Abstract
Stem cell fate and specification is largely controlled by extrinsic cues that comprise the 3D microenvironment. Biomaterials can serve to control the spatial and temporal presentation of morphogenic molecules in order to direct stem cell fate decisions. Here we describe a microparticle (MP)-based approach to deliver growth factors within multicellular aggregates to direct pluripotent stem cell differentiation. Compared to conventional soluble delivery methods, gelatin MPs laden with BMP4 or noggin induced efficient gene expression of mesoderm and ectoderm lineages, respectively, despite using nearly 12-fold less total growth factor. BMP4-laden MPs increased the percentage of cells expressing GFP under the control of the Brachyury-T promoter as visualized by whole-mount confocal imaging and quantified by flow cytometry. Furthermore, the ability to localize MPs laden with different morphogens within a particular hemisphere of stem cell aggregates allowed for spatial control of differentiation within 3D cultures. Overall, localized delivery of growth factors within multicellular aggregates from microparticle delivery vehicles is an important step towards scalable differentiation technologies and the study of morphogen gradients in pluripotent stem cell differentiation.
Keywords: Embryonic stem cells, Differentiation, Bone morphogenic protein, Mesoderm, Embryoid body, Microparticle
1. Introduction
Transfer of pluripotent stem cells from adherent monolayer to three-dimensional suspension culture of cell aggregates, referred to as embryoid bodies (EBs), is frequently used to promote differentiation towards cell types of all three germ layers [1–3]. In many instances, limiting differentiation within aggregate cultures to a particular cell type has proven difficult, but the addition of specific morphogenic growth factors to the culture medium often enhances the production efficiency of desired cell types. For example, the addition of bone morphogenetic protein 4 (BMP4) to the culture medium at early time points after aggregate formation enhances mesoderm differentiation by activation of the transcription factor Brachyury-T through SMAD 1/5/8 signaling, a mechanism conserved in mouse and human development [4,5]. BMP4 signaling can also be inhibited to promote ectoderm differentiation by the addition of noggin, which directly binds BMP4, or other small molecule inhibitors of the SMAD pathway [6,7].
Due to the three-dimensional nature of multicellular aggregates, inherent barriers exist to the free diffusion of molecules throughout the aggregate and we, in addition to several other labs, have demonstrated diffusion limitations encountered with several different types of molecules [8–10]. Therefore, concentration gradients of molecules created throughout cell spheroids may be, at least in part, responsible for the general difficulty in controlling the homogeneity of differentiation within three-dimensional aggregates. The challenge of precise dosing control is further compounded by the fact that, in terms of scalability, growth factor delivery methods have not kept pace with recent advances in stem cell technologies allowing for scalable formation and culture of homogeneous pluripotent stem cell aggregates [11–14].
We present here a scalable method for integrating biomaterial, microparticle (MP)-based growth factor delivery vehicles that, because the growth factor is delivered from within the aggregate itself, is independent of the volume of the medium in the culture vessel and can circumvent barriers to diffusion of molecules from the culture medium throughout the aggregate. Delivery of BMP4 and noggin from gelatin-based MPs was separately tested to direct early pluripotent lineage commitment in three-dimensional aggregates. The ability to localize MPs laden with different morphogens within a particular hemisphere of multicellular aggregates was also investigated as a method to spatially control differentiation within a model of mammalian development. MP delivery of growth factors does not require medium manipulation for directed differentiation and is an important step towards scalable differentiation of pluripotent stem cells for cell biomanufacturing and tissue engineering purposes.
2. Methods
2.1. Fabrication and loading of gelatin microparticles
Microparticles (MPs) of gelatin type B (G9391, Sigma–Aldrich, St. Louis, MO) were generated using a water-in-oil emulsion method and fluorescently labeled as previously described [2]. Heparin sodium salt (CalBiochem, San Diego, CA) was conjugated to MPs after MP formulation in the following manner: EDC and S-NHS (Thermo Scientific, Waltham, MA) were added to heparin at 10:1 and 25:1 m ratios respectively, relative to heparin dissolved in 800 µL activation buffer (0.1 m MES, 0.5 m NaCl, pH 6.0) and allowed to react for 15 min at room temperature to modify the carboxyl groups of heparin to amine reactive S-NHS esters. The EDC/NHS reaction was quenched with 20 mm 2-mercaptoethanol and the activated heparin was added to 400 µL of MP in PBS at a 5:1 m ratio of heparin to gelatin and agitated for 4 h at 37 °C. Prior to cell culture, MPs were treated in 70% ethanol for a minimum of 30 min before washing 3× with ddH2O. Each MP batch was lyophilized and stored at −20 C until further use. Growth factor solutions were added to lyophilized MPs at 5 µL/mg overnight at 4 C to allow for rehydration of the MPs and uptake of the growth factor. Growth factors were added at either 50 or 125 ng/mg of MPs. After growth factor loading, all of the loaded MPs were suspended in differentiation media (500 µL) and then 10 µL of the solution was counted on a hemocytometer to determine the concentration of MPs.
2.2. Albumin release from MPs
Bovine serum albumin (Sigma–Aldrich, St. Louis, MO) was labeled with Alexa- Fluor 555 using EDC/S-NHS chemistry. Free dye was removed from the protein solution using an Amicon Ultra-15 centrifugal filter unit with a 30 kDa cutoff (Millipore, Billerica, MA). Labeled BSA was loaded into gelatin MPs as described above at a 1 mg/mL loading concentration.
