Abstract
Rheumatoid arthritis (RA) is a common inflammatory disease characterized by progressive bone and cartilage destruction, resulting in severe functional limitations, shortened lifespan, and increased mortality rates. Recent advances and new treatment approaches have significantly delayed disease progression and improved the quality of life for many patients. Yet few patients attain or can be maintained in disease remission without continuous immunosuppressive therapy. In addition a sizable portion of patients also fails to respond or eventually develops tolerance to current therapies. Thus there is a continued need for the development of new therapeutic strategies for the treatment of RA. Unlike conventional drugs, nanosystems are designed to deliver therapeutic agents specifically to the site of inflammation, therefore avoiding potential systemic and off-target unwanted effects. They allow investigators to consider or reconsider therapeutic agents that were previously deemed too toxic to deliver through a systemic route. This article reviews recent nanotechnology-based strategies that are being developed for the treatment of inflammatory arthritis.
RA is a chronic systemic inflammatory disease affecting approximately 1% of the general population worldwide. It is characterized by persistent polyarticular inflammation of the synovial tissues, leading ultimately to the destruction of articular cartilage and bone of the affected joints. Left untreated the progressive damage leads to severe functional deterioration and premature death.(1)
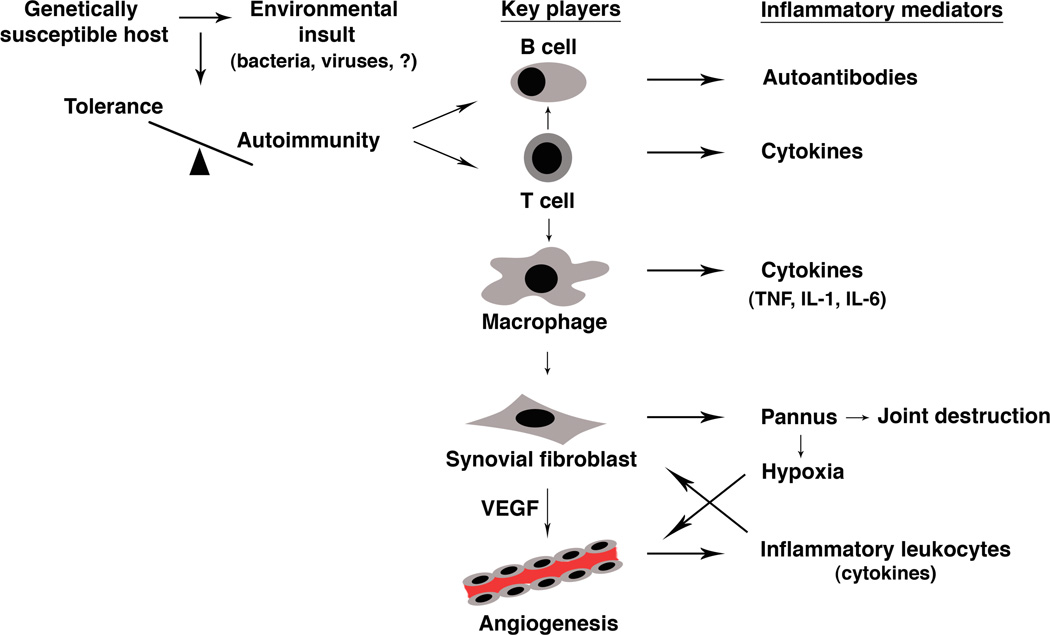
RA is a complex disease with multiple interacting mechanisms, including genetic components and environmental influences that shape the subsequent immune response (Figure 1). Studies over the years have identified multiple cell types (including B cells, T cells, macrophages/ synoviocytes) as key regulators of immunologic events in RA.(2) The role of B cells recently gained significant attention, as it became evident that B-cell-depleting therapy (anti-CD20 monoclonal antibodies or rutiximab) is effective in RA.(3) Similarly, T cells have also been implicated as primary mediators in the pathogenesis of RA. T cells are prominent in RA synovium and they contribute to the inflammatory response through the elaboration of cytokines as well as the interaction with other cells that perpetuate the inflammation and joint destruction.
Figure 1. Pathogenesis of RA.
