One Sentence Summary
The central light atom in the iron-molybdenum cofactor of nitrogenase is identified as carbon.
Nitrogenase is a complex enzyme that catalyzes the reduction of dinitrogen to ammonia. Despite insight from structural and biochemical studies, its structure and mechanism await full characterization. An iron-molybdenum cofactor (FeMoco) is thought to be the site of dinitrogen reduction, but the identity of a central atom in this cofactor remains unknown. Fe Kß X-ray emission spectroscopy (XES) of intact nitrogenase MoFe protein, isolated FeMoco, and the FeMoco-deficient ∆nifB protein indicates that among the candidate atoms O, N, and C, it is C that best fits the XES data. The experimental XES is supported by computational efforts, which shows that oxidation and spin states do not affect the assignment of the central atom to C4-. Identification of the central atom will drive further studies on its role in catalysis.
Nitrogenase (N2ase), found in symbiotic and free-living diazotrophs, catalyzes the reduction of dinitrogen (N2) to ammonia (NH3), using eight electrons, eight protons and sixteen MgATPs (1). Industrially, the same reaction is performed by the Haber-Bosch process that produces more than 100 million tons of NH3 each year, thereby accounting for ~1.4% of global energy consumption. Understanding how Nature activates the strongest bond in chemistry, the triple bond of N2, is the key for the future design of molecular catalysts.
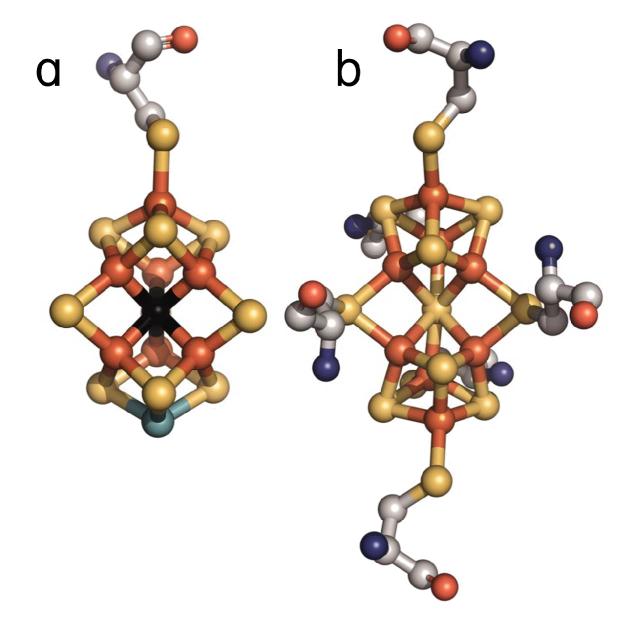
The high-resolution crystal structure of N2ase by Einsle et al.(2) showed that the active site of the molybdenum iron (MoFe) protein component of N2ase binds a complex cluster consisting of seven iron ions, one molybdenum ion and nine sulfides (Figure 1a); this cluster is referred to as the iron-molybdenum cofactor (FeMoco) and is thought to be the site of dinitrogen activation. For each FeMoco (of which there are two in the α2ß2 tetrameric MoFe protein) there is an additional cluster that consists of eight irons and seven sulfides (Figure 1b); this cluster is referred to as the P-cluster. The P-clusters serve as electron transfer sites. Several reaction intermediates in nitrogenase catalysis have recently been observed (3, 4). However, despite the progress in the experimental and theoretical analysis of the FeMoco (4-7), neither the reaction that occurs at the FeMoco nor the structure of FeMoco has been fully clarified. In 2002, Einsle et al. identified a light atom in the center of FeMoco that could be attributed to a single, fully ionized carbon, nitrogen or oxygen atom (2). No consensus has emerged concerning the nature of this key atom. Study of FeMoco by EPR and related techniques is complicated by complex spin-couplings between the open-shell ions, which are not fully understood. Mössbauer spectroscopy suffers from spectral crowding, and neither nuclear resonance vibrational spectroscopy (NRVS) nor extended X-ray absorption fine structure (EXAFS) are sufficiently conclusive (8).