2.3. Cell culture
Undifferentiated D3 ESCs were maintained on 0.1% gelatin-coated tissue culture dishes in DMEM media supplemented with 15% fetal bovine serum, 2 mm l-glutamine (Mediatech), 1× MEM non-essential amino acid solution (Mediatech), antibiotic/ antimycotics (Mediatech), 0.1 mm β-mercaptoethanol (MP Biomedicals, LLC), and 103 U/ml leukemia inhibitory factor (LIF) (Millipore, Billerica, MA). Mouse Brachyury-T GFP cells (E14.1, 129/Ola) [15] were maintained on 0.5% gelatin coated petri dishes in a humidified 5% CO2 atmosphere using modified serum-free maintenance media and base differentiation media. The defined media was composed of a DMEM/F12 (50/50) (Thermo Scientific) media supplemented with N2 (Gibco) and 50 µg/mL BSA (Millipore) in a 1:1 combination with B27 (Gibco) supplemented Neurobasal™ medium (Gibco) with 100 U/ml penicillin, 100 g/mL streptomycin, 0.25 g/mL amphotericin (Mediatech) and 2 mm l-glutamine (Mediatech) [16]. Media was routinely exchanged in ESC cultures every 1–2 days, and cells were passaged every 2–3 days as needed before reaching 80% confluency. ESGRO complete basal medium (Millipore) was used for all differentiation cultures.
2.4. Aggregate formation and culture
ESCs were trypsinized into a single cell suspension and aggregates were formed by forced aggregation in AggreWell™ 400 inserts (Stem Cell Technologies, Vancouver, CA) [13]. Briefly, 1.2 × 106 cells in 0.5 mL of ESGRO complete basal medium were added to each insert, containing approximately 1200 wells, and centrifuged at 200× g for 5 min to cluster cells in the wells. Gelatin MPs were incorporated within EBs using a second centrifugation of the culture plates after addition of 200 µL of a MP solution. In all cases, the MP:cell seed ratio was 1:3. After 24 h of culture, cell aggregates were removed from the wells using a wide-bore pipette and transferred to suspension culture on a rotary orbital shaker (40 rpm) to maintain the homogeneity of the aggregate population and prevent EB agglomeration. In the case of soluble growth factor addition, BMP4 (10 ng/mL) or noggin (50 ng/mL) was added during the initial 24 h of formation, again when transferred to suspension culture, and then supplemented every other day when the spent medium was exchanged until day 4 of EB culture. In some cases, EBs were plated onto 0.1% gelatin-coated culture vessels at day 7 of differentiation to allow attachment and EB cell spreading. After attachment, spent medium was exchanged every other day.
For EB merging studies, after 24 h of initial aggregate formation, one population of EBs (population A) was added to a second distinct EB population (population B) formed in a separate microwell insert. After an additional 24 h of culture, EBs from the two populations would merge to form single larger aggregates in the individual microwells. EBs from population A were added at a 1:2 ratio (A:B) to decrease the probability of adding more than one EB from population A to microwells containing fully formed aggregates from population B.
2.5. Spheroid morphology analysis
At days 4 and 7 of differentiation, EBs were collected from rotary culture, fixed in 10% formalin for 30 min, and suspended in Histogel (Richard–Allan Scientific, Kalamazoo, MI). The samples were then embedded in paraffin and cut into 5 µm-thick sections (MICROM HM 310, Global Medical Instrumentation Inc., Ramsey, MN). After the sections were deparaffinized, they were stained with hematoxylin and eosin (H&E). Histological samples were imaged via a Nikon 80i upright microscope equipped with a SPOT Flex camera (15.2 64 MP Shifting Pixel, Diagnostic Instruments).
2.6. Confocal microscopy
The presence of GFP expressing cells within EBs was analyzed using a LSM 510 NLO confocal microscope (Zeiss, Thornwood, NY). EBs were removed from suspension culture, fixed in 4% paraformaldehyde, and stained with Hoechst (1:100) before imaging on glass slides. Visualization of GFP signal was performed using an argon laser with a 488 nm excitation filter and a 510 emission filter.
2.7. Gene expression analysis
RNA was extracted from spheroids after 4 days of differentiation with the RNeasy Mini kit (Qiagen Inc, Valencia, CA). RNA was converted to complementary DNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) and analyzed using real time PCR (MyIQ cycler, BioRad). Forward and reverse primers for Oct4, Brachyury- T, Pax6, Flk1, and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) were designed with Beacon Designer software and purchased from Invitrogen (Table 1). Gene expression was calculated with respect to expression levels of EBs without MPs using the Pfaffl method [17].
Table 1.
Primers and conditions used for PCR.
| Gene | Forward sequence | Reverse sequence | Melt temperature |
|---|---|---|---|
| GAPDH | GCCTTCCGTGTTCCTACC | GCCTGCTTCACCACCTTC | 55.0 |
| Oct-4 | CCGTGTGAGGTGGAGTCTGGAG | GCGATGTGAGTGATCTGCTGTAGG | 64.0 |
| Pax-6 | ACGGCATGTATGATAAACTAAG | GCTGAAGTCGCATCTGAG | 58.0 |
| Brachyury-T | CACACCACTGACGCACAC | GAGGCTATGAGGAGGCTTTG | 55.0 |
| Flk-1 | GGCGGTGGTGACAGTATC | TGACAGAGGCGATGAATGG | 64.3 |
2.8. Flow cytometry
Spheroids were rinsed thoroughly with PBS and dissociated to a single cell suspension with 0.25% trypsin-EDTA and trituration for 10 min. The single-cell suspension was rinsed 3 times in PBS and pelleted at 1000 rpm for 5 min between rinses. Flow cytometry was performed with an Accuri C6 cytometer (Accuri Cytometers, Ann Arbor, MI), with a minimum of 20,000 events per sample collected within the FSC/SSC gate for live cell populations (n = 3 independent experimental samples per condition). Heparin–gelatin MPs, gelatin MPs, and undifferentiated Brachyury-T GFP cells alone were used for to establish appropriate gates and compensation. Within the FSC/SSC gate, polygonal gating was used on the (FSC)/FL-1 (480 nm excitation; 530 ± 15 nm emission) plots to limit 1% of undifferentiated negative control population via FlowJo software (Tree Star, Inc., Ashland, OR). The whole cell population was gated to include less than 2% heparin–gelatin and 5% gelatin MPs (Supplemental Fig. 4).