In a genetically susceptible individual an environmental insult leads to a breach of immune tolerance, tipping the balance toward autoimmunity. This is usually heralded by the production of autoantibodies (rheumatoid factor, anti-citrullinated protein antibody) by B cells with the help of T cells. Recruitment of activated T cells to the synovium leads to macrophage activation and the overproduction of inflammatory cytokines, including TNF-α, IL-1 β, and IL-6. Cytokines also stimulate the proliferation of synovial fibroblasts, forming a pannus that is capable of invading cartilage and bone, leading to joint destruction. In addition, production of vascular endothelial growth factor (VEGF) by synovial fibroblasts and other cells stimulate angiogenesis, which perpetuates the inflammation by recruiting more inflammatory leukocytes. Growth of the pannus also induces a state of relative hypoxia that further promotes angiogenesis through the elaboration of hypoxia-inducible factor 1 (HIF-1).
Traditional paradigm for RA has also implicated a variety of cytokines in the pathogenesis of RA. Tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and IL-6, among others, have been found to be consistently elevated in synovial fluid of patients with RA.(4) Furthermore, TNF-α and IL-1β both induce synovial cells to release tissue degrading matrix metalloproteases and TNF-α stimulates the development of osteoclasts, which are responsible for bone erosions. Animal models provide further evidence of the importance of these cytokines in RA. Mice expressing a dysregulated and modified human TNF transgene developed spontaneous arthritis.(5) Treatment of these arthritic mice with a monoclonal antibody against human TNF-α completely abrogated the development of this disease. Also, administration of neutralizing antibodies to IL-1β ameliorated bone loss and cartilage destruction in a model of collagen-induced arthritis (CIA).(6) In fact, the understanding of cytokine actions in animal models led to clinically effective treatments of RA, as demonstrated by the use of biological agents, such as TNF-α, IL-1β, and IL-6 inhibitors. Yet, despite these advances in medical treatment over recent years, many patients with RA fail to respond to these new biological agents.(7) In addition, studies show that around half of the initial responders to anti-TNF therapy stop treatment in the first year due to loss of efficacy or side effects.(8) Many of these patients will ultimately require joint replacement to improve or maintain their daily activities. Although other biological therapies are beginning to emerge,(9) the complexity of RA, the heterogeneity of the patients, and previous experience with biologics suggest that targeting a single receptor or cytokine pathway will not lead to a predictable response in every patient.
Nanotechnology is a multidisciplinary approach that employs a diverse array of tools and techniques aimed at the diagnosis of disease and the delivery of therapeutic agents with the use of submicrometer size carriers, nanocarriers. Unlike conventional drugs, these nanocarriers allow targeted delivery of therapeutic agents specifically to the desired site of inflammation and can potentially be adjusted individually according to alterations in disease expression. In addition to their therapeutic actions, these nanocarriers potentially permit non-invasive and quantitative image-based readouts of drug effects, which may one day allow the practitioners to monitor and optimize therapies based on individual responses. This review focuses on the emerging nanotechnology that is being developed for the treatment of RA.
Current therapeutic strategies for RA
Advances in the understanding of RA pathogenesis have led to revisions in the standard treatment recommended for RA over the past decade. Although non-steroidal anti-inflammatory drugs (NSAIDs) are still widely used to lessen pain, they are no longer considered first-line treatment because of their limited effectiveness, inability to modify disease course in the long-term, and adverse effects.(10) Current strategies and recommendations for RA are modeled after other disciplines, oncology in particular, and centered on early, intensive treatment with disease-modifying anti-rheumatic drug (DMARD) monotherapy or, more increasingly, combination therapy together with analgesics such as NSAIDs to suppress disease as soon as possible or to induce remission.(2) The cornerstone of conventional DMARD therapy is methotrexate (MTX), an antimetabolite that inhibits dihydrofolate reductase. MTX is effective in approximately 30% of patients as monotherapy but also serves as an important anchor drug for successful combinations with other conventional DMARDs or biologics.(2) The introduction of biologics has revolutionized the treatment of RA. This class thus far includes cytokine antagonists (TNF, IL-1 and IL-6 inhibitors or receptor antagonists), B cell depleting agents, and T cell co-stimulation modulator.(2, 11) The efficacy of biologics, especially TNF inhibitors in combination with MTX, is well documented, with the probability of 60–70% of patients achieving positive response when therapy is initiated early in the course of the disease.(12) Yet despite these impressive statistics, there are several drawbacks to current biologic therapy, including high costs, the occurrence of infectious complications, and the loss or failure to maintain response overtime. Glucocorticoids (GCs) represent an affordable class of anti-inflammatory agents that are widely used in active RA as co-therapy with other DMARDs. Unlike conventional DMARDs and biologics, GCs have rapid effects and are also frequently used as “bridging therapy” at the initiation of RA treatment. More recent studies show that systemic GCs as co-therapy has disease-modifying effects in patients with RA during the first two years of treatment.(13) In addition, intra-articular GC therapy in combination with MTX has been shown to prevent progression of joint damage.(14) However long-term use of GCs is associated with a number of unwanted side effects, including increased risks of cardiovascular diseases, osteoporosis, infections, and impaired glucose metabolism, just to name a few.(13)
In summary, despite increasing therapeutic options (Table 1), RA remains a challenging disease as current recommended regimens rarely lead to a cure (remission) and are often associated with development of drug resistance and adverse events. Moreover, the optimal and effective therapeutic approach to an individual cannot be predicted at this point due to the heterogeneity of the patients, thus necessitating “trials and errors” that increase costs and delay clinical response.
