.05 | 15.2 ± 9.53 | 0.942 |
| Iron, μmol/L | 4.81 ± 4.12 | 5.33 ± 4.39 | 0.507 |
| Transferrin, g/L | 1.68 ± 0.49 | 1.72 ± 0.48 | 0.582 |
| Transferrin saturation, % | 11.08 ± 10.07 | 11.65 ± 8.36 | 0.716 |
| Ferritin, mg/L | 313.1 ± 244.9 | 298.9 ± 283.3 | 0.778 |
| Vitamin B12, pmol/L | 482.6 ± 321.0 | 376.0 ± 256.0 | 0.027 |
| Folic acid (serum), nmol/L | 25.2 ± 16.7 | 26.1 ± 15.4 | 0.747 |
| Urea, mmol/L | 7.9 ± 4.30 | 7.8 ± 3.67 | 08.37 |
| Creatinine, mmol/L | 92.0 ± 43.86 | 93.6 ± 57.24 | 0.873 |
| eGFR, mL/min/1.73 m2 | 62.5 ± 25.2 | 64.9 ± 23.2 | 0.561 |
| Albumin, g/L | 36.6 ± 6.0 | 37.0 ± 6.4 | 0.723 |
| Bilirubin, μmol/L | 13.0 ± 5.95 | 12.2 ± 7.62 | 0.503 |
| ALT, U/L | 20.4 ± 12.8 | 21.4 ± 22.3 | 0.801 |
| ALP, U/L | 103.8 ± 44.3 | 105.9 ± 85.8 | 0.882 |
| GGT, U/L | 55.8 ± 90.5 | 54.3 ± 98.6 | 0.928 |
| TSH, µU/L | 1.25 ± 1.47 | 1.60 ± 2.31 | 0.361 |
| T4, pmol/L | 16.02 ± 3.61 | 15.98 ± 3.54 | 0.949 |
| CRP, mg/L | 137.1 ± 76.7 | 129.2 ± 83.1 | 0.583 |
The values are expressed as mean ± standard deviation (SD). HF: hip fracture; MCV: mean corpuscular volume; MCH: mean corpuscular haemoglobin; MCHC: mean corpuscular haemoglobin concentration; RDW: red-cell distribution width; eGFR: estimated glomerular filtration rate; ALT: alanine aminotransferase; ALP: alkaline phosphatase; GGT: gamma glutamyltransferase; TSH: thyroid-stimulating hormone; T4: total thyroxin.
The prevalence of anaemia (haemoglobin < 120 g/L: 32.0% versus 36.7%, P = 0.456), renal impairment (eGFR < 60 mL/min/1.73 m2: 47.0% versus 4.3.4%, P = 0.650), hypoalbuminemia (<33 g/L: 32.0% versus 27.9%, P = 0.637), low total lymphocyte count (<12 × 109: 40.0% versus 44.7%, P = 0.548), low transferrin (<1.5 g/L: 33.0% versus 25.9%, P = 0.356), and low vitamin B12 levels (<250 pmol/L: 33.0% versus 40.8%, P = 0.395) was similar in both groups. In other wards, about one-third of HF patients with or without history of stroke were anaemic and undernourished, and nearly half have renal impairment (CKD stage ≥ 3).
On the other hand, the proportion of subjects with higher vitamin B12 levels (>350 pmol/L) was significantly higher among the poststroke HF patients (60.0% versus 39.4%, P = 0.032), although none of the patients had solid or haematological malignancies, serious liver diseases, or received parenteral nutrition, which might have been associated with elevated vitamin B12 serum levels [51–53].
3.2. Mineral and Bone Metabolism in Poststroke Hip Fracture Patients
History of stroke was associated with higher levels of serum PTH (Pearson correlation coefficient r = 0.155, P = 0.010), vitamin B12 (r = 0.139, P = 0.027), urinary bone resorption marker DPD/Cr (r = 0.183, P = 0.004), and lower BSI (r = −0.170, P = 0.009). In the poststroke group compared to the rest of the cohort (Table 3), the mean serum PTH levels (+34.3%) and the incidence of SPTH (+15.8%) were significantly higher, although the mean serum 25(OH)D concentrations were similar, and the incidence of vitamin D insufficiency (<50 nmol/L) was even slightly lower (−13.7%, P = 0.044). Interestingly, among the poststroke patients 56.0% of those with 25(OH)D < 50 nmol/L had SHPT, whereas in the nonstroke group elevated PTH was observed only in 36.0% of subjects with vitamin D insufficiency (P = 0.007, χ 2 test), indicating that in the former group in addition to low vitamin D levels other mechanisms contribute to the excess of PTH. In an age- and sex-adjusted model, serum PTH levels were significantly associated with history of stroke (OR = 1.058, 95%CI 1.00–1.11, P = 0.025) indicating that risk for poststroke HF relative to 1 pmol/L increase in PTH increases by 5.8%. Of note, the proportion of subjects with SHPT among HF patients without CVD was 23.2%. The odds ratio (adjusted for sex, age, dementia, 25(OH)D < 50 nmol/L, and DPD/Cr > 7.5 nmol/μmol) for SHPT in the poststroke HF subjects compared to the later group was 4.34 (95%CI 1.63–11.52, P = 0.003).
Table 3.
Parameters of mineral and bone metabolism in older hip fracture patients with and without history of stroke.
| Parameter | Poststroke HF | Without stroke HF | P value |
|---|---|---|---|
| Serum calcium*, mmol/L | 2.27 ± 0.12 | 2.28 ± 0.13 | 0.730 |
| Serum phosphate, mmol/L | 0.89 ± 0.30 | 0.96 ± 0.50 | 0.428 |
| Serum magnesium, mmol/L | 0.77 ± 0.12 | 0.78 ± 0.13 | 0.887 |
| 25(OH)vitamin D, nmol/L | 40.1 ± 20.05 | 36.8 ± 17.97 | 0.319 |
| PTH, pmol/L | 9.0 ± 6.66 | 7.6 ± 5.41 | 0.019 |
| Osteocalcin, ng/L | 13.8 ± 8.44 | 18.0 ± 16.18 | 0.025 |
| BAP, IU/L | 25.5 ± 9.76 | 26.8 ± 14.97 | 0.624 |
| Osteocalcin/BAP ratio | 0.59 ± 0.37 | 0.78 ± 0.78 | 0.026 |
| Urinary DPD/Cr, nmol/μmol | 16.0 ± 14.02 | 12.0 ± 5.53 | 0.004 |
| Urinary NTx/Cr, nmol/μmol | 207.9 ± 279.0 | 149.7 ± 141.3 | 0.056 |
| BSI | −12.5 ± 14.8 | −8.6 ± 6.1 | 0.009 |
| PTH > 6.8 pmol/L, % | 50.0 | 34.2 | 0.046 |
| 25(OH)D < 50 nmoL/L, % | 68.0 | 81.7 | 0.044 |
| 25(OH) vitamin D < 25 nmol/L, % | 27.0 | 31.0 | 0.626 |
| Osteocalcin < 14.0 ng/L, % | 57.0 | 51.9 | 0.582 |
| BAP < 14.0 IU/L, % | 11.0 | 9.3 | 0.734 |
| Urinary DPD/Cr > 7.5 nmol/μmol,% | 90.0 | 81.9 | 0.189 |
| Urinary NTx/Cr > 65 nmol/μmol, % | 78.0 | 71.6 | 0.444 |
The values are expressed as mean ± standard deviation (SD) or percentage.
*Adjusted for serum albumin. PTH: parathyroid hormone; BAP: bone specific alkaline phosphate; DPD/Cr: deoxypyridinoline corrected for urinary creatinine concentration; NTx/Cr: cross-linked N-telopeptide of type 1 collagen corrected for urinary creatinine concentration; BSI: bone balance status index.
The mean serum concentrations of calcium (albumin corrected), phosphate, and magnesium were within normal ranges and similar in both groups. Regarding bone turnover status, compared to the rest of the cohort, patients with poststroke HF demonstrated a lower mean serum level of osteocalcin and higher mean levels of urinary bone resorption markers DPD/Cr (P = 0.004) and NTx/Cr (P = 0.056) resulting in a significantly higher negative BSI (resorption exceeds formation). The mean serum OC/BAP ratio, an indicator of osteoblastic differentiation [54], in the poststroke HF patients was also significantly lower. DPD/Cr was elevated (>7.5 nmol/μmol) in 90.0.0% of poststroke patients, in 81.9% of the total nonstroke group and in 74.8% of the non-CVD group. NTx/Cr was elevated (>65 nmol/μmol) in 78.0%, 71.6%, and 70.4%, respectively; osteocalcin was below the low limit of normal range (<14 ng/mL) in 57.0%, 51.9%, and 50.1%, respectively. In multiple regression analyses adjusted for age, sex, HF type, 25(OH)D, PTH, eGFR, calcium, phosphate, and magnesium, history of stroke was an independent determinant of both higher DPD/Cr levels (β = 3.87, 95%CI 1.11–6.64, P = 0.006) and lower BSI (β = −4.17, 95%CI −7.29–1.06, P = 0.027).
In the total cohort, higher B12 levels (>350 pmol/L) were associated with significantly lower mean serum OC/BAP ratio (0.63 ± 0.45 versus 0.78 ± 0.65, P = 0.036) and serum albumin concentrations (36.0 ± 6.5 versus 38.2 ± 5.7 g/L, P = 0.003). Poststroke patients with higher vitamin B12 levels had significantly higher BAP levels (28.4 ± 10.9 versus 21.0 ± 6.0 IU/L, P = 0.028). Compared to nonstroke subjects, the poststroke group with higher vitamin B12 levels had a lower OC/BAP ratio (0.54 ± 0.36 versus 0.65 ± 0.47, P = 0.025).
3.3. Metabolic Relationships in Hip Fracture Patients with and without History of Stroke
The relationships between parameters of mineral and bone metabolism in HF patients with and without history of stroke were further analysed by Pearson correlation coefficients, and the results are displayed in Table 4. These demonstrate similarities as well as remarkable differences between the groups. In both groups, there was a significant negative correlation between log-transformed serum PTH levels and calcium, calcium correlated positively with osteocalcin, serum phosphate levels were associated positively with urinary DPD/Cr and negatively with eGFR, BAP correlated with vitamin B12, osteocalcin correlated negatively with eGFR, which inversely correlated with phosphate and age, the two urinary resorption markers were significantly interrelated, and both strongly correlated to BSI. However, only in poststroke HF patients, serum phosphate levels correlated positively with serum calcium, osteocalcin, and urinary NTx/Cr and negatively with BSI, and PTH correlated with vitamin B12 levels. No association was observed between 25(OH)D concentrations and any of bone turnover markers and BSI in the poststroke group, while in the nonstroke group 25(OH)D inversely correlated with each of the four bone markers and positively with BSI (all P < 0.03, not shown).
Table 4.