2.9. Statistical analysis
Unless otherwise indicated, all data are reported as mean ± standard error for a minimum of triplicate experimental samples. All data was normalized to a Gaussian distribution using a Box–Cox power transformation before statistical analysis. Statistical significance was assessed using student’s t-test or one-way ANOVA with Tukey’s post hoc analysis after verifying variance equality from Levene’s equality of variances test. p-values of less than 0.05 were considered statistically significant.
3. Results
3.1. Morphogen delivery via incorporated MPs
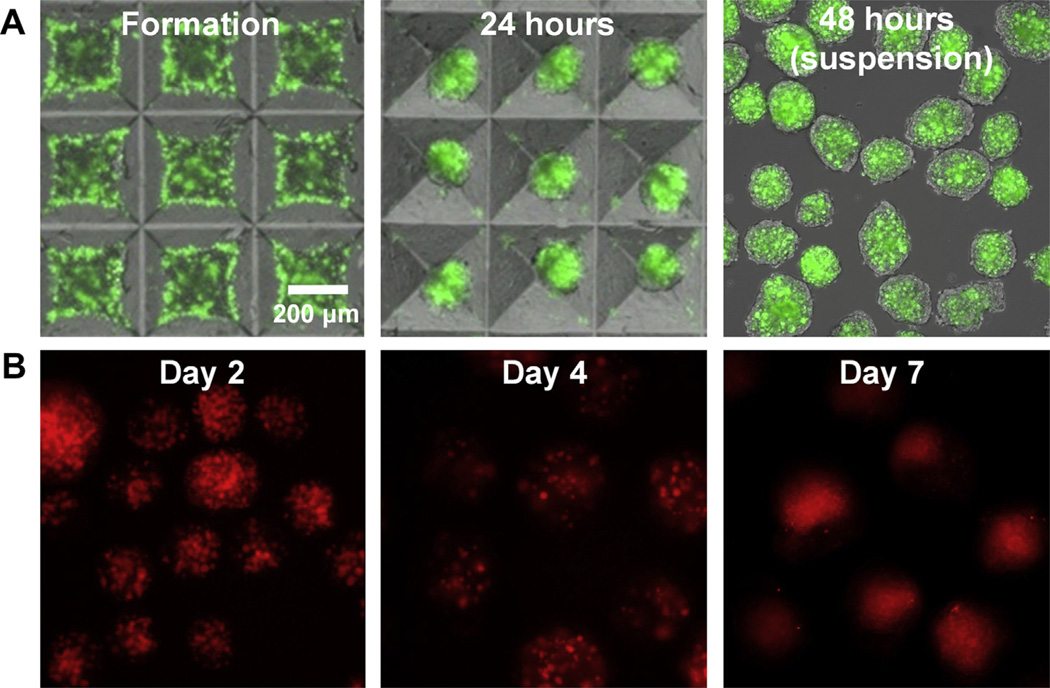
Gelatin MPs were incorporated within embryonic stem cell aggregates using forced aggregation within microwells at a 1:3 (MP:cell) seeding ratio. The number of gelatin MPs incorporated was below the level at which material incorporation was previously observed to alter differentiation within stem cell aggregates [2]. Fluorescently labeled MPs were easily identifiable within the aggregate during formation and after transfer to suspension culture (Fig. 1A). In order to visualize protein release within the aggregates, fluorescently labeled albumin was loaded within the gelatin MPs prior to incorporation within ESC aggregates (Fig. 1B). Punctate fluorescence patterns within EBs were observed through the first 4 days of differentiation, indicating protein association primarily with the MPs during this time period. However, punctuate fluorescence was replaced by a more diffuse fluorescent pattern suggesting protein release from the MPs over a total period of 7 days of examination.
Fig 1.
Gelatin microparticles are efficiently incorporated within embryoid bodies and release albumin over 7 days. A) Alexa Fluor® 488-labeled gelatin MPs were incorporated within stem cell aggregates using forced aggregation. After 24 h of culture, the MPs remained visible within the aggregates in the microwells and after transfer to suspension culture. B) Alexa Fluor® 546-labeled albumin was incorporated within gelatin MPs to model protein release within EBs. Punctate fluorescent signals demonstrated protein localization within the MPs up to 4 days, after which a diffuse fluorescence signal suggestive of protein release was observed. Scale bar = 200 µm.