Table 1.
Current therapeutic options for RA
| Drugs | Mechanisms of action |
|---|---|
| NSAIDs | Analgesia |
| Immunomodulation and anti-angiogenesis? | |
| Glucocorticoids | Immunosuppression |
| Disease-modifying activity? | |
| Methotrexatea | Immunosuppression |
| Disease-modifying activity | |
| Biologics | |
| a) Anti-cytokines | Antagonism of cytokine actions |
| • Anti-TNF | |
| • Anti-IL-6 | |
| • Anti-IL-1 | |
| b) Anti-T cell | Down-regulation of T cell activation |
| • CTLA4-lg | |
| c) Anti-B cell | B cell depletion |
| • Anti-CD20 | |
| Kinase inhibitors | |
| a) Syk inhibitors (Phase II trials) | Inhibition of spleen tyrosine kinase Antagonism of cytokine actions |
| b) Jak inhibitors (Phase II trials) | Inhibition of janus kinase Antagonism of cytokine actions |
| c) IκB inhibitors (Preclinical trials) | Inhibition of IκB kinase of NF-κB pathway Antagonism of cytokine actions |
Other disease-modifying drugs routinely used in RA therapy include sulfasalazine, hydroxychloroquine, and leflunomide.4
Nanotherapeutic approaches to RA treatment
Adverse effects due to the nonselective activity of the drugs often limit dose escalation in current RA therapy. By encapsulating bioactive agents into a nanocarrier, selective drug delivery to the desired sites of action (i.e. the joints) may be achieved through a process known as targeted approach that avoids high or frequent dosing to attain effective drug concentration locally. Although intra-articular injection offers the most direct targeted therapy, this treatment option is invasive (requiring repeated joint needling and increasing the risk of infection) and often short-lived. The development of a targeted nanocarrier system for sustained drug delivery in RA is thus highly desirable. In addition, nanocarrier systems may increase the solubility of certain drugs and protect them against degradation in the circulation, further increasing their local bioavailability. Therefore, the use of nanocarriers promises to increase drug specificity and bioavailability while reducing unwanted off-target side effects.
A survey of the literature suggests that the use of targeted nanocarriers to deliver therapeutic agents (nanotherapeutics) in the treatment of inflammatory arthritis remains largely unexplored. Targeting of macrophages using a nanocarrier system was an approach that was investigated early on as it was known that the presence of macrophages is increased in inflamed joints and nanoparticles can be efficiently phagocytosed by macrophages even without surface modifications. This approach is known as passive targeting. One of the first studies used low-dose clodronate, a bisphophonate that induces phagocytic cell apoptosis,(15) encapsulated within liposomes (unilamellar or multilamellar lipid vesicles of 100 nm mean size) to modulate macrophage-mediated production of pro-inflammatory cytokines.(16) Weekly intra-articular injections of low dose liposomal clodronate in rabbits led to temporary reduction and delay in joint swelling but the effect was not sustained after the first week.(17) As higher dose of liposomal clodronate had pro-inflammatory effects and induced synovitis,(18) it is unlikely that this approach will find practical use in the actual treatment of arthritis. Targeting of macrophages through parenteral (systemic) administration of nanocarrier systems has also been actively pursued. These approaches take advantage of the fact that macrophages are central players in RA (by producing pro-inflammatory cytokines) and nanocarriers can be taken up by macrophages into arthritic joints through inflamed leaky capillaries, an effect known as enhanced permeability and retention (EPR). However, systemically administered nanocarriers can also be cleared quickly by macrophages residing in the reticulo-endothelial system (RES), thereby decreasing the availability of drugs reaching the inflamed joints. Thus, surface modifications of nanocarriers to retard RES interaction and selective (active) targeting of other organ systems or immune cells and pathways are constantly being explored. In the following sections, examples of more recent nanotechnology-based approaches to RA treatment will be reviewed.