Pearson correlation coefficients among selected parameters of mineral and bone metabolism and their relation to age and renal function in older hip fracture (HF) patients with and without history of prefracture stroke.
| Poststroke HF | HF without history of stroke | |||
|---|---|---|---|---|
| r | P | r | P | |
| Calcium-PTH | −0.328 | 0.047* | −0.273 | <0.001 |
| Calcium-osteocalcin | 0.3226 | 0.052* | 0.139 | 0.034* |
| Calcium-BAP | 0.137 | 0.434 | 0.16 | 0.015 |
| Osteocalcin-eGFR | −0.525 | 0.001 | −0.346 | <0.001 |
| Calcium-eGFR | −0.464 | 0.004 | −0.075 | 0.250 |
| DPD/Cr-NTx/Cr | 0.779 | <0.001 | 0.380 | <0.001 |
| DPD/Cr-BSI | −0.926 | <0.001 | −0.690 | <0.001 |
| NTx/Cr-BSI | −0.952 | <0.001 | −0.876 | <0.001 |
| Age-eGFR | −0.443 | 0.005 | −0.337 | <0.001 |
| Phosphate-calcium | 0.415 | 0.011 | 0.055 | 0.399 |
| Phosphate-DPD/Cr | 0.580 | 0.001 | 0.208 | 0.003 |
| Phosphate-osteocalcin | 0.489 | 0.002 | 0.123 | 0.061 |
| Phosphate-BAP | 0.215 | 0.216 | 0.169 | 0.01 |
| Phosphate-NTx/Cr | 0.478 | 0.006 | 0.079 | 0.263 |
| Phosphate-BSI | −0.531 | 0.003 | −0.109 | 0.126 |
| Phosphate-eGFR | −0.32 | 0.054* | −0.173 | 0.008 |
| PTH-vitamin B12 | 0.365 | 0.028* | 0.016 | 0.817 |
| BAP-vitamin B12 | 0.442 | 0.009 | 0.188 | 0.006 |
Values for all variables other than age were log transformed before analysis.
PTH: parathyroid hormone; eGFR: estimated glomerular filtration rate; DPD/Cr: urinary deoxypyridinoline corrected for urinary creatinine concentration; NTx/Cr: cross-linked N-telopeptide of type 1 collagen corrected for urinary creatinine concentration; BSI: bone balance status index. *Nonsignificant (P > 0.005) correlations when Bonferroni and Sidak adjustments for multiplicity were adopted.
3.4. Clinical and Laboratory Variables Independently Associated with History of Stroke in Older Hip Fracture Patients
In multiple regression models, poststroke HF was designated as the dependent variable and all clinical, and laboratory characteristics with P ≤ 0.20 on univariate analysis were designed as the independent variables (Table 5). A multivariate logistic regression model which included only clinical characteristics revealed that the six following variables were independent indicators for history of stroke in HF patients: female sex, history of TIA, dementia, hypertension, CAD, and use of a walking aid. The mean variance inflation factor (VIF) of 1.19 indicates that multicollinearity was not significant in this model.
Table 5.
Clinical and laboratory characteristics independently associated with poststroke hip fracture.
| Characteristic | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Female sex | 3.73 | 1.11–12.59 | 0.034 | 3.64 | 1.17–11.42 | 0.026 | 3.58 | 1.03–12.45 | 0.045 |
| TIA | 5.17 | 1.62–16.48 | 0.005 | 5.17 | 1.59–16.79 | 0.006 | |||
| Dementia | 4.16 | 1.56–11.05 | 0.004 | 4.14 | 1.79–9.59 | 0.001 | |||
| Hypertension | 3.34 | 1.37–8.14 | 0.008 | 3.20 | 1.29–7.91 | 0.012 | |||
| CAD | 2.70 | 1.03–7.07 | 0.043 | 2.41 | 0.97–5.97 | 0.057 | |||
| Use of walking aid | 2.20 | 1.03–5.09 | 0.043 | 2.47 | 1.08–5.65 | 0.032 | |||
| Vitamin B12 > 350 pmol/L | 2.35 | 1.05–5.49 | 0.039 | 2.33 | 1.03– 5.25 | 0.042 | |||
| R 2 | 0.221 | 0.126 | 0.225 | ||||||
| Mean V1F | 1.19 | 1.25 | 1.03 | ||||||
OR: odds ratio; CI: confidence interval; TIA: transient ischaemic attack; CAD: coronary artery disease; VIF: variance inflation factor.
Model 1 (clinical parameters) adjusts for age, sex, dementia, hypertension, CAD, history of myocardial infarction, TIA: atrial fibrillation, renal impairment (eGFR < 60 mL/min/1.73 m2), use of walking aid, and living in a long-term residential care facility.
Model 2 (laboratory parameters) adjusts for age, sex, vitamin D insufficiency (25(OH)D < 50 nmol/L), elevated PTH (>6.8 pmol/L), and high bone resorption markers (DPD/Cr > 7.5 mmol/μmol, NTX/Cr > 65 mmol/μmol).
Model 3 (combined clinical and laboratory parameters) adjusts for al variables in Models 1 and 2.
When the laboratory parameters were analysed as continuous variables and adjusted for age and sex, the significant independent associations with poststroke HF showed only vitamin B12 (P = 0.006), DPD/Cr (P = 0.035), and female sex (P = 0.011). In a similar model with laboratory parameters as categorical variables corrected for age and sex, higher vitamin B12 levels and female sex were the only significant independent indicators of history of stroke (Table 5, Model 2). The other variables identified in the univariate analysis dropped out of the model, presumably because of their strong correlations with these two factors.
In multiple regression analysis using both clinical and categorical laboratory characteristics as independent variables, all clinical factors as well as higher serum vitamin B12 levels retained substantial independent value for poststroke HF, except CAD which becomes borderline significant (P = 0.057). Of note, relation of higher serum vitamin B12 to poststroke HF in the final model adjusted for both clinical and laboratory confounding variables did not change. This suggests that higher levels of B12, in contrast to vitamin D insufficiency, SHPT, and excess bone resorption, contain information not captured in and complementary to clinical characteristics. The mean variance inflation factor (VIF) of 1.03 in the final model indicates that multicollinearity was not significant.
3.5. Predicting Poststroke Hip Fracture
To examine which parameters could be clinically useful for assessing the risk of poststroke HF, we evaluated the predictive properties of all variables independently associated with poststroke HF as well as of SPTH (a significant independent determinant when subjects with history of stroke are compared with patients without CVD) and living in a permanent RCF (although not an independent but a significant and practically important indicator of poststroke HF). The relative risk (RR) estimates of characteristics associated with poststroke HF are depicted in Figure 1. Among elderly HF patients, risk of having a poststroke HF is greater 3.7 times in subjects with history of TIA and in permanent RCF residents, 3.2 times higher in females, 2.9 times in patients with dementia or hypertension, 2.2 times in those with SHPT, 2.1 times in subjects with higher serum vitamin B12 (>350 pmol/L), 2 times in patients with CAD, and 1.8 times in walking aid users. The sensitivity of these variables ranges between 83% (female sex) and 16% (TIA history, RCF resident) and the specificity between 90.5% (TIA history) and 30.0% (female sex), positive predictive value between 17% (female sex)—45.2% (TIA history, SHPT, and RCF resident) and 60% (vitamin B12 > 350 pmol/L), and negative predictive value between 79.1% (SHPT) and >90% (female sex, hypertension, and vitamin B12 > 350 pmol/L).
Figure 1.
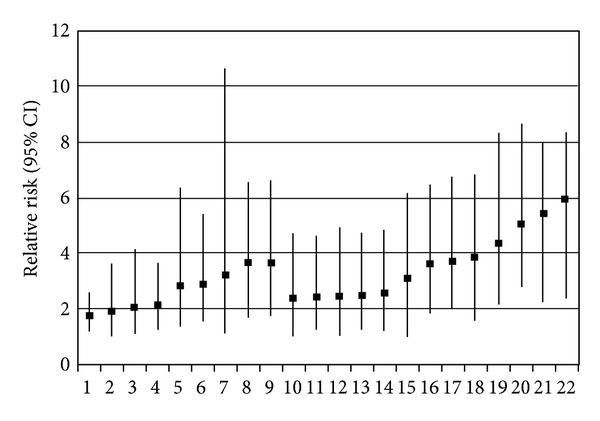
Estimated relative risk and 95% confidence intervals (CI) for poststroke hip fracture in older persons by selected clinical and laboratory characteristics. 1—Use of a walking aid; 2—coronary artery disease (CAD); 3—serum vitamin B12 > 350 pmol/L; 4—elevated serum PTH level (<6.8 pmol/L); 5—hypertension; 6—dementia; 7—female sex; 8—resident of a long-term care facility; 9—history of transient ischaemic attack; 10—CAD and serum vitamin B12 > 350 pmol/L; 11—hypertension and serum vitamin B12 > 350 pmol/L; 12—CAD and use of a walking aid; 13—CAD and hypertension; 14—use of a walking aid and elevated serum PTH level (<6.8 pmol/L); 15—transient ischaemic attack and elevated serum PTH level (<6.8 pmol/L); 16—dementia and use of a walking aid; 17—dementia and female sex; 18—transient ischaemic attack and hypertension; 19—dementia and serum vitamin B12 > 350 pmol/L; 20—dementia and hypertension; 21—transient ischaemic attack and serum vitamin B12 > 350 pmol/L; 22—transient ischaemic attack and dementia.
The probability of poststroke HF increases, understandably, when two or more factors coexist. Measurement of serum PTH and vitamin B12 may provide additional prognostic information when results are combined with selected clinical factors. The highest RR demonstrates the following combinations: dementia and TIA (RR = 5.9), TIA and higher vitamin B12 level (RR = 5.4), dementia and hypertension (RR = 5.1), dementia and higher vitamin B12 level (RR = 4.4), and TIA and SHPT (RR = 3.1). The specificity of each of the pairs of variables shown in Figure 1 is 93% or higher, and the negative predictive value is >88.0%, while the sensitivity ranges only between 13.3% and 39.5%, and the positive predictive value ranges between 40.0% and 71.4%.
3.6. Short-Term Outcomes in Poststroke Hip Fracture Patients
Data summarised in Table 6 shows that, in HF patients, history of stroke was associated with 2.7 higher incidence of postoperative myocardial injury defined as cTnI rise and being discharge to a long-term RCF but did not influence significantly the length of hospital stay (LOS) and the in-hospital mortality (5.0% versus 4.8% in subjects with and without history of stroke, resp.).
Table 6.
Effect of history of stroke on short-term outcomes in older hip fracture patients.
| Outcome | OR | 95% CI | P value |
|---|---|---|---|
| Postoperative myocardial injury* | 2.68 | 1.31–5.47 | 0.007 |
| LOS ≤ 10 days | 0.92 | 0.44–1.90 | 0.816 |
| LOS ≥ 20 days | 1.35 | 0.66–2.77 | 0.413 |
| New discharges to long-term RCF | 2.69 | 1.11–6.54 | 0.028 |
| In-hospital death | 1.07 | 0.23–5.00 | 0.927 |
Adjusted for age and sex.
*Cardiac troponin I rise (>0.06 μg/L). LOS: length of hospital stay.