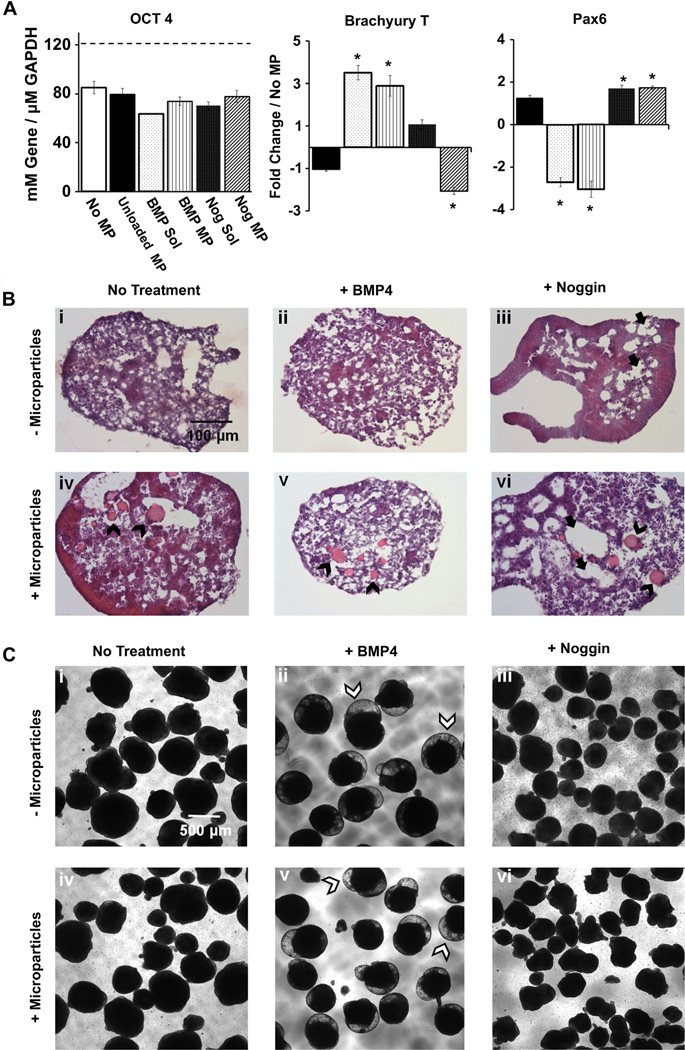
Gelatin MPs were next used to deliver either BMP4 or noggin within EBs for directed differentiation. Efficacy of MP-based delivery was tested at two loading concentrations of each molecule, 50 or 125 ng of growth factor per mg of MPs. The corresponding total amounts of growth factor delivered via MPs to ~ 1200 EBs per 10 cm plate of were 6 and 17 ng respectively; more than an order of magnitude less than the total amount of 210 ng of growth factor added over 5 days of soluble supplementation with a 10 ng/mL growth factor solution. After 4 days of culture, gene expression of early lineage commitment markers, Brachyury-T, Flk1 and Pax6, was analyzed (Supplemental Fig. 1). EBs containing both concentrations of BMP4-loaded particles exhibited increased expression of early mesoderm markers Brachyury-T and Flk1 compared to untreated and noggin treated samples. Pax6, a marker for neural ectoderm, was increased in noggin MP samples and decreased in BMP4 MP samples compared to untreated EBs. No significant differences were observed between noggin loading conditions; however, loading of 125 ng BMP4 per mg MP resulted in significantly greater gene expression of Brachyury-T and less Pax6 compared to the lower loading concentration (50 ng/mg MP). Therefore, for all subsequent studies of morphogen delivery from microparticles, a loading concentration of 125 ng of BMP4/mg MP was used, whereas 50 ng/mg MP was used for noggin delivery.
Directed differentiation by MP delivery of BMP4 and noggin was then compared to soluble delivery of the same molecules based upon gene expression analysis after 4 days of treatment (Fig. 2A). As expected, soluble delivery of BMP4 resulted in increased Brachyury-T expression and decreased Pax6 expression, while the opposite was true for soluble delivery of noggin. MP-based delivery of BMP4 and noggin resulted in similar changes in Brachyury-T and Pax6, with no significant differences in gene expression between soluble or MP-based delivery of growth factors. The presence of unloaded gelatin MPs within EBs did not significantly influence the expression of the specific genes examined compared to aggregates lacking MPs. Similarly, none of the experimental groups differed significantly from untreated controls in the decreased expression of the pluripotent transcription factor Oct4.
Fig 2.
Growth factor-laden MPs incorporated within stem cell aggregates can direct early lineage commitment. A) Directed differentiation by MP delivery of BMP4 and noggin induced comparable levels of lineage-specific gene expression compared to soluble delivery of the same molecules. Gene expression analysis indicated no change in the decrease of OCT4 in any of the groups. BMP4 delivered from MPs or added to the medium induced Brachyury-T (mesoderm) expression while noggin delivered by MPs or by soluble addition induced Pax6 (neuroectoderm) expression in day 4 EBs. * =significantly different from untreated samples p < 0.05. B) EBs treated with noggin possessed numerous cavitations (denoted by black full arrows), visible in histological analysis of day 7 H&E stained sections. EBs treated with BMP4 displayed areas of mesenchymal-like phenotypes, with a lower cell density organization compared to the non-cavitated regions of noggin treated EBs. No significant differences in morphology were observed between soluble or MP-based delivery. Scale bar = 100 µm. C) Morphological changes in day 10, suspension-culture EBs were observed. BMP4 treated EBs contained translucent, dome-like outgrowths (denoted by white arrowheads), absent in the other groups. In contrast, thin, cellular protrusions were observed in noggin treated EBs. MPs are indicated with black arrowheads. Scale bar = 500 µm.
3.2. Spheroid morphology
Although no differences in spheroid cross-section morphology were observed after 4 days of differentiation, gelatin MPs were visible in fixed sections of MP-containing EBs stained with hematoxylin and eosin (Supplemental Fig. 2). However, by 7 days of differentiation the differentially treated groups developed distinguishing morphological traits (Fig. 2B). In particular, EBs treated with noggin possessed numerous cavitations, while EBs treated with BMP4 displayed areas of mesenchymal-like phenotypes, with a lower cell density organization compared to the non-cavitated regions of noggin-treated EBs. No obvious differences in morphology were observed between EBs treated with soluble or MP-based growth factor delivery for either noggin or BMP4. Early differences in lineage commitment resulted in differences in aggregate morphology after 10 days of suspension culture (Fig. 2C). In both BMP4 MP and soluble BMP4 treated spheroids, optically translucent, bubble shaped outgrowths were observed in the majority of spheroids; these morphological features were not observed in untreated or noggin treated EBs. Similar differences in morphology due to BMP4 and noggin treatment were also observed in day 10 spheroids plated onto gelatin-coated substrates (Supplemental Fig. 3). Hollow, ring-like structures were commonly observed in plated BMP4 treated EBs with either MP or soluble delivery, but these structures were not observed in untreated or noggin treated EBs, where neurite outgrowths were frequently observed instead (Supplemental Fig. 3). Neurite outgrowths were consistent with early gene expression results for Pax6, a neuroectoderm marker, that was increased in noggin samples and decreased in BMP4 samples (Fig. 2, Supplementary Fig. 3). The stark differences in EB and cell morphology after 10 days of differentiation suggested that MP-based growth factor delivery impacted the trajectory of ES cell differentiation from an earlier period of time.