New delivery systems for NSAIDs
NSAIDs are widely used in RA mainly for their analgesic effects. NSAIDs work through the inhibition of cyclooxygenase (COX) enzymes that play an important physiological and pathological role in many pathways, including inflammation, pain, bone and cartilage erosion, and angiogenesis.(19) However, their disease modifying potentials in long-term use are often overlooked due to variable differences in efficacy and side effects at high doses, especially in the more susceptible pediatric and elderly populations. Although the discovery of selective cyclooxygenase-2 (COX-2) inhibitors initially promised exciting alternatives to the frequently encountered gastrointestinal side effects associated with the more permissive COX-1 inhibition, it was soon discovered that long-term use of COX-2 inhibitors led to serious increase in cardiovascular risks, including myocardial infarction and stroke,(20) which resulted in the suspension and eventual withdrawal of several COX-2 inhibitors from the market.(21)
Recent data suggest that aside from their analgesic and anti-inflammatory effects, NSAIDs also possess immunomodulatory and anti-angiogenic properties that have recently been explored for cancer therapy.(22) Coupled with recent advances in drug delivery technology these studies have prompted researchers to reconsider the usefulness of NSAIDs in the treatment of arthritis. The current approaches aim at decreasing NSAID-related adverse effects through site-specific delivery and controlled release. For lipophilic drugs such as NSAIDs, several groups of investigators found that lipid microspheres (LM), lipid-based preparations with an internal oil phase surrounded by a phospholipid monolayer, offer better loading capacity compared with conventional liposomes. Early studies with LM preparations encapsulating the NSAID indomethacin indicated that encapsulation improved the anti-inflammatory activity of NSAID while lowering the gastrointestinal side effects.(23) However, LM are rapidly cleared by the RES. The incorporation of polyethylene glycol (PEG) to LM delayed their uptake by the RES system by three-fold, prolonging their circulation time and increasing the bioavailability of the encapsulated NSAID.(24, 25) Systemic administration of PEG-LM encapsulating the NSAID indomethacin led to significantly higher accumulation of the drug in paws of arthritic rats, 7.5-fold higher compared with conventional LM. (25) The accumulation of drug is likely due to extravasation of LM through the EPR effect and/or uptake by circulating monocytes with subsequent delivery to the arthritic joints.(25) Limitations of lipid-based preparations, however, include variable encapsulation efficiency and poor control of drug release.(26) Nanoencapsulation of NSAIDs can also be achieved using biodegradable polymers. Bernardi et al. described polymeric nanocapsules prepared with polysorbate 80, a hydrophilic coating that delays the binding of plasma proteins, thus prolonging the circulation time of these nanocapsules. When loaded with indomethacin these nanocapsules exhibited potent anti-inflammatory effects in an adjuvant-induced model of chronic arthritis, as evidenced by markedly depressed serum levels of pro-inflammatory cytokines TNF-α and IL-6 and enhanced levels of the anti-inflammatory cytokine IL-10.(27) In this model, it is thought that indomethacin-nanocapsules accumulate at sites of inflammation mainly through the EPR effect. Dendrimers are another emerging class of biocompatible nanoparticles that promise to be effective vectors for the delivery of NSAIDs due to their versatile surface functionalities. The branching structure of dendrimers can either entrap small drug molecules or their many end functional groups can be covalently attached to NSAIDs, thus increasing the solubility of these hydrophobic drugs. Poly(amidoamine) (PAMAM) dendrimers were shown to significantly increase the solubility of the NSAID flurbiprofen and prolong the inhibition of carrageenan-induced paw edema after intravenous administration to Sprague-Dawley rats.(28) In a separate study, Chandrasekar et al. exploited the overexpression of folate receptor on activated macrophages in RA as a targeting strategy. They conjugated folate to PEG-PAMAM dendrimers and loaded them with indomethacin. They showed that folate-PEG-PAMAM dendrimers have 10–20 fold increases in drug loading efficiency and circulatory half-life. Loaded drug displayed an initial rapid release followed by a slower more sustained release, leading to enhanced and more controlled delivery of indomethacin to the joints while lowering uptake by the gastrointestinal tract.(29) Thus dendrimers promise better targeting efficiency of NSAIDs with potentially reduced side effects. Targeted drug delivery can also be achieved through an external stimulus (i.e. ultrasound, light, magnetic field, etc.). For example, Arias et al. developed an iron/ethylcellulose (core/shell) nanoparticle that when loaded with the NSAID diclofenac resulted in a nanoplatform with high drug loading capacity and more prolonged drug release.(30) The iron core also offers another potential level of targeting by enhancing the local delivery of diclofenac to inflamed joints under the guidance of an external magnetic field. Taken together, these recent findings suggest that new nanosystems may represent a relevant alternative approach for the targeted delivery of NSAIDs in the treatment of RA that promises to decrease off-target undesirable effects. Whether higher local levels of NSAIDs will have the same immunodulatory effects seen in animal models of experimental arthritis remain to be determined.