In the total cohort, SHPT was an independent predictor of poor outcomes. Among the poststroke HF patients, SHPT was present in all subjects who died, in 65.8% with myocardial injury, in 71.7% with prolonged hospital stay (>20 days), and in 73.2% discharged to a long-term RCF (all P < 0.01). Higher vitamin B12 levels (>350 pmol/L) in the total cohort were also associated with in-hospital death (OR = 7.33, 95%CI 1.57–34.14, P = 0.011) and prolonged LOS (OR = 1.98, 95%CI 1.16–3.37, P = 0.012). Higher vitamin B12 levels had all poststroke HF patients who died and 71.4% with prolonged LOS (P = 0.027).
3.7. Prefracture Use of Antiosteoporotic Therapy by Poststroke Hip Fracture Patients
Compared to the rest of the cohort in the poststroke HF group, the prefracture use of a bisphosphonate (15.0% versus 8.5%) and supplementation with vitamin D (15.0% versus 9.3%) and calcium (21.0% versus 11.0%) were slightly but not significantly higher (all P > 0.1) and far from adequate.
4. Discussion
4.1. Main Findings
This cross-sectional analysis of data from an observational, prospective study demonstrates a high prevalence of poststroke in elderly subjects among HF patients (1), describes the differences in the clinical profile, mineral-bone metabolism characteristics, and short-term outcomes in poststroke compared to nonstroke HF patients (2), emphasises the prefracture underuse of antiosteoporotic therapy (3), and presents prognostic indicators of having a poststroke HF (4). While some of our findings corroborate previous work, others are novel and will be discussed in more detail.
4.2. Prevalence and Clinical Profile
We found that among 761 consecutive older patients with osteoporotic HF, the prevalence of poststroke HF was 13.1%; it occurred on average 2.4 years after the stroke and in 68% of patients on the side of hemiplegia. Our findings on stroke prevalence are consistent with most reported data showing that stroke account for 7.3% to 15.3% of all HFs [35, 41, 43, 45], but higher than 3.9% in some studies [55] and lower than 38.5% in 1997 in a Swedish study [4]. Such differences might be attributable to age (our cohort was approximately 11 years older than the Swedish study: 84.1 versus 73 years, resp.), incidence rates of stroke survivors, environmental factors, and activity levels. Our data are in agreement with observations that most HFs occurred ipsilateral to the side of hemiplegia within 2-3 years after the stroke [4, 28, 30, 35, 37]. In concordance with other studies, we did not find differences between the poststroke and nonstroke groups with regard to patient age [35, 37], type of HF [35], smoking history, alcohol consumption, diabetes, AF [27], Parkinson's disease, COPD, and renal impairment nor most (22 of 25) routine laboratory parameters.
The poststroke HF patients demonstrated a number of clinical and laboratory characteristics different from those in the rest of the cohort. The poststroke group showed a female predominance (independent of age), higher proportion of subjects living in long-term RCF, using walking aids, with dementia, having a history of TIA, hypertension, CAD, prior myocardial infarction, and ASA score ≥ 3. Among 25 laboratory parameters, higher serum vitamin B12 levels, leukocyte count, and lower mean corpuscular volume (MCV) in poststroke subjects were the only variables differing the groups. Our results are consistent with most previous reports of up to a 4-fold female predominance among poststroke HF persons [4, 25, 27, 28, 37, 45, 56], despite the age-specific incidence rates of stroke in women being lower than in men [57]. Only one study found a prevalence of men among poststroke HF patients [35]. Living in institutions [4], dementia [4], and ASA score ≥ 3 [35] is known to be associated with poststroke HF. The associations of TIA, hypertension, and CAD, established risk factors for stroke, with osteoporotic fractures [58–65] have also been documented. Our observations extend findings noted by others by providing data on a population with poststroke HF.
An unexpected finding was higher mean vitamin B12 concentrations in the poststroke HF patients, while the MCV was lower, and all other erythrocyte-related parameters were similar in both groups, suggesting the significant contribution of multiple factors (blood loss, inflammation, and malnutrition) other than vitamin B12 on erythropoiesis and haemoglobin status. Serum vitamin B12 levels were positively associated with history of stroke in crude and multivariate adjusted models irrespective of whether vitamin B12 was treated as a continuous or categorical variable (>350 pmol/L). Although some previous studies suggested that vitamin B12 deficiency by causing increased homocysteinaemia is a risk factor for osteoporosis and fragility fractures [66–71], recurrent stroke, vascular dementia, and Alzheimer's disease, existing data are inconclusive [72–76]. A reduction in HF rates in elderly Japanese stroke patients with residual hemiplegia treated with mecobalamine and folate was reported [68]. However, in general, causal relationships of low levels of vitamin B12 with reduced bone quality [77–80] and developing dementia [81, 82] are currently not supported. In poststroke patients no favourable effects of treatment with vitamin B12 (plus folic acid and vitamin B6) on risk of recurrent stroke, carotid intima-media thickness, myocardial infarction, or death have been observed [83–86]. Moreover, elevated vitamin B12 serum levels were associated, especially in the elderly, with symptomatic venous thromboembolism following major orthopaedic surgery of the low limb [87], mortality [88–90], carcinomas, haematological malignancies, and parenteral nutrition [51–53]. Vitamin B12 therapy increased cardiovascular risk in patients with diabetic neuropathy [91], cancer outcomes, and all-cause mortality in patients with CAD [92]. To the best of our knowledge, ours is the first study to show an association between higher vitamin B12 serum levels and poststroke HF. Understanding the underlying mechanisms will be a future research area of great interest.
4.3. Mineral and Bone Metabolism
With respect to indices of mineral and bone metabolism, we found that vitamin D insufficiency and excessive bone resorption were highly prevalent in both groups, but the poststroke group showed significantly higher mean levels of serum PTH and urinary bone resorption markers, lower values for osteocalcin, osteocalcin/BAP ratio, and a more pronounced imbalance between bone resorption and formation with resorption significantly exceeding formation.
These results are in line with reports showing (1) a high prevalence of hypovitaminosis D and SHPT in poststroke patients [15, 69, 93–97] and HF patients [98–100], (2) an association of vitamin D insufficiency and especially elevated serum PTH levels with cardiovascular disease [31, 101–111] including stroke [69, 93, 112], hypertension [94, 104, 113–124], CAD [125], and carotid artery intima-media thickness (IMT) [126, 127], and (3) a high prevalence of osteoporosis in stroke patients [128] and a negative balance between bone formation and resorption in poststroke HF subjects [15–17, 21, 46, 47, 93, 129, 130].
Other researchers, however, reported no association between vitamin D status and stroke risk [131, 132], between PTH and blood pressure/hypertension [116, 118, 133] and between PTH and IMT [134]. In some studies, poststroke patients demonstrated significant BMD loss, while serum PTH and vitamin D levels and bone turnover markers were within normal range [26].
Not surprisingly, altered vitamin D and PTH levels, two pleiotropic factors with multiple physiological functions in calcium homeostasis, morphogenesis, cell proliferation, differentiation, and apoptosis, affect both bone and vascular health [135, 136]. The role of PTH as an indicator of poststroke HF deserves additional comment to avoid misinterpretation. In this study of HF subjects, serum PTH levels were associated with an increased risk of poststroke HF in a continuous fashion after adjusting for age and sex. The mean PTH levels and prevalence of SHPT were significantly higher in poststroke patients compared to the rest of the cohort (despite similar mean 25(OH)D levels and even slightly lower prevalence of hypovitaminosis D), and elevated PTH levels independently predicted history of stroke when comparison was made with patients without CVD but not with all nonstroke patients among which a significant proportion had CVD. These findings are in agreement with previous studies reporting that higher serum PTH levels are associated with stroke, hypertension, CAD, AF, and congestive heart failure [31, 69, 101, 106, 107, 109, 110, 125, 137–140]. PTH receptors have been found on cardiomyocytes [141] and endothelial cells [142, 143]. Serum PTH levels were shown to be an independent risk factor for cardiovascular events, cardiovascular mortality, and all-cause mortality, even in individuals with PTH within the normal range [109, 137, 140, 144, 145]. Altogether these data support the hypothesis that elevated PTH concentration is an abnormality of mineral metabolism affecting both the skeleton and vascular system (particularly the function of medium- and large-sized arteries and the myocardium), and therefore the presence of even mild PTH elevation is a condition that should be taken into account for the prevention of both CVD and fracture risks.
Although vitamin D deficiency is known as the main cause of SHPT, clinical practice shows that the vitamin D-PTH relationship is complex. In elderly patients with long-standing hemiplegic stroke, hypovitaminosis D occurs without hyperparathyroidism [15, 16, 93] possibly because of immobilization-induced hypercalcaemia which may inhibit the compensatory PTH elevation; no correlation between serum calcium and PTH concentrations was seen in these patients [97]. In contrast, we found in poststroke HF subjects higher mean PTH levels and a higher SHPT prevalence despite similar mean 25(OH)D concentration and slightly lower prevalence of hypovitaminosis D compared to the nonstroke group, as well as a significant correlation between serum calcium and PTH levels in both groups. Taken together, these data indicate that the group of poststroke patients is clinically and biologically heterogeneous and in addition to hypovitaminosis D other factors attributable to SHPT must be taken into consideration.
In both groups no significant correlation between markers of bone turnover, except urinary markers of bone resorption, was present suggesting an imbalance between resorption and formation. However, uncoupling of these two processes, as indicated by the BSI index, was more prominent in the poststroke patients. Consistent with previous studies [47], we found that excess bone resorption in poststroke HF patients was near universal. Increased sclerostin production by osteocytes in response to skeletal unloading/immobilization has been proposed as an important molecular mechanism for acceleration of bone remodelling, with bone resorption exceeding formation [46, 146]. Reports on association between CVD and bone turnover markers are inconsistent [147, 148]. Increased bone resorption (high DPD) has been shown to be an independent predictor of CVD, including stroke [31, 33], and decreased bone formation, measured by bone histometry, was independently associated with increased coronary calcification [149].
Compared to the rest of the cohort, in poststroke HF patients, the mean BAP serum levels did not differ, but the mean OC concentration and the ratio OC/BAP were significantly lower. These may suggest impaired osteoblastic maturation, as BAP is expressed in the early period of osteoblastic differentiation, whereas OC is expressed in the later period [150]. This abnormality was associated with higher vitamin B12 levels. Indeed, vitamin B12 positively correlated with BAP in both groups, but patients with higher vitamin B12 levels had significantly lower mean OC/BAP ratio compared to those with vitamin B12 < 350 pmol/L. The poststroke subjects with higher vitamin B12 concentrations had significantly higher BAP levels and the lowest OC/BAP ratio, indicating that vitamin B12 may influence maturation of osteoblasts, which in turn may contribute to bone fragility and fractures. In vitro, vitamin B12 in low concentrations dose dependently increased alkaline phosphatase (ALP) activity of osteoblastic cells [151], while vitamin B12 deficiency did not affect the onset of osteoblast differentiation, maturation, and matrix mineralization [152] and had no influence on ALP expression in human osteoblasts [153] but increased homocysteine induced osteoclastogenesis in a dose-dependent manner [152]. These data indicates that vitamin B12 deficiency (when causing hyperhomocysteinaemia) may increase osteoclast formation and bone resorption, while higher B12 levels may affect osteoblast maturation. Reduction in serum OC/BAP ratio is associated with vertebral fractures independent of BMD in diabetic men [54]. In our study, vitamin B12 levels correlated with PTH. It appears, therefore, that higher serum B12 levels among poststroke HF patients in addition to SHPT and excess bone resorption may in part mediate the link between stroke and HF. The multivariate logistic regression analysis showed that higher serum vitamin B12 level was associated with poststroke HF independently of parameters of mineral and bone metabolism indicating that bone remodelling status, including osteoblast dysfunction, is incorporated in the model; this suggests that vitamin B12 is an important regulator of bone cell function and higher serum vitamin B12 levels may be directly linked to bone fragility in poststroke HF.