Figure 1.
The FeMoco (a) and P-cluster (b) of nitrogenase (adapted from PDB: 1MIN). Orange = Fe; Yellow= S; Cyan = Mo; Black = C4-, N3-, or O2-. For clarity, the homocitrate and histidine ligands to the Mo have been omitted.
Herein, we report iron Kß valence-to-core (V2C) X-ray emission spectroscopy (XES) of N2ase and demonstrate that these data provide a signature for the presence and identity of the central atom. Kα and Kβ XES monitor the emission of photons following ionization of a metal 1s electron. The Kβ1,3 emission line (~7040-7070 eV) corresponds to an electric dipole allowed 3p → 1s transition. To higher emission energies, valence-electron transitions into the metal 1s core hole are observed (referred to as the Kβ2,5/Kβ” or V2C region). These transitions have been previously assigned as ligand np → metal 1s (Kβ2,5, ~7102-7112 eV) and ligand ns → metal 1s (Kβ” or “satellite,” ~7090-7102 eV) transitions (9). V2C XES studies of Cr and Mn complexes have shown that the Kß” features provide a signature for the identity of the directly coordinating ligands, since energies of the observed features depend primarily on the ligand 2s ionization energies (10, 11). Recently, we have developed an experimental and theoretical protocol for the analysis of V2C XES spectra and applied it to mono-(12-14) and multinuclear(15) iron complexes. Of particular relevance is a study of a six iron cluster with a central μ6-C4-(15). These data show a feature at 7099 eV that is attributed to a transition originating from the μ6-C4- 2s orbital. Computationally this feature is predicted to shift to 7094 eV for a μ6-N3-, and 7088 eV for a μ6-O2-. These trends closely parallel what has been observed for infinite lattice complexes and mononuclear molecular complexes, thus highlighting the general applicability of this method (11, 12, 16).
Figure 2a presents the normalized V2C XES data of the isolated FeMoco of N2ase, together with a representative fit to the data. Based on previous studies, the features observed with maxima at ~7108 eV and ~7100 eV are assigned to ligand np and ns contributions respectively (12). In order to assess the contribution of the sulfur ligands relative to the interstitial atom X, data were also obtained for the ∆nifB MoFe protein (Figure 2b). This mutant contains only the two P-clusters (17, 18). Based on their structural similarity, it can be assumed that the P-cluster and the FeMoco have similar sulfur contributions to their XES spectra. This assumption is also supported computationally (see below). Data were also obtained for the intact MoFe protein (containing both clusters, Figure 2b). The MoFe protein spectra map well onto an average of the spectra of the P-cluster (represented by the ∆nifB MoFe protein) and the isolated FeMoco (Figure S1).
Figure 2.
(a) Normalized V2C XES spectra of isolated FeMoco (red) and a representative fit to the data (black dotted line). (b) Comparison of the normalized V2C XES data for FeMoco (red), the MoFe protein (gray), and the ∆nifB MoFe protein (black). Inset: V2C satellite region for Fe2O3 (red), Fe3N (blue), and MoFe protein (gray).