New delivery systems for glucocorticoids (GCs)
Although the efficacy of GCs as a fast-acting anti-inflammatory agent has been confirmed, their prolonged use is still discouraged due to well-known potential side effects.(13) As GCs are rapidly cleared following systemic administration, high and frequent dosing are often needed to achieve the desired anti-inflammatory effects locally, thus further increasing the risk of adverse effects. To improve risk:benefit ratios, new preparations of GCs aimed at targeted delivery, with sustained release of active drug at the inflamed sites, while minimizing dose frequency and systemic exposure are being sought. Several reports of PEG-liposomes containing different GC preparations so far look promising in rodent models of experimental arthritis. By encapsulating the GC prednisolone into small PEG-liposomes (~100 nm in size) Metselaar et al. showed that these nanocarriers remained in the circulation with a half-life of 50 hrs and a single systemic administration of this preparation led to complete reversal of paw inflammation within 2 days of injection, with the effect lasting for 2 weeks.(31) The small PEG-liposomes can selectively extravasate into inflamed joints while larger liposomes (~450–500 nm in size) and non-PEG liposomes localized mainly to the RES. In addition, the investigators observed therapeutic activity with these liposomes at GC doses 100-fold lower than that of unencapsulated GC. The same group also explored different GC formulations that have pharmacokinetic behaviour more comparable to human pharmacokinetics; however, sustained free GC levels, presumably due to unstable incorporation of the drug, continued to hinder the development of liposomal GC.(32) More recently, polymer conjugates of GCs have gained interest for therapeutics GC applications. Contrary to liposomes, drugs are bound to polymer conjugates instead of being trapped in the cavity of the particles and are thus released more slowly and hence necessitating less frequent administration. Ishihara et al. developed biocompatible and biodegradable blended nanoparticles of poly(D,L-lactic/glycolic) (PLGA)/poly(D,L-lactic acid) (PLA) homopolymers and PEG-PLGA/PLA block copolymers encapsulating betamethasone to examine the therapeutic activity of GCs in adjuvant arthritis in rats and antibody-induced arthritis in mice.(33, 34) The polymeric nanoparticles accumulated at the target sites (paws) through EPR effect and were phagocytosed by resident inflammatory macrophages after the loss of PEG, allowing gradual release of GCs over a period of 14 days. The investigators observed no changes in body weight or serum glucose levels, evidence of decreased systemic side effects. In addition, self-assembled, linear cyclodextrin polymer (CDP)-based nanoparticles conjugated to methylprednisolone (MP) have been shown to be effective in reducing the symptoms of collagen-induced arthritis in mice at doses up to 100-fold lower using weekly injections.(35) MP was conjugated to CDP through an ester linker that can undergo pH-dependent or enzyme-induced cleavage in the RA synovium, thus providing another mechanism for site-specific release GC. However, regardless of the preparations (liposomes versus polymers) a significant amount of GCs still accumulates in the RES following systemic administration, likely through macrophage uptake, prompting some investigators to examine local delivery strategies. Although intra-articular GCs represent a useful adjunct for local control of arthritis, especially when used in combination with systemic therapy with DMARDs or biologics, rapid clearance and systemic absorption of intra-articular GCs are well documented and represent major drawbacks for this approach.(36) To address these shortcomings, Butoescu et al. investigated superparamagnetic iron oxide microparticles as potential GC carriers for intra-articular delivery.(37, 38) These microparticles (1–10 µm in size) were efficiently internalized by synovial fibroblasts, did not induce any local inflammatory reaction, and could theoretically be maintained in the joint for longer periods of time with the help of an external magnet. In summary, liposomal and polymeric preparations may improve the safety profile of GCs by decreasing dosing and frequency of administration. However, the fact that disease usually recrudesces upon therapy withdrawal suggests that GCs by themselves have limited long-term immunomodulatory effects.