The next interesting finding of the study relates to phosphate homeostasis. As in other studies [47], the mean serum concentrations of calcium (albumin corrected), phosphate, and magnesium were within normal ranges and similar in both groups. However, upon classification of patients into two groups, with and without history of stroke, Pearson correlation results differed significantly. In the group with poststroke HF, serum phosphate level, though within the normal range, significantly and positively correlated with serum calcium and bone formation marker osteocalcin, as well as with both urinary bone resorption markers (DPD/Cr and NTx/Cr) and negatively with BSI. In the nonstroke group phosphate correlated positively only with BAP and DPD/Cr. In poststroke subjects, even small increases in phosphatemia are associated with increased bone resorption and decreased BSI, despite higher serum osteocalcin and calcium concentrations, both of which are negatively associated with eGFR. Notably, the phosphate levels correlated positively with in-hospital death. Even small increments in serum phosphate have been found to be associated with arterial calcification [154], adverse cardiovascular, and renal events and mortality [107, 155–158]. In the light of the above, our observation that, in the poststroke HF subjects, higher phosphate levels (without hyperphosphatemia) are associated with BSI, bone resorption markers, and osteocalcin, which also exerts a profound effect on energy metabolism [159, 160], can be interpreted as an additional factor contributing to the link between vascular dysfunction/calcification, subsequent stroke, and HF. However, whether higher phosphate levels (within the normal range) are directly implicated in the pathogenesis of poststroke HF or merely reflect mineral and bone metabolism status warrants further clarification.
Among several specific biological mechanisms that may underlie these associations of particular interest are fibroblast growth factor 23 (FGF-23) and Klotho proteins. FGF-23, an osteocytes-derived circulating hormone, that controls serum levels of phosphate, 1,25-dihydroxy vitamin D3, and PTH, interacts with RAAS [161] and may, therefore, affect both the cardiovascular system and skeleton. Elevated FGF-23 levels contribute to decreased 1,25-dihydroxy vitamin D3 levels [162], secondary hyperparathyroidism [163], suppression of osteoblast differentiation, and matrix mineralization in vitro and in vivo [122] and are associated with vascular calcification [157, 164], endothelial dysfunction [165, 166], cardiovascular disease [167], mortality [163, 167, 168], HF, and nonvertebral fractures in elderly men [169]. However, in some studies [170, 171] the relationship between FGF-23 and vascular calcification was not observed.
Our results further support the link between mineral-bone metabolism abnormalities and stroke (and other CVD) and demonstrate that some metabolic interactions, particularly between phosphate and bone resorption markers, between vitamin B12 and OC/BAP ratio, are prevalent in poststroke HF subjects. Circulating 25(OH)D, PTH, phosphate, and vitamin B12 levels are linked to both stroke and HF, further accentuating their important underlying role in vascular and bone biology.
Our final multivariate regression analysis demonstrated that six clinical characteristics (female sex, history of TIA, dementia, hypertension, CAD, and use of a walking aid) and higher serum B12 level are independent indicators/predictors for the development of HF. Because hypovitaminosis D was near universal among the HF patients, regardless history of stroke, and it is only one of the determinants of SHPT, it did not appear as an independent indicator of poststroke HF in our model.
4.4. Clinical Applications: Outcomes, Prediction, and Prevention Therapies
This study demonstrates that compared to the rest of the cohort, poststroke HF patients had approximately a 3-fold higher incidence of postoperative myocardial injury (as evidenced by cardiac troponin I rise) and need to be discharge to a long-term RCF. Our observations regarding the discharge status are consistent with some studies [4], but not others [35]. Similarly, in agreement with some [35] but not all [4, 172] previous reports, we found that in-hospital mortality [35] and LOS did not differ significantly between patients with and without history of stroke. Of note, SHPT and higher vitamin b12 levels were associated with in-hospital death and duration of hospital stay.
Approximately one in 7 older stroke survivors will suffer a HF which is associated with serious short- and long-term health consequences. To design preventive strategies, it is important to identify which persons are at the greatest risk. Our data suggests that compared with nonstroke HF patients, the relative risk of poststroke HF is 1.8–3.7 times higher in females, subjects with history of TIA, dementia, hypertension, CAD, using a walking aid, living in a permanent RCF, and having SHPT or higher vitamin B12 levels (>350 pmol/L). Presence of any two of these characteristics increases the RR to 2.5–5.9. SHPT and higher vitamin B12 levels are promising biomarkers for identification of older adults at increased risk of poststroke HF, and their measurement provides additional prognostic information when combined with clinical factors. These findings suggest that the combined effect of having a stroke, being a female and one of the above mentioned conditions, might significantly contribute to and predict an HF.
It should, however, be pointed out that, in general, the specificity of analysed factors was higher than the sensitivity, and the negative predictive value (usually above 88%) was higher than the positive predictive value with exception for female sex and hypertension which have sensitivity of 83% and 76.3%, respectively. That means that these two factors identify more poststroke persons at risk for HF, while the probability that the complication will not occur is low only when any of the studied characteristics is not present. Antiosteoporotic therapy for stroke survivors has not been adopted into current guidelines and routine clinical practice possibly because accurate predictive indicators were not available. Although the prognostic tools for poststroke HF need further improvement, the above-listed factors may be helpful in evaluating the risk of developing HF and deciding who is most likely to benefit from intervention.
Only a small proportion of our poststroke patients (15%) received antiosteoporotic medications prior to their HF. In concordance with our observations, use of bisphosphonates was low (2.1%–2.7%) and did not differ between poststroke and nonstroke groups in other countries [30, 36]. Many HF are preventable, and the effectiveness of antiresorptive agents and vitamin D in fracture risk reduction in stroke survivors has been demonstrated [24, 96, 97, 173–175], but the gap between existing evidence-based and actual care remains huge. The results of this study in keeping with previous reports [56, 176] emphasise the importance of assessment for and prevention of poststroke osteoporosis and falls. Currently recommended measures in addition to bisphosphonates, vitamin D, and calcium supplementation include sunlight exposure [177], improving physical activity, exercises aimed at preventing falls [178–181], and hip protectors [182, 183]. In poststroke patients with immobilization-induced hypercalcaemia, calcitonin (in combination with vitamin D) may be helpful [97, 184]. Antiosteoporosis medications are generally safe and well tolerated, but the extraskeletal risks of bisphosphonates and calcium supplements, although rare, should also be considered [185–187]. To date there have been no studies on the use of bone anabolic agents (PTH 1–34, teriparatide), strontium ranelate, or denosumab in stroke survivors.
It should be emphasized that CVDs, especially history of TIA, hypertension, and CAD, are not only important and modifiable hazards to the brain but also independent indicators/predictors of poststoke HF. Therefore, correction of hypovitaminosis D and SHPT, which plays a specific mechanistic role in the pathogenesis of osteoporosis and CVD, and adequate antiosteoporotic therapy in addition to antihypertensive, antithrombotic, and cholesterol lowering treatment may markedly contribute to both stroke and fracture prevention. Our data supports reports challenging the existing approach to homocysteine lowering with vitamin B therapy and suggests a need to consider the association of higher serum vitamin B12 levels and adverse effects in stroke patients. Although the serum B12 level below which total homocysteine and methylmalonic acid levels become elevated is 400 pmol/L [188], we found that the serum vitamin B12 concentration > 350 pmol/L is associated with adverse clinical outcomes. Vitamin B therapy may thus be beneficial in persons with B12 deficiency but may be harmful when the serum B12 concentration is higher (even within the reference range), especially in subjects with impaired renal function.
Over recent decades, stroke mortality was falling, and the population of stroke survivors was steadily increasing. To reduce the public health burden of the substantial proportion of poststroke subjects with HF secondary prevention programs directed on progression of osteoporosis should be included in the guidelines for management of these patients.
5. Limitations
Our study has several limitations. First, owing to the cross-sectional study design, causal effects cannot be determined. Second, in this single-centre study there were only 100 poststroke HF patients; therefore, its statistical power might be insufficient for detecting all relationships. Third, serum levels of ionized calcium, 1,25(OH)2D, FGF-23, and Klotho were not examined, and the dietary phosphorus and calcium intake were not assessed, although these variables contribute to mineral-bone metabolism and can provide additional insights to underlying mechanisms. Fourth, the cohort was comprised predominantly of Caucasians, and the findings may not be applicable to other racial groups. The strengths of this study include a comprehensive assessment of a relatively large well-characterized cohort of consecutive older patients with osteoporotic HF representative of real-life practice and consideration of multiple potential biologic factors, some of which have not been studied extensively previously.