Comparison between the data of the isolated FeMoco and those of the P-clusters in the ∆nifB MoFe protein allowed us to assess the relative contributions of these clusters to the spectra. The V2C XES data of the P-clusters showed only a weak satellite at 7098.8 with 0.30 ± 0.03 units of integrated intensity. By contrast, isolated FeMoco exhibited a well-resolved satellite feature to higher energy (7100.2 eV) with an approximately six-fold increase in the integrated intensity of the satellite feature (1.78 ± 0.18 units). In order to better understand the origin of these satellite features, the data for ∆nifB MoFe protein were also compared to the XES data for an [Fe4S4(SPh)4]2- model complex (19). Both the P-clusters and the Fe4S4 cubane have very similar XES spectra (Figs. S2-S3). Thus, the weak 7098.8 eV feature must be attributed to an S 3s → Fe 1s transition. Under the plausible assumption that the S 3s contributions to the P-cluster and FeMoco V2C XES spectra are similar, we can model the satellite region with two features: one fixed at 7098.8 eV (corresponding to the S 3s contributions) and a second to higher energy (7100.2 eV) with increased intensity (1.6 units) (Table 1 and Figure 2a). The higher energy feature is attributed to the presence of the interstitial light atom. Comparison of the energy of the 7100.2 eV satellite feature in FeMoco to the O 2s to Fe 1s (~7092 eV) and N 2s to 1s (~7095 eV) transitions observed in Fe2O3 and Fe3N, respectively (Figure 2b, inset), indicates that this feature arises from a ligand with 5 and 8 eV lower ionization potential than O or N, respectively. It therefore argues against either N or O 2s contributions and strongly supports a C 2s → Fe 1s assignment.
Table 1.
V2C XES Fit Parameters
| ΔnifB MoFe Protein | FeMoco | MoFe Protein | ||||
|---|---|---|---|---|---|---|
| E (eV) | Integrated Intensity |
E (eV) | Integrated Intensity |
E (eV) | Integrated Intensity |
|
| Kß" peak 1 | 7098.8 | .30 | 7098.8 | 0.18 | 7098.8 | 0.30 |
| Kß" peak 2 | n/a | n/a | 7100.2 | 1.60 | 7100.5 | 0.58 |
|
Total Kß"
Integrated Intensity |
0.30 | 1.78 | 0.88 | |||
|
Total V2C
Integrated Intensity |
7.73 | 10.45 | 7.61 | |||
Fe V2C XES spectra can be predicted surprisingly well within a simple scheme based on density functional theory (DFT) (12). To complement the experimental data, we performed detailed calculations on the FeMoco, the P-cluster, as well as the [Fe4S4(SPh)4]2- model complex. The FeMoco was modelled by a structure containing 152 atoms obtained from the high-resolution crystal structure of Einsle et al.(2), which incorporates the key structural and electronic features of the system. The oxidation state of the iron sulfur cluster in the resting state of the enzyme has not been determined unambiguously. Thus, calculations were done for the two oxidation states that have been shown to be most likely (20-22). Although these two states differ by two units of charge, the differences in the calculated V2C XES transition energies and intensities are much smaller than the experimental resolution (Figure S6). The effects of magnetic coupling of the various spin centers in the FeMoco calculations were approximated using the broken symmetry approach (23). However, calculations reveal that the ligand to metal crossover region of the predicted V2C XES is largely unaffected by magnetic coupling (Figure S7). This finding is understandable considering the large line width of the experimental spectra and the rather subtle differences in orbital energies arising from different magnetic coupling schemes. More importantly, the predicted V2C spectra were highly sensitive to the identity of the interstitial ion. Figure 3a presents the calculated spectra of the FeMoco assuming interstitial O2-, N3- and C4- ions together with the calculated spectrum for the P-cluster. As expected, all four spectra exhibit a relatively strong feature at around 7099.3 eV corresponding to transitions from S 3s orbitals to the Fe 1s orbitals. The only exception is FeMoco with a central C4- (Figure 3b), where the maximum is slightly shifted to higher energies due to contributions from C4--related transitions in the same region. Hence, our presented data, along with analogous calculations on [Fe4S4(SPh)4]2- (Figure S8-S9) support the aforementioned assumption that the S peak in the V2C region appears at the same position of the spectrum for all measured species.
Figure 3.
(a) Comparison of the calculated V2C XES spectra of FeMoco with an interstitial C4- (black), N3- (blue) and O2- (red) and of the spectra of the P-clusters (gray). (b) Calculated V2C XES spectra of FeMoco with an interstitial C4- (black), the P-clusters (gray). (c) Experimental difference spectrum of FeMoco with the P-clusters (gray), as well as calculated difference spectra of the P-cluseters with FeMoco containing interstitial C4- (black), N3- (blue), and O2- (red).