3.3. Mesoderm induction
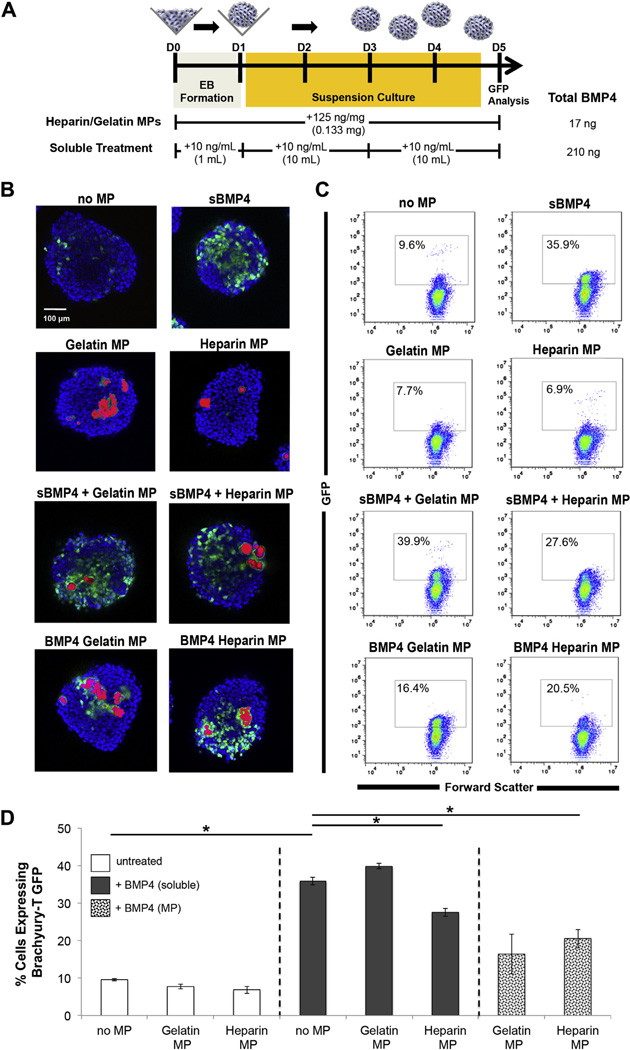
ESCs genetically engineered to express green fluorescent protein (GFP) under the control of the Brachyury-T promoter have been previously used to investigate hemogenic mesoderm specification during EB differentiation [15,18]. The Brachyury T-GFP reporter ES cells serve as a convenient tool to observe the spatial patterns of differentiation in response to a growth factor such as BMP4, that is known to promote early mesoderm lineage commitment in both human and mouse pluripotent cell lines [18–22]. BMP4 was delivered to Brachyury-T GFP EBs through MP-based delivery and soluble delivery, and GFP expression was analyzed by confocal microscopy and flow cytometry at day 5 of differentiation (Fig. 3) due to the fact that detectable numbers of GFP+ cells were first observed on day 4 of differentiation and reached a maximum level after 5 days, after which the % of GFP+ cells declined (Supplemental Fig. 5).
Fig 3.
MP delivery of BMP4 induces efficient mesoderm differentiation. A) Brachyury-T driven GFP expression was compared between MP and soluble delivery of BMP4 to EBs. MPs laden with BMP4 were added during formation, whereas soluble BMP4 was supplemented during EB formation, upon transfer to suspension culture and again with subsequent media changes up to day 5. B) Few GFP+ cells were observed in untreated spheroids or in spheroids with unloaded MPs. Compared to unmodified MPs, heparin modification of gelatin MPs resulted in increased numbers of GFP+ cells surrounding the incorporated particles. C,D) Flow cytometry for GFP+ cells was performed on dissociated aggregates on day 5. Spheroids treated with soluble BMP4 had the highest percentage of GFP+ cells (27.6 ± 1.1%), however BMP loaded MP groups had greater Brachyury-T GFP+ cells (20.5 ± 2.5% for Hep MP and 16.4 ± 5.3% for Gel MP) than the untreated and unloaded MP control groups (9.6 ± 0.3%). p < 0.05 denotes significance. Scale bar = 100 µm.
Few or no GFP expressing cells were visually observed in untreated EBs or in EBs containing unloaded gelatin or heparin–gelatin MPs by confocal analysis, but when soluble BMP4 (10 ng/ mL) was added to the culture medium, GFP+ cells were observed throughout EBs (Fig. 3B). Similarly, when BMP4 was added solubly to EBs containing unloaded MPs, GFP expression was again detected throughout EBs. On the other hand, incorporation of BMP4- laden MPs resulted in increased GFP expression in the vicinity of the observed MPs, with heparin–gelatin MPs having a more pronounced localization effect than simply gelatin MPs.