Nanotechnology-based gene therapy in the treatment of RA
Synovial cytokines play a key role in RA pathophysiology and their destructive effects on bones and articular cartilage provide the rationale for current RA treatment strategies, namely monoclonal antibodies against TNF-α, IL-1β, and IL-6. Despite their success, several limitations persist for the use of these biologics. Systemic immunosuppression due to repeated injections of biologics increases the risks of serious opportunistic infections and certain malignancies.(39) Moreover, a significant segment of the patient population does not respond to biologics, even in combination with DMARDs, or stop responding to therapy along the way. Gene therapy, the delivery of nucleic acids into the cell to silence, repress or override the aberrant expression of a protein, represents a promising therapeutic approach for many human disorders. In RA, gene therapy promises local and joint-specific targeted approach to either silence the expression of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) or overexpress anti-inflammatory cytokines (IL-1ra, IL-4, IL-10, IFN-β) in the hope that long-term expression of these anti-arthritic agents will lead to sustained anti-inflammatory effects while avoiding systemic adverse reactions. The use of viral vectors, including retroviral (RV), adenoviral (AdV), and adeno-associated virus (AAV) vectors for gene therapy in RA has been explored both in animal models and a few clinical trials. These studies have been extensively reviewed elsewhere.(40) Although the strategy usually entails local injections (direct intra-articular or intramuscular), systemic humoral immune responses to viral antigens, unwanted ectopic transgene expression (due to spreading of the vector), and oncogenic effects continue to be concerns for the use of viral-based vectors, prompting the search for alternative gene transfer methods. The search for an ideal non-viral, nanotechnology-based vector for gene therapy is part of an ever-growing field. Non-viral, nanotechnology-based vectors for gene therapy present many advantages to viral-based vectors, including low immunogenicity, no risk of infection, and no insertional mutagenesis. Many of these nanothechologic approaches have been extensively reviewed elsewhere.(41–44) This segment of the review will concentrate on some examples of gene therapy for the treatment of RA. One of the earliest investigations employed the use of in vivo transfection that is based on the method for in vitro electroporation to locally introduce naked double-stranded RNA molecules known as small interfering RNA (siRNA) designed to silence TNF-α that, when combined with electroporation, led to inhibition of paw inflammation in a collagen-induced arthritis (CIA) model.(45) However, this approach may be too cumbersome to translate to the clinical setting. Systemic delivery of siRNA on the other hand requires a carrier system that will protect the siRNA against degradation and rapid clearance. To enhance the stability and potency of siRNA when injected systemically, another group of investigators mixed siRNA with carrier DNA before complexation with cationic liposomes (lipolexes) for in vivo silencing of pro-inflammatory cytokines. Carrier DNA, by presenting positive charge density (zeta potential) on the surface, favors interaction with cells, making lipoplexes ideally suited for more efficient siRNA delivery. Using a mouse CIA model, the investigators showed that siRNA lipoplexes directed against TNF-α or a cocktail of siRNA lipoplexes directed against IL-1, IL-6 and IL-18 simultaneously led to significant reduction in the incidence and severity of arthritis.(46, 47) TNF-α levels in the animals treated with the siRNA/DNA lipoplexes were reduced by 50–70%.