6. Conclusions
In the elderly, approximately one in seven HF occurs in stroke survivors, usually within the first 2.4 years after the stroke. Poststroke HFs are prevalent in women and associated with dementia, history of TIA, hypertension, CAD, hypovitaminosis D, SHPT, high bone resorption, higher serum vitamin B12 levels, and poorer outcomes (postoperative myocardial injury and RCF need). In stroke survivors prefracture antiosteoporotic therapy is rarely used; early implementation of fracture prevention strategies is urgently needed.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Ashburn A, Hyndman D, Pickering R, Yardley L, Harris S. Predicting people with stroke at risk of falls. Age and Ageing. 2008;37(3):270–276. doi: 10.1093/ageing/afn066. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen L, Engstad T, Jacobsen BK. Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke. 2002;33(2):542–547. doi: 10.1161/hs0202.102375. [DOI] [PubMed] [Google Scholar]
- 3.Kerse N, Parag V, Feigin VL, et al. Falls after stroke: results from the auckland regional community stroke (ARCOS) study, 2002 to 2003. Stroke. 2008;39(6):1890–1893. doi: 10.1161/STROKEAHA.107.509885. [DOI] [PubMed] [Google Scholar]
- 4.Ramnemark A, Nilsson M, Borssén B, Gustafson Y. Stroke, a major and increasing risk factor for femoral neck fracture. Stroke. 2000;31(7):1572–1577. doi: 10.1161/01.str.31.7.1572. [DOI] [PubMed] [Google Scholar]
- 5.Weerdesteyn V, De Niet M, Van Duijnhoven HJR, Geurts ACH. Falls in individuals with stroke. Journal of Rehabilitation Research and Development. 2008;45(8):1195–1214. [PubMed] [Google Scholar]
- 6.English C, McLennan H, Thoirs K, Coates A, Bernhardt J. Loss of skeletal muscle mass after stroke: a systematic review. International Journal of Stroke. 2010;5(5):395–402. doi: 10.1111/j.1747-4949.2010.00467.x. [DOI] [PubMed] [Google Scholar]
- 7.Pang MY, Ashe MC, Eng JJ. Muscle weakness, spasticity and disuse contribute to demineralization and geometric changes in the radius following chronic stroke. Osteoporosis International. 2007;18(9):1243–1252. doi: 10.1007/s00198-007-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamdy RC, Moore SW, Cancellaro VA, Harvill LM. Long-term effects of strokes on bone mass. American Journal of Physical Medicine and Rehabilitation. 1995;74(5):351–356. [PubMed] [Google Scholar]
- 9.Jørgensen L, Engstad T, Jacobsen BK. Bone mineral density in acute stroke patients: low bone mineral density may predict first stroke in women. Stroke. 2001;32(1):47–51. doi: 10.1161/01.str.32.1.47. [DOI] [PubMed] [Google Scholar]
- 10.Jørgensen L, Jacobsen BK. Functional status of the paretic arm affects the loss of bone mineral in the proximal humerus after stroke: a 1-Year prospective Study. Calcified Tissue International. 2001;68(1):11–15. doi: 10.1007/BF02684997. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen L, Jacobsen BK. Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: a 1 year prospective Study. Bone. 2001;28(6):655–659. doi: 10.1016/s8756-3282(01)00434-3. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Tsuji T, Higuchi Y, Domen K, Tsujiuchi K, Chino N. Osteoporosis in hemiplegic stroke patients as studied with dual-energy X-ray absorptiometry. Archives of Physical Medicine and Rehabilitation. 1999;80(10):1219–1226. doi: 10.1016/s0003-9993(99)90019-9. [DOI] [PubMed] [Google Scholar]
- 13.Ramnemark A, Nyberg L, Lorentzon R, Englund U, Gustafson Y. Progressive hemiosteoporosis on the paretic side and increased bone mineral density in the nonparetic arm the first year after severe stroke. Osteoporosis International. 1999;9(3):269–275. doi: 10.1007/s001980050147. [DOI] [PubMed] [Google Scholar]
- 14.Ramnemark A, Nyberg L, Lorentzon R, Olsson T, Gustafson Y. Hemiosteoporosis after severe stroke, independent of changes in body composition and weight. Stroke. 1999;30(4):755–760. doi: 10.1161/01.str.30.4.755. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Fujimatsu Y, Honda Y, Kunoh H, Kikuyama M, Oizumi K. Accelerated bone remodeling in patients with poststroke hemiplegia. Journal of Stroke and Cerebrovascular Diseases. 1998;7(1):58–62. doi: 10.1016/s1052-3057(98)80022-0. [DOI] [PubMed] [Google Scholar]
- 16.Sato Y, Kuno H, Kaji M, Ohshima Y, Asoh T, Oizumi K. Increased bone resorption during the first year after stroke. Stroke. 1998;29(7):1373–1377. doi: 10.1161/01.str.29.7.1373. [DOI] [PubMed] [Google Scholar]
- 17.Sato Y, Kuno H, Kaji M, Etoh K, Oizumi K. Influence of immobilization upon calcium metabolism in the week following hemiplegic stroke. Journal of the Neurological Sciences. 2000;175(2):135–139. doi: 10.1016/s0022-510x(00)00298-7. [DOI] [PubMed] [Google Scholar]
- 18.Beaupre GS, Lew HL. Bone-density changes after stroke. American Journal of Physical Medicine and Rehabilitation. 2006;85(5):464–472. doi: 10.1097/01.phm.0000214275.69286.7a. [DOI] [PubMed] [Google Scholar]
- 19.Carda S, Cisari C, Invernizzi M, Bevilacqua M. Osteoporosis after stroke: a review of the causes and potential treatments. Cerebrovascular Diseases. 2009;28(2):191–200. doi: 10.1159/000226578. [DOI] [PubMed] [Google Scholar]
- 20.Lazoura O, Groumas N, Antoniadou E, et al. Bone mineral density alterations in upper and lower extremities 12 months after stroke measured by peripheral quantitative computed tomography and DXA. Journal of Clinical Densitometry. 2008;11(4):511–517. doi: 10.1016/j.jocd.2008.05.097. [DOI] [PubMed] [Google Scholar]
- 21.Levendoglu F, Ugurlu H, Gurbilek M, et al. Increased bone resorption in the proximal femur in patients with hemiplegia. American Journal of Physical Medicine & Rehabilitation. 2004;83(11):835–841. doi: 10.1097/01.phm.0000140802.91648.57. [DOI] [PubMed] [Google Scholar]
- 22.Marsden J, Gibson LM, Lightbody CE, Sharma AK, Siddiqi M, Watkins C. Can early onset bone loss be effectively managed in post-stroke patients? An integrative review of the evidence. Age and Ageing. 2008;37(2):142–150. doi: 10.1093/ageing/afm198. [DOI] [PubMed] [Google Scholar]
- 23.Myint PK, Poole KES, Warburton EA. Hip fractures after stroke and their prevention. QJM. 2007;100(9):539–545. doi: 10.1093/qjmed/hcm067. [DOI] [PubMed] [Google Scholar]
- 24.Poole KES, Reeve J, Warburton EA. Falls, fractures, and osteoporosis after stroke: time to think about protection. Stroke. 2002;33(5):1432–1436. doi: 10.1161/01.str.0000014510.48897.7d. [DOI] [PubMed] [Google Scholar]
- 25.Whitson HE, Pieper CF, Sanders L, Horner RD, Duncan PW, Lyles KW. Adding injury to insult: fracture risk after stroke in veterans. Journal of the American Geriatrics Society. 2006;54(7):1082–1088. doi: 10.1111/j.1532-5415.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 26.Yavuzer G, Ataman S, Suldur N, et al. Bone mineral density in patients with stroke. International Journal of Rehabilitation Research. 2002;25(3):235–239. doi: 10.1097/00004356-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Dennis MS, Lo KM, McDowall M, West T. Fractures after stroke: frequency, types, and associations. Stroke. 2002;33(3):728–734. doi: 10.1161/hs0302.103621. [DOI] [PubMed] [Google Scholar]
- 28.Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001;32(3):702–706. doi: 10.1161/01.str.32.3.702. [DOI] [PubMed] [Google Scholar]
- 29.Ramnemark A, Nyberg L, Borssén B, Olsson T, Gustafson Y. Fractures after stroke. Osteoporosis International. 1998;8(1):92–95. doi: 10.1007/s001980050053. [DOI] [PubMed] [Google Scholar]
- 30.Brown DL, Morgenstern LB, Majersik JJ, Kleerekoper M, Lisabeth LD. Risk of fractures after stroke. Cerebrovascular Diseases. 2008;25(1-2):95–99. doi: 10.1159/000111997. [DOI] [PubMed] [Google Scholar]
- 31.Fisher A, Srikusalanukul W, Davis M, et al. Cardiovascular diseases in older patients with osteoporotic hip fracture: prevalence, disturbances in mineral and bone metabolism, and bidirectional links. Journal of Clinical Interventions in Aging. 2013;8:239–256. doi: 10.2147/CIA.S38856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerber Y, Melton LJ, III, McNallan SM, et al. Cardiovascular and noncardiovascular disease associations with hip fractures. The American Journal of Medicine. 2013;126(2):169.e19–169.e26. doi: 10.1016/j.amjmed.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szulc P, Maurice C, Marchand F, Delmas PD. Increased bone resorption is associated with higher mortality in community-dwelling men ≥50 years of age: the MINOS Study. Journal of Bone and Mineral Research. 2009;24(6):1116–1124. doi: 10.1359/jbmr.081251. [DOI] [PubMed] [Google Scholar]
- 34.Tanko LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. Journal of Bone and Mineral Research. 2005;20(11):1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 35.Youm T, Aharonoff G, Zuckerman JD, Koval KJ. Effect of previous cerebrovascular accident on outcome after hip fracture. Journal of Orthopaedic Trauma. 2000;14(5):329–334. doi: 10.1097/00005131-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Pouwels S, Lalmohamed A, Leufkens B, et al. Risk of hip/femur fracture after stroke: a population-based case-control study. Stroke. 2009;40(10):3281–3285. doi: 10.1161/STROKEAHA.109.554055. [DOI] [PubMed] [Google Scholar]
- 37.Wu C-H, Liou T-H, Hsiao P-L, Lin Y-C, Chang K-H. Contribution of ischemic stroke to hip fracture risk and the influence of gender difference. Archives of Physical Medicine and Rehabilitation. 2011;92(12):1987–1991. doi: 10.1016/j.apmr.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 38.Colón-Emeric CS, Pieper CF, Artz MB. Can historical and functional risk factors be used to predict fractures in community-dwelling older adults? Development and validation of a clinical tool. Osteoporosis International. 2002;13(12):955–961. doi: 10.1007/s001980200133. [DOI] [PubMed] [Google Scholar]
- 39.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. New England Journal of Medicine. 1995;332(12):767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 40.Melton LJ, III, Brown RD, Jr., Achenbach SJ, O’Fallon WM, Whisnant JP. Long-term fracture risk following ischemic stroke: a population-based Study. Osteoporosis International. 2001;12(11):980–986. doi: 10.1007/s001980170028. [DOI] [PubMed] [Google Scholar]
- 41.Chiu KY, Pun WK, Luk KDK, Chow SP. A prospective study on hip fractures in patients with previous cerebrovascular accidents. Injury. 1992;23(5):297–299. doi: 10.1016/0020-1383(92)90171-n. [DOI] [PubMed] [Google Scholar]
- 42.Peszczynski M. The fractured hip in hemiplegic patients. Geriatrics. 1957;12(12):687–690. [PubMed] [Google Scholar]
- 43.Stavrou ZP, Erginousakis DA, Loizides AA, Tzevelekos SA, Papagiannakos KJ. Mortality and rehabilitation following hip fracture. A study of 202 elderly patients. Acta Orthopaedica Scandinavica, Supplementum. 1997;68(275):89–91. doi: 10.1080/17453674.1997.11744754. [DOI] [PubMed] [Google Scholar]
- 44.Kramer AM, Steiner JF, Schlenker RE, et al. Outcomes and costs after hip fracture and stroke: a comparison of rehabilitation settings. Journal of the American Medical Association. 1997;277(5):396–404. [PubMed] [Google Scholar]
- 45.Poplingher A-R, Pillar T. Hip fracture in stroke patients. Epidemiology and rehabilitation. Acta Orthopaedica Scandinavica. 1985;56(3):226–227. doi: 10.3109/17453678508993000. [DOI] [PubMed] [Google Scholar]
