Abstract
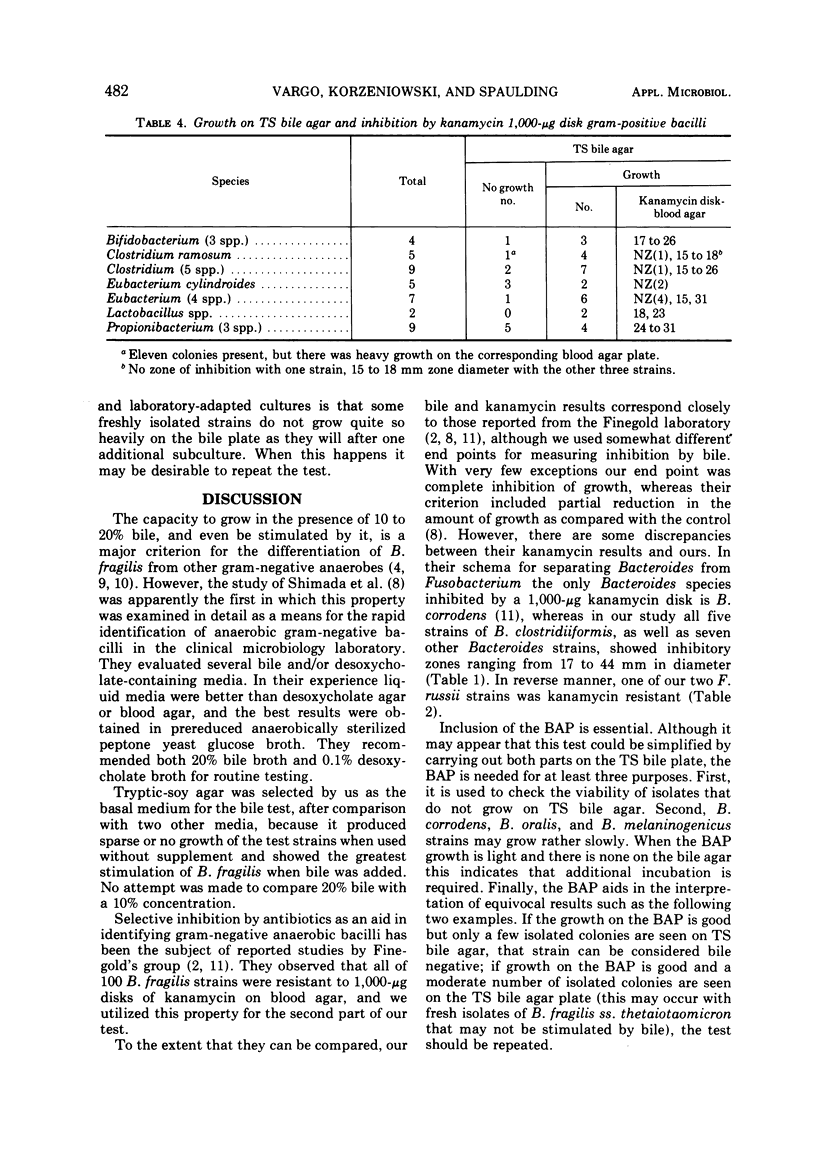
A simple and practical test for the identification of Bacteroides fragilis is described. It utilizes two well-known properties of this species, i.e., stimulation of growth by bile and resistance to kanamycin. The test media are a tryptic-soy bile agar plate and a supplemented blood agar plate on which a kanamycin 1,000-μg/ml disk is placed. Incubation is for 24 h at 37 C in GasPak. The results of screening 190 strains, mostly clinical isolates, indicate that B. fragilis can be easily and reliably distinguished from other Bacteroides and from Fusobacterium species by its growth on tryptic-soy bile agar and resistance to kanamycin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Felner J. M., Dowell V. R., Jr "Bacteroides" bacteremia. Am J Med. 1971 Jun;50(6):787–796. doi: 10.1016/0002-9343(71)90187-2. [DOI] [PubMed] [Google Scholar]
- Finegold S. M., Harada N. E., Miller L. G. Antibiotic susceptibility patterns as aids in classification and characterization of gram-negative anaerobic bacilli. J Bacteriol. 1967 Nov;94(5):1443–1450. doi: 10.1128/jb.94.5.1443-1450.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., Gardner M., Washington J. A., 2nd In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother. 1972 Feb;1(2):148–158. doi: 10.1128/aac.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J. Practical method for isolation of anerobic bacteria in the clinical laboratory. Appl Microbiol. 1971 Dec;22(6):1168–1171. doi: 10.1128/am.22.6.1168-1171.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Cato E. P., Holdeman L. V. Anaerobic bacteria of the gastrointestinal flora and their occurrence in clinical infections. J Infect Dis. 1969 Jun;119(6):641–649. doi: 10.1093/infdis/119.6.641. [DOI] [PubMed] [Google Scholar]
- Shimada K., Sutter V. L., Finegold S. M. Effect of bile and desoxycholate on gram-negative anaerobic bacteria. Appl Microbiol. 1970 Nov;20(5):737–741. doi: 10.1128/am.20.5.737-741.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Finegold S. M. Antibiotic disc susceptibility tests for rapid presumptive identification of Gram-negative anaerobic bacilli. Appl Microbiol. 1971 Jan;21(1):13–20. doi: 10.1128/am.21.1.13-20.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. M., Michaelson T. C., Daly M. J., Spaulding E. H. Anaerobic bacterial infections of the female genital tract. Obstet Gynecol. 1973 Oct;42(4):538–541. doi: 10.1097/00006250-197310000-00009. [DOI] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Martin W. J. Comparison of three blood culture media for recovery of anaerobic bacteria. Appl Microbiol. 1973 Jan;25(1):70–71. doi: 10.1128/am.25.1.70-71.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Thiel T. Modified broth-disk method for testing the antibiotic susceptibility of anaerobic bacteria. Antimicrob Agents Chemother. 1973 Mar;3(3):350–356. doi: 10.1128/aac.3.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabransky R. J. Isolation of anaerobic bacteria from clinical specimens. Mayo Clin Proc. 1970 Apr;45(4):256–264. [PubMed] [Google Scholar]
- Zabransky R. J., Johnston J. A., Hauser K. J. Bacteriostatic and bactericidal activities of various antibiotics against Bacteroides fragilis. Antimicrob Agents Chemother. 1973 Feb;3(2):152–156. doi: 10.1128/aac.3.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]