Decrease in TNF-α correlated with decrease in the levels of IL-6 and monocyte chemotactic protein 1(MCP-1), two pro-inflammatory cytokines that are secreted abundantly by monocytes/macrophages and macrophage-like synovial fibroblasts. Likewise, injection of anti-IL-1/IL-6/IL-18 mixed siRNA/DNA lipoplexes led to significant reduction in the incidence and severity of arthritis, with therapeutic indexes surpassing those obtained from animals treated with anti-TNFα siRNA/DNA lipoplexes. As lipoplexes are internalized by macrophages through passive targeting, they can potentially induce an IFN response through stimulation of Toll-like receptors. However, the investigators found no elevation of IFN levels in mice treated with siRNA/DNA lipoplexes. The major drawbacks to the use of lipoplexes include the short-lived duration of silencing (approximately one week), necessitating repeating injections to maintain therapeutic effect, in addition to serum protein-induced aggregation of the polyplexes and rapid clearance by the RES.(48, 49) To circumvent these later problems the investigators sought an intraperitoneal route of injection to deliver siRNA chitosan nanoparticles into a serum-free, macrophage-rich environment.(50) The approach not only silenced approximately 44% of TNF-α expression in peritoneal macrophages but also arrested the progression of arthritis in a CIA model. Although the intraperitoneal route provides a potentially relevant approach to RA therapy in humans, the transient nature of this gene silencing approach (requiring injections every 2–3 days) coupled with the rapid rise in joint inflammation, a so-called “bounce” effect, upon discontinuation of therapy suggests that this delivery system requires considerable optimizations before translation to the clinical phase could be considered. Other examples of gene therapy in animal models of arthritis include the systemic delivery of siRNA lipoplexes that silence cytosolic phospholipase A2alpha (cPLA2α, a key molecule involved in inflammation and pro-inflammatory cytokine production)(51) and the over-expression of IL-1 receptor antagonist (IL-1ra, a naturally occurring anti-inflammatory molecule) by chitosan DNA nanoparticles.(52) Other potential targets for gene therapy in RA include microRNAs (miRNAs), noncoding RNA molecules that interact with the RNA-induced silencing complex (RISC) and modulate gene expression post-transcriptionally.(53) A recent study by Nagata et al. demonstrates that intra-articular injection of miR-15a leads to synovial cell apoptosis through the down-regulation of Bcl-2,(54) suggesting that in the future targeted over-expression of miRNAs may offer a novel therapeutic approach for RA treatment. In summary, nanotechnology-based non-viral delivery systems for gene therapy looks promising in pre-clinical animal models. However, the challenge will be to overcome the transient character of the silencing effect while controlling potential off-target side effects.
Nanotechnology-based anti-angiogenic approaches
Angiogenesis is the development of new blood vessels from pre-existing vessels. It is one of the earliest histological findings in RA and is found mainly in areas of inflammation. Angiogenic blood vessels supply nutrients for the RA synovium and enable leukocyte recruitment to the site of inflammation. Whether angiogenesis causes or is the result of inflammation remains a highly debated topic.(55) Many key pro-angiogenic molecules are produced in the synovium, including growth factors [i.e. vascular endothelial growth factor (VEGF), transforming growth factor (TGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), platelet derived growth factor (PDGF), etc.], cytokines (TNF-α, IL-1β, IL-6, IL-17, IL-18), chemokines (CXCL8, CXCL12, CCL2, CXC3L1), proteases [metalloproteases (MMPs)], adhesion molecules [αvα3 and αvα5 integrins, vascular adhesion molecule 1 (VCAM-1), intercellular adhesion molecules (ICAM-1)]. In addition, there are many endogenous anti-angiogenic factors including angiostatin, endostatin, thrombospondin, IL-4, IL-13, just to name a few. It is generally accepted that in RA, an imbalance between anti- and pro-angiogenic factors leads to the perpetuation of new vessel formation and inflammation.(56) In fact, over-expression of VEGF in the joint increased vascularization and exacerbated inflammation in a mouse CIA model, providing a direct link between angiogenesis and arthritis.(57) Angiogenesis also drives the proliferation and hyperplasia of synovial cells, increasing the local demand of oxygen and inducing a relative state of hypoxia. A hypoxic environment upregulates the expression of hypoxia-inducible factor 1 (HIF-1), a transcription factor that promotes glycolytic metabolism and regulates angiogenesis.(58) Given the direct link between angiogenesis and inflammation, there have been increasing efforts to target synovial angiogenesis in the treatment of RA.