- 46.Gaudio A, Pennisi P, Bratengeier C, et al. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. Journal of Clinical Endocrinology and Metabolism. 2010;95(5):2248–2253. doi: 10.1210/jc.2010-0067. [DOI] [PubMed] [Google Scholar]
- 47.Paker N, Bugdayci D, Tekdos D, Dere C, Kaya B. Relationship between bone turnover and bone density at the proximal femur in stroke patients. Journal of Stroke and Cerebrovascular Diseases. 2009;18(2):139–143. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clinical Chemistry. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 49.Kushida K, Takahashi M, Kawana K, Inoue T. Comparison of markers for bone formation and resorption in premenopausal and postmenopausal subjects, and osteoporosis patients. Journal of Clinical Endocrinology and Metabolism. 1995;80(8):2447–2450. doi: 10.1210/jcem.80.8.7629240. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi M, Naitou K, Ohishi T, Nagano A. Comparison of biochemical markers of bone turnover and bone mineral density between hip fracture and vertebral fracture. Journal of Clinical Densitometry. 2003;6(3):211–218. doi: 10.1385/jcd:6:3:211. [DOI] [PubMed] [Google Scholar]
- 51.Elkhatib I, Cao W, Rao S, Fryer J, Buchman AL. Serum B12 concentration is elevated in patients receiving chronic parenteral nutrition, but is not a marker of intestinal failure-associated liver disease. Journal of Clinical Gastroenterology. 2010;44(8):571–574. doi: 10.1097/MCG.0b013e3181d7723b. [DOI] [PubMed] [Google Scholar]
- 52.Ermens AAM, Vlasveld LT, Lindemans J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clinical Biochemistry. 2003;36(8, part 1):585–590. doi: 10.1016/j.clinbiochem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Serraj K, Mecili M, Housni I, Andrès E. Hypervitaminemia B12 (high level of cobalamin): physiopathology, role and interest in clinical practice. Presse Médicale. 2011;40(12, Part 1):1120–1127. doi: 10.1016/j.lpm.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Yano S, Sugimoto T. Serum osteocalcin/bone-specific alkaline phosphatase ratio is a predictor for the presence of vertebral fractures in men with type 2 diabetes. Calcified Tissue International. 2009;85(3):228–234. doi: 10.1007/s00223-009-9272-4. [DOI] [PubMed] [Google Scholar]
- 55.Mulley G, Espley AJ. Hip fracture after hemiplegia. Postgraduate Medical Journal. 1979;55(642):264–265. doi: 10.1136/pgmj.55.642.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lisabeth LD, Morgenstern LB, Wing JJ, et al. Poststroke fractures in a bi-ethnic community. Journal of Stroke & Cerebrovascular Diseases. 2012;21(6):471–477. doi: 10.1016/j.jstrokecerebrovasdis.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haast RA, Gustafson DR, Kiliaan AJ. Sex differences in stroke. Journal of Cerebral Blood Flow and Metabolism. 2012;32(12):2100–2107. doi: 10.1038/jcbfm.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. Journal of Internal Medicine. 2006;259(6):598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 59.Collins TC, Ewing SK, Diem SJ, et al. Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation. 2009;119(17):2305–2312. doi: 10.1161/CIRCULATIONAHA.108.820993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.den Uyl D, Nurmohamed MT, van Tuyl LHD, Raterman HG, Lems WF. (Sub)clinical cardiovascular disease is associated with increased bone loss and fracture risk; A systematic review of the association between cardiovascular disease and osteoporosis. Arthritis Research and Therapy. 2011;13(1):p. R5. doi: 10.1186/ar3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hak AE, Pols HAP, Van Hemert AM, Hofman A, Witteman JCM. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(8):1926–1931. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- 62.Naves M, Rodriguez-Garcia M, Diaz-Lopez JB, et al. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporosis International. 2008;19(8):1161–1166. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- 63.Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. Journal of the American Medical Association. 2009;302(15):1666–1673. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- 64.Szulc P, Kiel DP, Delmas PD. Calcifications in the abdominal aorta predict fractures in men: MINOS Study. Journal of Bone and Mineral Research. 2008;23(1):95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- 65.Von Mühlen D, Allison M, Jassal SK, Barrett-Connor E. Peripheral arterial disease and osteoporosis in older adults: the Rancho Bernardo Study. Osteoporosis International. 2009;20(12):2071–2078. doi: 10.1007/s00198-009-0912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Meurs JBJ, Dhonukshe-Rutten RAM, Pluijm SMF, et al. Homocysteine levels and the risk of osteoporotic fracture. New England Journal of Medicine. 2004;350(20):2033–2041. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 67.McLean RR, Jacques PF, Selhub J, et al. Homocysteine as a predictive factor for hip fracture in older persons. New England Journal of Medicine. 2004;350(20):2042–2049. doi: 10.1056/NEJMoa032739. [DOI] [PubMed] [Google Scholar]
- 68.Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Effect of folate and mecobalamin on hip fractures in patients with stroke: a randomized controlled trial. Journal of the American Medical Association. 2005;293(9):1082–1088. doi: 10.1001/jama.293.9.1082. [DOI] [PubMed] [Google Scholar]
- 69.Sato Y, Kaji M, Metoki N, Satoh K, Iwamoto J. Does compensatory hyperparathyroidism predispose to ischemic stroke? Neurology. 2003;60(4):626–629. doi: 10.1212/01.wnl.0000047345.05818.8b. [DOI] [PubMed] [Google Scholar]
- 70.Dhonukshe-Rutten RAM, Pluijm SMF, De Groot LCPGM, Lips P, Smit JH, Van Staveren WA. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. Journal of Bone and Mineral Research. 2005;20(6):921–929. doi: 10.1359/JBMR.050202. [DOI] [PubMed] [Google Scholar]
- 71.Yang J, Hu X, Zhang Q, et al. Homocysteine level and risk of fracture: a meta-analysis and systematic review. Bone. 2012;51(3):376–382. doi: 10.1016/j.bone.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 72.Gerdhem P, Ivaska KK, Isaksson A, et al. Associations between homocysteine, bone turnover, BMD, mortality, and fracture risk in elderly women. Journal of Bone and Mineral Research. 2007;22(1):127–134. doi: 10.1359/jbmr.061003. [DOI] [PubMed] [Google Scholar]
- 73.Gjesdal CG, Vollset SE, Ueland PM, Refsum H, Meyer HE, Tell GS. Plasma homocysteine, folate, and vitamin B12 and the risk of hip fracture: the hordaland homocysteine study. Journal of Bone and Mineral Research. 2007;22(5):747–756. doi: 10.1359/jbmr.070210. [DOI] [PubMed] [Google Scholar]
- 74.Stone KL, Bauer DC, Sellmeyer D, Cummings SR. Low serum vitamin B-12 levels are associated with increased hip bone loss in older women: a prospective Study. Journal of Clinical Endocrinology and Metabolism. 2004;89(3):1217–1221. doi: 10.1210/jc.2003-030074. [DOI] [PubMed] [Google Scholar]
- 75.Tucker KL, Hannan MT, Qiao N, et al. Low plasma vitamin B12 is associated with lower BMD: the framingham osteoporosis Study. Journal of Bone and Mineral Research. 2005;20(1):152–158. doi: 10.1359/JBMR.041018. [DOI] [PubMed] [Google Scholar]
- 76.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A., III High homocysteine and low B vitamins predict cognitive decline in aging men: the veterans affairs normative aging Study. American Journal of Clinical Nutrition. 2005;82(3):627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 77.Herrmann M, Wildemann B, Wagner A, et al. Experimental folate and vitamin B12 deficiency does not alter bone quality in rats. Journal of Bone and Mineral Research. 2009;24(4):589–596. doi: 10.1359/jbmr.081211. [DOI] [PubMed] [Google Scholar]
- 78.Holstein JH, Herrmann M, Splett C, et al. Hyperhomocysteinemia is not associated with reduced bone quality in humans with hip osteoarthritis. Clinical Chemistry and Laboratory Medicine. 2010;48(6):821–827. doi: 10.1515/CCLM.2010.155. [DOI] [PubMed] [Google Scholar]
- 79.Kakehasi AM, Carvalho AV, Maksud FA, et al. Serum levels of vitamin B12 are not related to low bone mineral density in postmenopausal Brazilian women. Revista Brasileira de Reumatologia. 2012;52(6):863–869. [PubMed] [Google Scholar]
- 80.Cagnacci A, Bagni B, Zini A, Cannoletta M, Generali M, Volpe A. Relation of folates, vitamin B12 and homocysteine to vertebral bone mineral density change in postmenopausal women. A five-year longitudinal evaluation. Bone. 2008;42(2):314–320. doi: 10.1016/j.bone.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 81.Ho RCM, Cheung MWL, Fu E, et al. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. American Journal of Geriatric Psychiatry. 2011;19(7):607–617. doi: 10.1097/JGP.0b013e3181f17eed. [DOI] [PubMed] [Google Scholar]
- 82.Van Dam F, Van Gool WA. Hyperhomocysteinemia and Alzheimer’s disease: a systematic review. Archives of Gerontology and Geriatrics. 2009;48(3):425–430. doi: 10.1016/j.archger.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 83.Hankey GJ, Eikelboom JW, Yi Q, et al. Treatment with B vitamins and incidence of cancer in patients with previous stroke or transient ischemic attack: results of a randomized placebo-controlled trial. Stroke. 2012;43:1572–1577. doi: 10.1161/STROKEAHA.111.641613. [DOI] [PubMed] [Google Scholar]
- 84.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. Journal of the American Medical Association. 2008;299(17):2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Potter K, Hankey GJ, Green DJ, Eikelboom J, Jamrozik K, Arnolda LF. The effect of long-term homocysteine-lowering on carotid intima-media thickness and flow-mediated vasodilation in stroke patients: a randomized controlled trial and meta-analysis. BMC Cardiovascular Disorders. 2008;8, article 24 doi: 10.1186/1471-2261-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. Journal of the American Medical Association. 2004;291(5):565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 87.Grossfeld A, Dekel S, Lerman Y, et al. Symptomatic venous thromboembolism in elderly patients following major orthopedic surgery of the lower limb is associated with elevated vitamin B12 serum levels. Clinical Biochemistry. 2013;46(1-2):54–58. doi: 10.1016/j.clinbiochem.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 88.Geissbühler P, Mermillod B, Rapin C-H. Elevated serum vitamin B12 levels associated with CRP as a predictive factor of mortality in palliative care cancer patients: a prospective study over five years. Journal of Pain and Symptom Management. 2000;20(2):93–103. doi: 10.1016/s0885-3924(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 89.Sviri S, Khalaila R, Daher S, et al. Increased Vitamin B12 levels are associated with mortality in critically ill medical patients. Clinical Nutrition. 2012;31(1):53–59. doi: 10.1016/j.clnu.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 90.Tal S, Shavit Y, Stern F, Malnick S. Association between vitamin B12 levels and mortality in hospitalized older adults. Journal of the American Geriatrics Society. 2010;58(3):523–526. doi: 10.1111/j.1532-5415.2010.02721.x. [DOI] [PubMed] [Google Scholar]
- 91.House AA, Eliasziw M, Cattran DC, et al. Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. Journal of the American Medical Association. 2010;303(16):1603–1609. doi: 10.1001/jama.2010.490. [DOI] [PubMed] [Google Scholar]