Flow cytometry of dissociated EBs indicated that a low percentage of GFP+ cells were present in untreated EBs (9.6 ± 0.3%) after 5 days, as well as EBs with unloaded heparin–gelatin (6.9 ± 1.0%) or gelatin particles (7.7 ± 0.7%) (Fig. 3C & D). Soluble addition of BMP4 resulted in the greatest number of GFP+ cells (35.9 ± 1.0%) at day 5 of differentiation. EBs with BMP4-loaded heparin–gelatin MPs had significantly higher Brachyury-T GFP+ cells (20.5 ± 2.5%) compared to untreated and unloaded MP spheroids, but not compared to spheroids containing gelatin MPs loaded with BMP4. Interestingly, the percentage of GFP+ cells was significantly lower when BMP4 was added to the culture medium of EBs containing unloaded heparin–gelatin MPs compared to soluble BMP4 added to untreated EBs or EBs with unmodified gelatin MPs, suggesting a possible interaction of the heparin-modified particles with exogenously added growth factor. The percentage of GFP+ cells within BMP4-loaded gelatin MP aggregates (16.4 ± 5.3%) was the most variable, with no significant difference in the number of GFP+ cells compared to untreated and unloaded MP spheroids, as well as EBs with BMP4-loaded heparin–gelatin MPs. Overall, the quantities of GFP+ cells obtained from different EB experimental groups were consistent with the observed patterns of GFP expression in intact EBs. These results demonstrate that MP-based delivery of morphogens can promote specific differentiation in a more localized manner within multi-cellular aggregates compared to conventional soluble delivery of the same molecules.
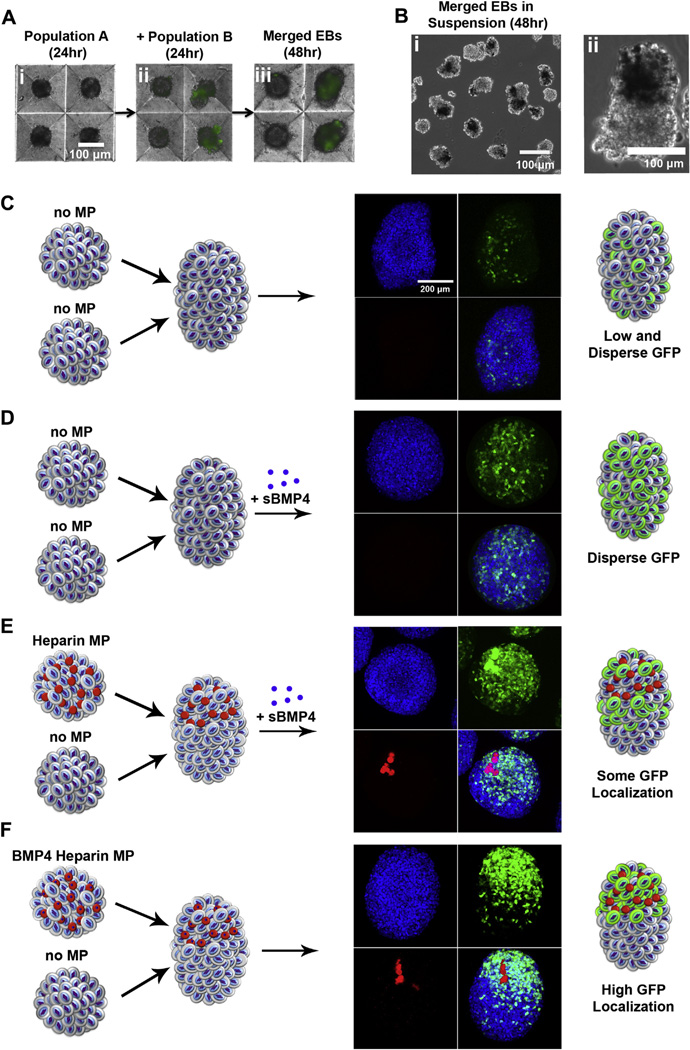
3.4. Aggregate co-culture for localized mesoderm induction
Having established that incorporation of growth factor-laden MPs within stem cell spheroids was capable of inducing efficient localized differentiation, we investigated the potential of using MP incorporation within single aggregates to spatially control differentiation within merged spheroids. This study was motivated by previous observations that MPs incorporated within opposite hemispheres of ESC aggregates maintained spatial separation throughout several days of differentiation [23]. We previously demonstrated that magnetic control of multicellular aggregate merging can be achieved to spatially pattern complex 3D structures; however, this method required tedious merging of single spheroids one-at-a-time. By simply adding a second spheroid population to microwells containing a pre-formed population of ESC aggregates after 24 h (Fig. 4Ai), we observed that the two populations of EBs would readily merge after an additional day of culture (Fig. 4Aii,iii). The second spheroid population was added at half the number of the first population (preformed in the micro-wells) to decrease the probability of multiple merging events in single microwells. This method allowed for the creation of single aggregates containing MPs localized within one hemisphere of the newly merged spheroid (Fig. 4B). EBs merged in the absence (Fig. 4C) or presence of BMP4 (Fig. 4D) resulted in similar GFP expression patterns to the respective single EB studies described above. When unloaded heparin–gelatin MPs were incorporated on one hemisphere and BMP4 was added to the culture medium, Brachyury-T GFP+ cells were observed throughout the aggregate, although the density of GFP+ cells appeared greater in the hemisphere containing the MPs (Fig. 4E). However, when BMP4 was delivered only from heparin–gelatin incorporated MPs, GFP+ cells were located predominantly within the hemisphere containing the originally incorporated MPs (Fig. 4F), suggesting the ability of morphogen-laden MPs to spatially direct differentiation within specific regions of multicellular stem cell aggregates.
Fig 4.