Subtraction of the calculated P-cluster spectrum from the calculated spectrum of the three FeMoco species yields the contributions from the respective interstitial ions (Figure 3c). The analysis of the difference spectra reveals that the interstitial ions give rise to two features in the V2C spectrum associated with transitions from the ligand 2s and 2p orbitals, respectively. These features occur at 7096.1 eV and 7105.1 eV for N3- and at 7091.0 eV and 7104.0 eV for O2-. When a C4- ion is placed in the center of the FeMoco, the two features are observed at 7100.2 eV and 7107.9 eV (Figure 3b). For N3- and C4-, the higher energy feature is not distinguishable from the large peak at ~7107 eV that is dominated by transitions originating from the S 3p orbitals.
The experimental and theoretical results taken together support assignment of the interstitial species as a C4-. The calculated position of the C4- 2s to Fe 1s peak matches the experimentally determined position at 7100.2 eV. Both N3- and O2- are unlikely since their respective calculated spectra show strong features at 7096.1 eV (N3- 2s) and 7091.0 eV (O2- 2s). In addition, the measured spectra do not exhibit any features at lower energies than the S 3s peak, whereas such features have been observed experimentally in other N3- and O2- systems (as shown in the inset of Figure 2b). The assignment is further supported by our previous studies that have shown that features with a calculated intensity of more than 10-15 units of intensity are experimentally observable (12). These studies also showed that the integrated intensities of experimental and calculated V2C agree strongly, with a 19% error for crystallographic structures. Even considering this error, the calculated low energy features related to the N3- (31 units of intensity) and O2- and (26 units of intensity) ions considerably exceed this threshold. In addition, several other studies on O2-and N3- have shown features at the corresponding energy offsets (9-12). This finding raises interesting questions about both the role of the central atom and the possible pathways for biosynthesis of such an organometallic cluster.
Supplementary Material
References and Notes
- 1.Hu YL, Ribbe MW. Decoding the Nitrogenase Mechanism: The Homologue Approach. Acc. Chem. Res. 2010;43:475. doi: 10.1021/ar900254x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Einsle O, et al. Nitrogenase MoFe protein at 1.16 Å resolution. Science. 2002;297:1696. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- 3.Barney BM, et al. Trapping an Intermediate of Dinitrogen (N-2) Reduction on Nitrogenase. Biochemistry. 2009;48:9094. doi: 10.1021/bi901092z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman BM, Dean DR, Seefeldt LC. Climbing nitrogenase: Toward a mechanism of enzymatic nitrogen fixation. Acc. Chem. Res. 2009;42:609. doi: 10.1021/ar8002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukoyanov D, et al. Testing if the interstitial atom, X, of the nitrogenase molybdenum-iron cofactor is N or C: ENDOR, ESEEM, and DFT studies of the S=3/2 resting state in multiple environments. Inorg. Chem. 2007;46:11437. doi: 10.1021/ic7018814. [DOI] [PubMed] [Google Scholar]
- 6.Neese F. The Yandulov/Schrock cycle and the nitrogenase reaction: Pathways of nitrogen fixation studied by density functional theory. Angew Chem. Int. Ed. 2006;45:196. doi: 10.1002/anie.200502667. [DOI] [PubMed] [Google Scholar]