- 92.Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. Journal of the American Medical Association. 2009;302(19):2119–2126. doi: 10.1001/jama.2009.1622. [DOI] [PubMed] [Google Scholar]
- 93.Fujimatsu Y. Role of the parathyroid gland on bone mass and metabolism in immobilized stroke patients. Kurume Medical Journal. 1998;45(3):265–270. doi: 10.2739/kurumemedj.45.265. [DOI] [PubMed] [Google Scholar]
- 94.Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM. 25-hydroxyvitamin D levels and the risk of stroke: a prospective Study and meta-analysis. Stroke. 2012;43:1470–1477. doi: 10.1161/STROKEAHA.111.636910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sato Y, Asoh T, Kondo I, Satoh K. Vitamin D deficiency and risk of hip fractures among disabled elderly stroke patients. Stroke. 2001;32(7):1673–1677. doi: 10.1161/01.str.32.7.1673. [DOI] [PubMed] [Google Scholar]
- 96.Sato Y, Iwamoto J, Honda Y. Beneficial effect of etidronate therapy in chronically hospitalized, disabled patients with stroke. Journal of Stroke and Cerebrovascular Diseases. 2010;19(3):198–203. doi: 10.1016/j.jstrokecerebrovasdis.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 97.Sato Y, Iwamoto J, Honda Y. An open-label trial comparing alendronate and alphacalcidol in reducing falls and hip fractures in disabled stroke patients. Journal of Stroke and Cerebrovascular Diseases. 2011;20(1):41–46. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 98.Dhanwal DK, Sahoo S, Gautam VK, Saha R. Hip fracture patients in India have vitamin D deficiency and secondary hyperparathyroidism. Osteoporosis International. 2012;24:553–557. doi: 10.1007/s00198-012-1993-y. [DOI] [PubMed] [Google Scholar]
- 99.Di Monaco M, Castiglioni C, Vallero F, et al. Parathyroid hormone response to severe vitamin D deficiency is sex associated: an observational study of 571 hip fracture inpatients. Journal of Nutrition Health and Aging. 2013;17(2):180–184. doi: 10.1007/s12603-012-0088-y. [DOI] [PubMed] [Google Scholar]
- 100.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocrine Reviews. 2001;22(4):477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 101.Bosworth C, Sachs MC, Duprez D, et al. Parathyroid hormone and arterial dysfunction in the Multi-Ethnic Study of Atherosclerosis. Clinical Endocrinology. 2013;79(3):429–436. doi: 10.1111/cen.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-Hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. Journal of the American Society of Nephrology. 2009;20(8):1805–1812. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Ballegooijen AJ, Visser M, Kestenbaum B, et al. Relation of vitamin D and parathyroid hormone to cardiac biomarkers and to left ventricular mass (from the cardiovascular dealth Study) American Journal of Cardiology. 2013;111(3):418–424. doi: 10.1016/j.amjcard.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao G, Ford ES, Li C, Kris-Etherton PM, Etherton TD, Balluz LS. Independent associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with blood pressure among US adults. Journal of Hypertension. 2010;28(9):1821–1828. doi: 10.1097/HJH.0b013e32833bc5b4. [DOI] [PubMed] [Google Scholar]
- 105.Andersen KK, Olsen TS. One-month to 10-year survival in the copenhagen stroke Study: interactions between stroke severity and other prognostic indicators. Journal of Stroke and Cerebrovascular Diseases. 2011;20(2):117–123. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 106.Bosworth C, de Boer IH. Impaired vitamin D metabolism in CKD. Seminars in Nephrology. 2013;33(2):158–168. doi: 10.1016/j.semnephrol.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kestenbaum B, Katz R, De Boer I, et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. Journal of the American College of Cardiology. 2011;58(14):1433–1441. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schottker B, Haug U, Schomburg L, et al. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large Cohort Study. American Journal of Clinical Nutrition. 2013;97(4):782–793. doi: 10.3945/ajcn.112.047712. [DOI] [PubMed] [Google Scholar]
- 109.Sugimoto T, Dohi K, Onishi K, et al. Interrelationship between haemodynamic state and serum intact parathyroid hormone levels in patients with chronic heart failure. Heart. 2013;99(2):111–115. doi: 10.1136/heartjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 110.Terrovitis J, Zotos P, Kaldara E, et al. Bone mass loss in chronic heart failure is associated with secondary hyperparathyroidism and has prognostic significance. European Journal of Heart Failure. 2012;14(3):326–332. doi: 10.1093/eurjhf/hfs002. [DOI] [PubMed] [Google Scholar]
- 111.Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Cardiovascular Quality and Outcomes. 2012;5(6):819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brondum-Jacobsen P, Nordestgaard BG, Schnohr P, et al. 25-Hydroxyvitamin D and symptomatic ischemic stroke: an original Study and meta-analysis. Annals of Neurology. 2013;73(1):38–47. doi: 10.1002/ana.23738. [DOI] [PubMed] [Google Scholar]
- 113.Giovannucci E. Vitamin D and cardiovascular disease. Current Atherosclerosis. 2009;11(6):456–461. doi: 10.1007/s11883-009-0068-9. [DOI] [PubMed] [Google Scholar]
- 114.Jorde R, Sundsfjord J, Haug E, Bønaa KH. Relation between low calcium intake, parathyroid hormone, and blood pressure. Hypertension. 2000;35(5):1154–1159. doi: 10.1161/01.hyp.35.5.1154. [DOI] [PubMed] [Google Scholar]
- 115.Jorde R, Svartberg J, Sundsfjord J. Serum parathyroid hormone as a predictor of increase in systolic blood pressure in men. Journal of Hypertension. 2005;23(9):1639–1644. doi: 10.1097/01.hjh.0000179764.40701.36. [DOI] [PubMed] [Google Scholar]
- 116.Jungert A, Roth HJ, Neuhauser-Berthold M. Serum 25-hydroxyvitamin D3, parathyroid hormone and blood pressure in an elderly cohort from Germany: a cross-sectional Study. Nutrition & Metabolism. 2012;9, article 20 doi: 10.1186/1743-7075-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li K, Kaaks R, Linseisen J, et al. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and nutrition Study (EPIC-Heidelberg) Heart. 2012;98(12):920–925. doi: 10.1136/heartjnl-2011-301345. [DOI] [PubMed] [Google Scholar]
- 118.Li L, Yin X, Yao C, et al. Vitamin D, parathyroid hormone and their associations with hypertension in a Chinese population. Plos One. 2012;7(8) doi: 10.1371/journal.pone.0043344.e43344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pilz S, Dobnig H, Fischer JE, et al. Low vitamin D levels predict stroke in patients referred to coronary angiography. Stroke. 2008;39(9):2611–2613. doi: 10.1161/STROKEAHA.107.513655. [DOI] [PubMed] [Google Scholar]
- 120.Reis JP, Von Mühlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30(6):1549–1555. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 121.Taylor EN, Curhan GC, Forman JP. Parathyroid hormone and the risk of incident hypertension. Journal of Hypertension. 2008;26(7):1390–1394. doi: 10.1097/HJH.0b013e3282ffb43b. [DOI] [PubMed] [Google Scholar]
- 122.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chan R, Chan D, Woo J, et al. Serum 25-hydroxyvitamin D and parathyroid hormone levels in relation to blood pressure in a cross-sectional study in older Chinese men. Journal of Human Hypertension. 2012;26(1):20–27. doi: 10.1038/jhh.2010.126. [DOI] [PubMed] [Google Scholar]
- 124.Snijder MB, Lips P, Seidell JC, et al. Vitamin D status and parathyroid hormone levels in relation to blood pressure: a population-based study in older men and women. Journal of Internal Medicine. 2007;261(6):558–565. doi: 10.1111/j.1365-2796.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 125.Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone levels predict coronary heart disease: the Tromsø Study. European Journal of Cardiovascular Prevention and Rehabilitation. 2004;11(1):69–74. doi: 10.1097/01.hjr.0000114706.27531.01. [DOI] [PubMed] [Google Scholar]
- 126.Choi HS, Kim SH, Rhee Y, Cho MA, Lee EJ, Lim S-K. Serum parathyroid hormone is associated with carotid intima-media thickness in postmenopausal women. International Journal of Clinical Practice. 2008;62(9):1352–1357. doi: 10.1111/j.1742-1241.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 127.Walker MD, Fleischer J, Rundek T, et al. Carotid vascular abnormalities in primary hyperparathyroidism. Journal of Clinical Endocrinology and Metabolism. 2009;94(10):3849–3856. doi: 10.1210/jc.2009-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saverino A, Del Sette M, Conti M, et al. Hyperechoic plaque: an ultrasound marker for osteoporosis in acute stroke patients with carotid disease. European Neurology. 2006;55(1):31–36. doi: 10.1159/000091423. [DOI] [PubMed] [Google Scholar]
- 129.Sato Y. Abnormal bone and calcium metabolism in patients after stroke. Archives of Physical Medicine and Rehabilitation. 2000;81(1):117–121. doi: 10.1016/s0003-9993(00)90231-4. [DOI] [PubMed] [Google Scholar]
- 130.Bainbridge NJ, Davie MWJ, Haddaway MJ. Bone loss after stroke over 52 weeks at os calcis: influence of sex, mobility and relation to bone density at other sites. Age and Ageing. 2006;35(2):127–132. doi: 10.1093/ageing/afj045. [DOI] [PubMed] [Google Scholar]
- 131.Bolland MJ, Bacon CJ, Horne AM, et al. Vitamin D insufficiency and health outcomes over 5 y in older women. American Journal of Clinical Nutrition. 2010;91(1):82–89. doi: 10.3945/ajcn.2009.28424. [DOI] [PubMed] [Google Scholar]
- 132.Drechsler C, Pilz S, Obermayer-Pietsch B, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. European Heart Journal. 2010;31(18):2253–2261. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52(5):828–832. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reis JP, von Mühlen D, Michos ED, et al. Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis. 2009;207(2):585–590. doi: 10.1016/j.atherosclerosis.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lips P. Vitamin D physiology. Progress in Biophysics & Molecular Biology. 2006;92(1):4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 136.Wysolmerski JJ. Parathyroid hormone-related protein: an update. Journal of Clinical Endocrinology & Metabolism. 2012;97(9):2947–2956. doi: 10.1210/jc.2012-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hagström E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119(21):2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 138.Schierbeck LL, Jensen TS, Bang U, Jensen G, Køber L, Jensen J-EB. Parathyroid hormone and vitamin D—markers for cardiovascular and all cause mortality in heart failure. European Journal of Heart Failure. 2011;13(6):626–632. doi: 10.1093/eurjhf/hfr016. [DOI] [PubMed] [Google Scholar]
- 139.Anderson JL, Vanwoerkom RC, Horne BD, et al. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: dependent or independent risk factors? American Heart Journal. 2011;162(2):331.e2–339.e2. doi: 10.1016/j.ahj.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 140.Pilz S, Tomaschitz A. Role of vitamin D in arterial hypertension. Expert Review of Cardiovascular Therapy. 2010;8(11):1599–1608. doi: 10.1586/erc.10.142. [DOI] [PubMed] [Google Scholar]