Spheroid merging enables local control of mesoderm induction. A) One day after aggregate formation, a second spheroid population was added containing fluorescently labeled MPs. After an additional day of culture, spheroids had merged in the microwells (arrows). B) A merged spheroid in suspension fabricated from an untreated spheroid and a spheroid containing optically dense MPs. C) The merging of untreated spheroids resulted in few GFP+ cells dispersed throughout individual aggregates, whereas D) addition of soluble BMP4 promoted GFP+ cells throughout merged aggregates. E) Soluble BMP4 addition to spheroids containing unloaded heparin–gelatin MPs resulted in some GFP localization mostly within the hemisphere containing the MPs. F) The merging of untreated aggregates with aggregates containing BMP4 loaded heparin–gelatin MPs yielded GFP+ cells localized predominantly within The hemisphere of the spheroid containing the MPs. Scale bars = 100 µm (A,B), 200 µm (C–F).
4. Discussion
In this study, we demonstrated that MP-mediated delivery can be used as an efficient alternative to soluble addition of growth factors for directed differentiation of pluripotent stem cell aggregates. The morphology of aggregates containing BMP4- or noggin-loaded MPs was markedly different between treatment groups after 7 days of EB differentiation and differences persisted throughout 10 days of culture, suggesting that the differentiation trajectory of ESCs can be controlled by early presentation of morphogens using MPs. Compared to soluble treatments, MP delivery of BMP4 and noggin within EBs resulted in similar induction of mesoderm and ectoderm gene expression, respectively, without significantly affecting loss of pluripotency, as denoted by decreased Oct4 expression. Brachyury-T gene expression was increased in EBs treated with soluble BMP4 or MPs loaded with BMP4 and the percentage of Brachyury-T-GFP positive cells was similarly increased by either method. In contrast to conventional soluble delivery methods, MP delivery concentrates the amount of delivered growth factor within the volume of the multicellular aggregates such that even over short periods of growth factor treatment (i.e. 5 days), an order of magnitude less total growth factor is needed to elicit a comparable phenotypic response. Therefore, MP-based growth factor delivery approaches are a particularly attractive alternative for more efficient scale-up production of differentiated cells from ESCs in suspension culture.
Interestingly, when BMP4 was delivered from gelatin MPs conjugated with heparin, Brachyury-T induction was localized near the MPs. This observation indicated that alterations in the affinity of the material to the delivered growth factor can result in changes to the microenvironment surrounding the MPs. For instance, our previous study on the release of BMP4 from MPs fabricated from gelatin types A and B confirmed the important role of the polyion complexation between the growth factor and the MP base materials [18]. BMP4, with an isoelectric point of approximately 9.0, carries a net positive charge at neutral pH and was almost entirely released from similar positively-charged, gelatin type A particles after just 6 h. Heparin modification of gelatin type A particles resulted in release kinetics mirroring those of negatively charged type B particles with most release occurring over a 4 day period and leaving residual growth factor associated with the MPs. Growth factor-MP affinity is likely responsible for stable localization of growth factor and subsequent mesoderm induction of the cells surrounding the MPs. Based on the ability of heparin to sequester exogenously delivered BMP4 [24,25], the low percentage of GFP+ cells in spheroids with unloaded heparin–gelatin MPs suggests a lack of endogenous BMP4 activity within EBs during the first several days of differentiation. Along the same line of thought, biomaterials with engineered affinity to molecules secreted endogenously during differentiation may present an opportunity to sequester and harness autocrine and paracrine signaling within three-dimensional aggregates for directed differentiation without the use of exogenous factors. Such an approach can target a specific molecule, such as vascular endothelial growth factor [26], or a group of growth factors with similar affinity, such as heparin-binding growth factors, for example [27,28]. Thus, engineering of MP properties and desired molecular affinities may be aided by studies of endogenous extracellular matrix production and growth factor expression profiles by ES cells undergoing differentiation [29].
We hypothesized that BMP4 delivered either by incorporation of loaded heparin–gelatin MPs or delivered solubly to spheroids with unloaded heparin–gelatin MPs may be sequestered by the MPs and locally concentrated. Sequestration of BMP4 by heparin-modified MPs, would, at least partially, account for the reduced frequency of Brachyury-T GFP+ cells using MP-based delivery compared to soluble delivery alone. This hypothesis prompted investigation of pairing morphogen and material interactions to spatially control morphogen delivery within ES cell aggregates. When BMP4-laden, heparin–gelatin MPs were localized to one hemisphere of a single EB, mesoderm differentiation, visualized by Brachyury-T GFP expression, was localized to the same hemisphere. The ability to spatially control delivered morphogens via MP-mediated delivery of growth factors addresses an inherent limitation and unavoidable shortcoming of traditional soluble treatment methods. The process of mammalian development is heavily dependent on spatially and temporally controlled presentation of morphogenic factors, and while EBs are frequently used to study early developmental events, such as gastrulation, primitive streak, and epithelial–mesenchymal transitions [30–32], study of more complicated phenomena, including tissue morphogenesis, requires finer spatiotemporal control of morphogen presentation, as demonstrated here. Overall, flexibility in the properties of MP-base materials permits control over the temporal release profile as well as the amount of growth factor delivered, two parameters which are difficult to control in classical mammalian developmental models.
5. Conclusions
In this study we have demonstrated how incorporation of biomaterial microparticles within stem cell aggregates can be used to deliver morphogens in order to differentially direct stem cell differentiation. MP-based delivery of growth factors is a more efficient method of presentation than soluble delivery that can aid directly in the scalable differentiation of pluripotent stem cells. Additionally, the ability to localize delivery of morphogens can result in spatially controlled differentiation within stem cell aggregates, an important enabling technology for engineering of complex tissues from pluripotent cells, as well as fundamental studies of morphogen gradients and their effects in EBs to model analogous processes that occur during mammalian embryonic development.