- 7.Harris TV, Szilagyi RK. Comparative Assessment of the Composition and Charge State of Nitrogenase FeMo-Cofactor. Inorg. Chem. 2011;50:4811. doi: 10.1021/ic102446n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao YM, et al. How nitrogenase shakes - Initial information about P-cluster and FeMo-cofactor normal modes from nuclear resonance vibrational Spectroscopy (NRVS) J. Am. Chem. Soc. 2006;128:7608. doi: 10.1021/ja0603655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glatzel P, Bergmann U. High resolution 1s core hole X-ray spectroscopy in 3d transition metal complexes—electronic and structural information. Coord. Chem. Rev. 2005;249:65. [Google Scholar]
- 10.Smolentsev G, et al. X-ray Emission Spectroscopy To Study Ligand Valence Orbitals in Mn Coordination Complexes. J. Am. Chem. Soc. 2009;131:13161. doi: 10.1021/ja808526m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eeckhout SG, et al. Cr local environment by valence-to-core X-ray emission spectroscopy. J Anal. Atom Spect. 2009;24:215. [Google Scholar]
- 12.Lee N, Petrenko T, Bergmann U, Neese F, DeBeer S. Probing Valence Orbital Composition with Iron K beta X-ray Emission Spectroscopy. J. Am. Chem. Soc. 2010;132:9715. doi: 10.1021/ja101281e. [DOI] [PubMed] [Google Scholar]
- 13.Pollock CJ, DeBeer S. Valence-to-Core X-ray Emission Spectroscopy: A Sensitive Probe of the Nature of a Bound Ligand. J. Am. Chem. Soc. 2011;133:5594. doi: 10.1021/ja200560z. [DOI] [PubMed] [Google Scholar]
- 14.Lancaster KM, Finkelstein KD, DeBeer S. Kß X-ray Emission Spectroscopy Offers Unique Chemical Bonding Insights: Revisiting the Electronic Structure of Ferrocene. Inorg. Chem. 2011;50:6767. doi: 10.1021/ic200822b. [DOI] [PubMed] [Google Scholar]
- 15.Delgado-Jaime MU, et al. Identification of Light Atoms within Multinuclear Metal Clusters using Valence-to-Core X-Ray Emission Spectroscopy. submitted. doi: 10.1021/ic201173j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergmann U, Horne CR, Collins TJ, Workman JM, Cramer SP. Chemical dependence of interatomic X-ray transition energies and intensities – a study of Mn Kß" and Kß2, 5 spectra. Chem. Phys. Lett. 1999;302:119. [Google Scholar]
- 17.Schmid B, et al. Structure of a cofactor-deficient nitrogenase MoFe protein. Science. 2002;296:352. doi: 10.1126/science.1070010. [DOI] [PubMed] [Google Scholar]
- 18.Allen RM, Chatterjee R, Ludden PW, Shah VK. Incorporation of Iron and Sulfur from Nifb Cofactor into the Iron-Molybdenum Cofactor of Dinitrogenase. J Biol. Chem. 1995 Nov 10;270:26890. doi: 10.1074/jbc.270.45.26890. [DOI] [PubMed] [Google Scholar]
- 19.Averill BA, Herskovi T, Holm RH, Ibers JA. Synthetic Analogs of Active-Sites of Iron-Sulfur Proteins .2. Synthesis and Structure of Tetra[Mercapto-Mu3-Sulfido-Iron] Clusters, [Fe4s4(Sr)4]2- J. Am. Chem. Soc. 1973;95:3523. doi: 10.1021/ja00792a013. [DOI] [PubMed] [Google Scholar]
- 20.Lee HI, Hales BJ, Hoffman BM. Metal-ion valencies of the FeMo cofactor in CO-inhibited and resting state nitrogenase by Fe-57 Q-band ENDOR. J. Am. Chem. Soc. 1997;119:11395. [Google Scholar]
- 21.True AE, Nelson MJ, Venters RA, Ormejohnson WH, Hoffman BM. Fe-57 Hyperfine Coupling Tensors of the Femo Cluster in Azotobacter vinelandii MoFe Protein - Determination by Polycrystalline ENDOR Spectroscopy. J. Am. Chem. Soc. 1988 Mar 16;110:1935. [Google Scholar]
- 22.Yoo SJ, Angove HC, Papaefthymiou V, Burgess BK, Munck E. Mossbauer study of the MoFe protein of nitrogenase from Azotobacter vinelandii using selective Fe-57 enrichment of the M-centers. J. Am. Chem. Soc. 2000 May 24;122:4926. [Google Scholar]
- 23.Noodleman L. Valence Bond Description of Anti-Ferromagnetic Coupling in Transition-Metal Dimers. J. Chem. Phys. 1981;74:5737. [Google Scholar]
- 24.SD thanks Cornell University for financial support FN acknowledges financial support from the University of Bonn, the Max Planck Society, and the SFB 813. MWR thanks the NIH for funding (R01-GM 67626) Portions of this research were carried out at SSRL, a DOE, BES user facility. The SSRL SMB Program is supported by DOE, BER, and NIH, NCRR, BMTP.