- 141.Tastan I, Schreckenberg R, Mufti S, Abdallah Y, Piper HM, Schlüter K-D. Parathyroid hormone improves contractile performance of adult rat ventricular cardiomyocytes at low concentrations in a non-acute way. Cardiovascular Research. 2009;82(1):77–83. doi: 10.1093/cvr/cvp027. [DOI] [PubMed] [Google Scholar]
- 142.Jiang B, Morimoto S, Yang J, Niinoabu T, Fukuo K, Ogihara T. Expression of parathyroid hormone/parathyroid hormone-related protein receptor in vascular endothelial cells. Journal of Cardiovascular Pharmacology. 1998;31(supplement 1):S142–S144. doi: 10.1097/00005344-199800001-00042. [DOI] [PubMed] [Google Scholar]
- 143.Rashid G, Bernheim J, Green J, Benchetrit S. Parathyroid hormone stimulates endothelial expression of atherosclerotic parameters through protein kinase pathways. American Journal of Physiology. Renal Physiology. 2007;292(4):F1215–F1218. doi: 10.1152/ajprenal.00406.2006. [DOI] [PubMed] [Google Scholar]
- 144.Grandi NC, Breitling LP, Brenner H. Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Preventive Medicine. 2010;51(3-4):228–233. doi: 10.1016/j.ypmed.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 145.Björkman M, Sorva A, Tilvis R. Parathyroid hormone as a mortality predictor in frail aged inpatients. Gerontology. 2009;55(6):601–606. doi: 10.1159/000239757. [DOI] [PubMed] [Google Scholar]
- 146.Lin C, Jiang X, Dai Z, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β-catenin signaling. Journal of Bone and Mineral Research. 2009;24(10):1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 147.Fahrleitner-Pammer A, Herberth J, Browning SR, et al. Bone markers predict cardiovascular events in chronic kidney disease. Journal of Bone and Mineral Research. 2008;23(11):1850–1858. doi: 10.1359/jbmr.080610. [DOI] [PubMed] [Google Scholar]
- 148.Manghat P, Souleimanova I, Cheung J, et al. Association of bone turnover markers and arterial stiffness in pre-dialysis chronic kidney disease (CKD) Bone. 2011;48(5):1127–1132. doi: 10.1016/j.bone.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 149.Tomiyama C, Carvalho AB, Higa A, Jorgetti V, Draibe SA, Canziani MEF. Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. Journal of Bone and Mineral Research. 2010;25(3):499–504. doi: 10.1359/jbmr.090735. [DOI] [PubMed] [Google Scholar]
- 150.Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocrine Reviews. 1993;14(4):424–442. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- 151.Kim GS, Kim C-H, Park JY, Lee K-U, Park CS. Effects of vitamin B12 on cell proliferation and cellular alkaline phosphatase activity in human bone marrow stromal osteoprogenitor cells and UMR106 osteoblastic cells. Metabolism. 1996;45(12):1443–1446. doi: 10.1016/s0026-0495(96)90171-7. [DOI] [PubMed] [Google Scholar]
- 152.Vaes BLT, Lute C, Blom HJ, et al. Vitamin B12 deficiency stimulates osteoclastogenesis via increased homocysteine and methylmalonic acid. Calcified Tissue International. 2009;84(5):413–422. doi: 10.1007/s00223-009-9244-8. [DOI] [PubMed] [Google Scholar]
- 153.Herrmann M, Peter Schmidt J, Umanskaya N, et al. The role of hyperhomocysteinemia as well as folate, vitamin B6 and B12 deficiencies in osteoporosis: a systematic review. Clinical Chemistry and Laboratory Medicine. 2007;45(12):1621–1632. doi: 10.1515/CCLM.2007.362. [DOI] [PubMed] [Google Scholar]
- 154.Ix JH, De Boer IH, Peralta CA, et al. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clinical Journal of the American Society of Nephrology. 2009;4(3):609–615. doi: 10.2215/CJN.04100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Archives of Internal Medicine. 2007;167(9):879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 156.Dominguez JR, Kestenbaum B, Chonchol M, et al. Relationships between serum and urine phosphorus with all-cause and cardiovascular mortality: the osteoporotic fractures in men (MrOS) Study. American Journal of Kidney Diseases. 2013;61(4):555–563. doi: 10.1053/j.ajkd.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakayama M, Kaizu Y, Nagata M, et al. Fibroblast growth factor 23 is associated with carotid artery calcification in chronic kidney disease patients not undergoing dialysis: a cross-sectional study. BMC Nephrology. 2013;14, article 22 doi: 10.1186/1471-2369-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Onufrak SJ, Bellasi A, Shaw LJ, et al. Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis. 2008;199(2):424–431. doi: 10.1016/j.atherosclerosis.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 159.Fisher AA, Srikusalanukul W, Davis MW, et al. Clinical profiles and risk factors for outcomes in older patients with cervical and trochanteric hip fracture: similarities and differences. Journal of Trauma Management & Outcomes. 2012;6(1):p. 2. doi: 10.1186/1752-2897-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends in Endocrinology and Metabolism. 2008;19(5):161–166. doi: 10.1016/j.tem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 161.De Borst MH, Vervloet MG, Ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. Journal of the American Society of Nephrology. 2011;22(9):1603–1609. doi: 10.1681/ASN.2010121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu S-Z, Tian L-F, Xu P, et al. Analysis of correlation between blood biochemical indicators and bone mineral density of post-menopausal women. Molecular Biology Reports. 2011;38(2):939–948. doi: 10.1007/s11033-010-0187-y. [DOI] [PubMed] [Google Scholar]
- 163.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. Journal of the American Society of Nephrology. 2005;16(7):2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 164.Desjardins L, Liabeuf S, Renard C, et al. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporosis International. 2011;23(7):2017–2025. doi: 10.1007/s00198-011-1838-0. [DOI] [PubMed] [Google Scholar]
- 165.Mirza MAI, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205(2):385–390. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 166.Bernheim J, Benchetrit S. The potential roles of FGF23 and klotho in the prognosis of renal and cardiovascular diseases. Nephrology Dialysis Transplantation. 2011;26(8):2433–2438. doi: 10.1093/ndt/gfr208. [DOI] [PubMed] [Google Scholar]
- 167.Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS, (Cardiovascular Health Study) Journal of the American College of Cardiology. 2012;60(3):200–207. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney International. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mirza MAI, Karlsson MK, Mellström D, et al. Serum fibroblast growth factor-23 (FGF-23) and fracture risk in elderly men. Journal of Bone and Mineral Research. 2011;26(4):857–864. doi: 10.1002/jbmr.263. [DOI] [PubMed] [Google Scholar]
- 170.Roos M, Lutz J, Salmhofer H, et al. Relation between plasma fibroblast growth factor-23, serum fetuin-A levels and coronary artery calcification evaluated by multislice computed tomography in patients with normal kidney function. Clinical Endocrinology. 2008;68(4):660–665. doi: 10.1111/j.1365-2265.2007.03074.x. [DOI] [PubMed] [Google Scholar]
- 171.Scialla JJ, Lau WL, Reilly MP, et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney International. 2013;83(6):1159–1168. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Di Monaco M, Vallero F, Di Monaco R, Mautino F, Cavanna A. Functional recovery and length of stay after hip fracture in patients with neurologic impairment. American Journal of Physical Medicine and Rehabilitation. 2003;82(2):143–148. doi: 10.1097/00002060-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 173.Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovascular Diseases. 2005;20(3):187–192. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 174.Sato Y, Iwamoto J, Kanoko T, Satoh K. Risedronate sodium therapy for prevention of hip fracture in men 65 years or older after stroke. Archives of Internal Medicine. 2005;165(15):1743–1748. doi: 10.1001/archinte.165.15.1743. [DOI] [PubMed] [Google Scholar]
- 175.Poole KES, Loveridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37(1):243–245. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 176.Chung ME, Lee JI, Im S, Park JH. Ischemic stroke in rats enhances bone resorption in vitro. Journal of Korean Medical Science. 2012;27(1):84–88. doi: 10.3346/jkms.2012.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Iwamoto J, Takeda T, Matsumoto H. Sunlight exposure is important for preventing hip fractures in patients with Alzheimer’s disease, Parkinson’s disease, or stroke. Acta Neurologica Scandinavica. 2012;125(4):279–284. doi: 10.1111/j.1600-0404.2011.01555.x. [DOI] [PubMed] [Google Scholar]
- 178.Batchelor F, Hill K, MacKintosh S, Said C. What works in falls prevention after stroke?: a systematic review and meta-analysis. Stroke. 2010;41(8):1715–1722. doi: 10.1161/STROKEAHA.109.570390. [DOI] [PubMed] [Google Scholar]
- 179.Batchelor FA, Mackintosh SF, Said CM, et al. Falls after stroke. International Journal of Stroke. 2012;7(6):482–490. doi: 10.1111/j.1747-4949.2012.00796.x. [DOI] [PubMed] [Google Scholar]
- 180.Borschmann K. Exercise protects bone after stroke, or does it? A narrative review of the evidence. Stroke Research and Treatment. 2012;2012:12 pages. doi: 10.1155/2012/103697.103697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Borschmann K, Pang MY, Bernhardt J, et al. Stepping towards prevention of bone loss after stroke: a systematic review of the skeletal effects of physical activity after stroke. International Journal of Stroke. 2012;7(4):330–335. doi: 10.1111/j.1747-4949.2011.00645.x. [DOI] [PubMed] [Google Scholar]
- 182.Van Schoor NM, Smit JH, Twisk JWR, Bouter LM, Lips P. Prevention of hip fractures by external hip protectors: a randomized controlled trial. Journal of the American Medical Association. 2003;289(15):1957–1962. doi: 10.1001/jama.289.15.1957. [DOI] [PubMed] [Google Scholar]
- 183.Runge M, Hunger G. Determinants of musculoskeletal frailty and the risk of falls in old age. Journal of Musculoskeletal Neuronal Interactions. 2006;6(2):167–173. [PubMed] [Google Scholar]
- 184.Turner AG, Tjahyono F, Chiu WSM, et al. The role of the calcitonin receptor in protecting against induced hypercalcemia is mediated via its actions in osteoclasts to inhibit bone resorption. Bone. 2011;48(2):354–361. doi: 10.1016/j.bone.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 185.Body J-J, Bergmann P, Boonen S, et al. Extraskeletal benefits and risks of calcium, vitamin D and anti-osteoporosis medications. Osteoporosis International. 2012;23(1, supplement):1–23. doi: 10.1007/s00198-011-1891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vestergaard P. Acute myocardial infarction and atherosclerosis of the coronary arteries in patients treated with drugs against osteoporosis: calcium in the vessels and not the bones? Calcified Tissue International. 2012;90(1):22–29. doi: 10.1007/s00223-011-9549-2. [DOI] [PubMed] [Google Scholar]
- 187.Rizzoli R, Reginster J-Y. Adverse drug reactions to osteoporosis treatments. Expert Review of Clinical Pharmacology. 2011;4(5):593–604. doi: 10.1586/ecp.11.42. [DOI] [PubMed] [Google Scholar]
- 188.Vogiatzoglou A, Oulhaj A, Smith AD, et al. Determinants of plasma methylmalonic acid in a large population: implications for assessment of vitamin B12 status. Clinical Chemistry. 2009;55(12):2198–2206. doi: 10.1373/clinchem.2009.128678. [DOI] [PubMed] [Google Scholar]