Supplementary Material
Acknowledgments
Financial support was provided by funding from the National Institutes of Health (GM088291) and the National Science Foundation (CBET 0651739). ABL was supported by an NIH training grant (GM008433) as well as funding from the Goizueta Foundation. AN was supported by an NSF Graduate Research Fellowship. Brachury-T GFP cells were kindly provided by Dr. Gordon Keller. We are grateful for use of core facilities provided by the Parker H. Petit Institute of Bioengineering and Bioscience and to Ms. Melissa Kinney for assistance with statistical analysis.
Footnotes
Appendix A. Supplementary material
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.biomaterials.2013.05.081.
References
- 1.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 2.Bratt-Leal AM, Carpenedo RL, Ungrin MD, Zandstra PW, McDevitt TC. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials. 2011;32:48–56. doi: 10.1016/j.biomaterials.2010.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 4.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 5.Czyz J, Wobus A. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation. 2001;68:167–174. doi: 10.1046/j.1432-0436.2001.680404.x. [DOI] [PubMed] [Google Scholar]
- 6.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, et al. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 8.Carpenedo RL, Bratt-Leal AM, Marklein RA, Seaman SA, Bowen NJ, McDonald JF, et al. Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials. 2009;30:2507–2515. doi: 10.1016/j.biomaterials.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachlos E, Auguste DT. Embryoid body morphology influences diffusive transport of inductive biochemicals: a strategy for stem cell differentiation. Biomaterials. 2008;29:4471–4480. doi: 10.1016/j.biomaterials.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Van Winkle AP, Gates ID, Kallos MS. Mass transfer limitations in embryoid bodies during human embryonic stem cell differentiation. Cells Tissues Organs. 2012;196:34–47. doi: 10.1159/000330691. [DOI] [PubMed] [Google Scholar]
- 11.Jing D, Parikh A, Tzanakakis ES. Cardiac cell generation from encapsulated embryonic stem cells in static and scalable culture systems. Cell Transplant. 2010;19:1397–1412. doi: 10.3727/096368910X513955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield and homogeneity of embryoid body differentiation. Stem Cells. 2007;25(9):2224–2234. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 13.Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS ONE. 2008;3:e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen D, Sa S, Pegan JD, Rich B, Xiang G, McCloskey KE, et al. Tunable shrink-induced honeycomb microwell arrays for uniform embryoid bodies. Lab Chip. 2009;9:3338–3344. doi: 10.1039/b914091c. [DOI] [PubMed] [Google Scholar]
- 15.Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 16.Rahman N, Purpura KA, Wylie RG, Zandstra PW, Shoichet MS. The use of vascular endothelial growth factor functionalized agarose to guide pluripotent stem cell aggregates toward blood progenitor cells. Biomaterials. 2010;31:8262–8270. doi: 10.1016/j.biomaterials.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 17.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purpura KA, Bratt-Leal AM, Hammersmith KA, McDevitt TC, Zandstra PW. Systematic engineering of 3D pluripotent stem cell niches to guide blood development. Biomaterials. 2012;33:1271–1280. doi: 10.1016/j.biomaterials.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 20.Pick M, Azzola L, Mossman A, Stanley EG, Elefanty AG. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. 2007;25:2206–2214. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- 21.Pearson S, Sroczynska P, Lacaud G, Kouskoff V. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development. 2008;135:1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- 22.Purpura KA, Morin J, Zandstra PW. Analysis of the temporal and concentration-dependent effects of BMP-4, VEGF, and TPO on development of embryonic stem cell-derived mesoderm and blood progenitors in a defined, serum-free media. Exp Hematol. 2008;36:1186–1198. doi: 10.1016/j.exphem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Bratt-Leal AM, Kepple KL, Carpenedo RL, Cooke MT, McDevitt TC. Magnetic manipulation and spatial patterning of multi-cellular stem cell aggregates. Integr Biol (Camb) 2011;3:1224–1232. doi: 10.1039/c1ib00064k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraushaar DC, Rai S, Condac E, Nairn A, Zhang S, Yamaguchi Y, et al. Heparan sulfate facilitates FGF and BMP signaling to drive mesoderm differentiation of mouse embryonic stem cells. J Biol Chem. 2012;287:22691–22700. doi: 10.1074/jbc.M112.368241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan SA, Nelson MS, Pan C, Gaffney PM, Gupta P. Endogenous heparan sulfate and heparin modulate bone morphogenetic protein-4 signaling and activity. Am J Physiol Cell Physiol. 2008;294:C1387–C1397. doi: 10.1152/ajpcell.00346.2007. [DOI] [PubMed] [Google Scholar]
- 26.Impellitteri NA, Toepke MW, Lan Levengood SK, Murphy WL. Specific VEGF sequestering and release using peptide-functionalized hydrogel micro-spheres. Biomaterials. 2012;33:3475–3484. doi: 10.1016/j.biomaterials.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudalla GA, Kouris NA, Koepsel JT, Ogle BM, Murphy WL. Harnessing endogenous growth factor activity modulates stem cell behavior. Integr Biol (Camb) 2011;3:832–842. doi: 10.1039/c1ib00021g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benoit DS, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005;1:461–470. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Nair R, Ngangan AV, Kemp ML, McDevitt TC. Gene expression signatures of extracellular matrix and growth factors during embryonic stem cell differentiation. PLoS ONE. 2012;7:e42580. doi: 10.1371/journal.pone.0042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen DR, Gustavsen C, Lindskog SR, Magnuson MA, Zaret KS, Serup P. Engineering artificial signaling centers to polarize embryoid body differentiation. Stem Cells Dev. 2012;21:647–653. doi: 10.1089/scd.2011.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharon N, Mor I, Golan-lev T, Fainsod A, Benvenisty N. Molecular and functional characterizations of gastrula organizer cells derived from human embryonic stem cells. Stem Cells. 2011;29:600–608. doi: 10.1002/stem.621. [DOI] [PubMed] [Google Scholar]
- 32.ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.