- 25.Burgess BK. The iron-molybdenum cofactor of nitrogenase. Chem. Rev. 1990;90:1377. SOM. [Google Scholar]
- 26.Ribbe MW, Hu YL, Guo ML, Schmid B, Burgess BK. The FeMoco-deficient MoFe protein produced by a nifHDeletion strain of Azotobacter vinelandii shows unusual P-cluster features. J. Biol. Chem. 2002;277:23469. doi: 10.1074/jbc.M202061200. SOM. [DOI] [PubMed] [Google Scholar]
- 27.Hu YL, Fay AW, Ribbe MW. Identification of a nitrogenase FeMo cofactor precursor on NifEN complex. Proc. Natl. Acad. Sci. USA. 2005;102:3236. doi: 10.1073/pnas.0409201102. SOM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fay AW, et al. Characterization of isolated nitrogenase FeVco. J. Am. Chem. Soc. 2010 Sep 15;132:12612. doi: 10.1021/ja1019657. SOM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Averill BA, Herskovitz T, Holm RH, Ibers JA. Synthetic analogs of the active sites of iron-sulfur proteins. II. Synthesis and structure of the tetra[mercapto-.mu.3-sulfido-iron] clusters, [Fe4S4(SR)4]2- J. Am. Chem. Soc. 1973;95:3523. doi: 10.1021/ja00792a013. SOM. [DOI] [PubMed] [Google Scholar]
- 30.Neese F, Becker U, Ganyushin D, Liakos DG, Kossmann S, Petrenko T, Riplinger C, Wennmohs F. ORCA, 2.7.0. University of Bonn; Bonn: 2009. SOM. [Google Scholar]
- 31.Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A. 1988;38:3098. doi: 10.1103/physreva.38.3098. SOM. [DOI] [PubMed] [Google Scholar]
- 32.Perdew JP. Accurate and simple density functional for the electronic exchange energy: generalized gradient approximation. Phys. Rev. B. 1986;33:8822. doi: 10.1103/physrevb.33.8800. SOM. [DOI] [PubMed] [Google Scholar]
- 33.Van Wüllen CJ. Molecular density functional calculations in the regular relativistic approximation: Method, application to coinage metal diatomics, hydrides, fluorides and chlorides, and comparison with first-order relativistic calculations. J. Chem. Phys. 1998;109:382. SOM. [Google Scholar]
- 34.Pantazis DA, Chen XY, Landis CR, Neese F. All-electron scalar relativistic basis sets for third-row transition metal atoms. J. Chem. Theory Comput. 2008;4:908. doi: 10.1021/ct800047t. SOM. [DOI] [PubMed] [Google Scholar]
- 35.Klamt A, Schüürmann G. COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin. Trans. 1993;2:799. SOM. [Google Scholar]
- 36.Grimme S. Accurate description of van der Waals complexes by density functional theory including empirical conditions. J. Comp. Chem. 2004;25:1463. doi: 10.1002/jcc.20078. SOM. [DOI] [PubMed] [Google Scholar]
- 37.Grimme S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comp. Chem. 2006;27:1787. doi: 10.1002/jcc.20495. SOM. [DOI] [PubMed] [Google Scholar]
- 38.Lukoyanov D, et al. Testing if the interstitial atom, X, of the nitrogenase molybdenum−iron cofactor is N or C: ENDOR, ESEEM, and DFT studies of the S = 3/2 resting state in multiple environments. Inorg. Chem. 2007;46:11437. doi: 10.1021/ic7018814. SOM. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.