Many of the current RA treatments indirectly affect angiogenesis through the modulation of cytokines and growth factors. For example, combination therapy that includes GCs and MTX modulates the production of VEGF and FGF in cultured cells and serum of RA patients.(59) Moreover, treatment with the anti-TNF biologic infliximab has been shown to reduce the levels of soluble ICAM-1 (slCAM-1), sVCAM-1, and VEGF in RA patients.(60) More recently, nanosystems aimed specifically at the blockade of pro-angiogenic mediators or pathways are beginning to emerge. Nanogold particles have recently been shown to have anti-angiogenic properties.(61) Gold salts (sodium gold thiomalate) were routinely used in the treatment of RA prior to the emergence of biologics although the precise mechanism of action was unknown and long-term accumulation of gold salts often resulted in serious nephrotoxicity.(62) In a proof of concept study intra-articular injection of 13 nm nanogold to rats with CIA significantly decreased the arthritic and radiographic scores when given prophylactically prior to onset of disease.(63) The disease amelioration was accompanied by a reduction in microvessel density as well as levels of TNF-α and IL-1β. Although the exact mechanism by which nanogold inhibits arthritis in vivo is unknown, in vitro studies suggest that nanogold bind to the sulfur/amines present in the heparin-binding domain of VEGF, thereby inhibiting VEGF-induced signaling. As nanogold monotherapy was not effective at halting the progression of established CIA, the findings in this study suggest that nanogold may be useful when combined with other biologics or DMARDs. Camptothecin (CPT) is a topoisomerase I inhibitor that has been shown to inhibit synoviocyte proliferation and angiogenesis in vitro.(64) However, CPT is poorly soluble and its use is limited by severe toxicities. To circumvent these problems, Koo et al. used sterically stabilized micelles (SSM) that increased the solubility and allowed targeted delivery of low dose CPT to arthritic joints in a CIA model.(65) They found that a single injection of CPT-SSM was more efficacious at reducing the severity of CIA when given at doses 3-fold lower than that required when using free CPT. In addition, conjugation of vasoactive intestinal peptide (VIP) to CPT-SSM for active targeting further reduced the therapeutic dose of CPT by 10-fold and effectively mitigated joint inflammation in CIA mice for at least 32 days without detectable systemic toxicities. As VIP receptor is only expressed on the abluminal side of microcirculation (away from circulating SSM), CPT-SSM-VIP can only extravasate at sites of inflammation where there is disruption in the endothelial layer, thus further increasing the selective delivery of CPT. Taken together, these results suggest that CPT-SSM-VIP may be developed into a safe platform for RA treatment.
Although VEGF plays a central role in angiogenesis and inhibition of VEGF is actively being explored for cancer therapy, direct targeting of this pathway from a nanotechnology standpoint has not been fully developed for the treatment of RA. Among the other specific mediators that are being explored for anti-angiogenic therapy, the integrin αvβ3 merits special consideration. Antagonism of αvβ3 integrin has been shown to decrease synovial angiogenesis and clinical disease in animal models. However, a phase II human RA trial using Vitaxin, a humanized antibody directed against αvβ3, was abandoned in 2004 due to lack of efficacy.(66) As an alternative, our group has explored the use of αvβ3 mainly as a targeting receptor for the delivery of anti-angiogenic drugs to suppress the progression of arthritis. We showed that αvβ3-targeted perfluorocarbon (PFC) nanoparticles administered systemically homed to the inflamed joints and suppressed inflammatory arthritis when conjugated to the anti-angiogenic drug fumagillin, a mycotoxin produced by Aspergillus fumagatus that inhibits the metalloprotease methionine aminopeptidase-2.(67) Furthermore we showed that a single systemic dose of fumagillin-PFC nanoparticles synergized with the conventional DMARD MTX to provide significant anti-inflammatory effects with a favourable safety profile in a mouse model of arthritis.(68) In summary, nanosystems that directly inhibit angiogenesis for the treatment of inflammatory arthritis have shown promises in several preclinical studies and merit further investigation to fully evaluate efficacy and toxicities in vivo. In addition new anti-angiogenic targets, such as HIF-α, continue to emerge that deserve considerations. Whether anti-angiogenic therapy targeted at a single factor or pathway will result in long-lasting effect or will have to be combined with other therapies to maintain effectiveness remains to be seen.
Induction of tolerance in the treatment of RA
The approaches detailed thus far target specific molecules and pathways in the inflammatory cascade for inhibition and the nanosystems being developed are aimed at capturing the segment of patient population that does not respond to current therapies and to circumvent toxicities. The induction of immune tolerance, not only as a treatment approach but ultimately as strategy to maintain RA patients in remission with minimal immunosuppression, represents a novel and promising next step toward the advancement of RA therapy.(69) Immune tolerance is a process by which the immune system does not mount an inflammatory response to an antigen, either “self” or external. It is generally believed that impairment in immune tolerance leads to autoimmunity. Immune tolerance involves multiple pathways and factors, including dendritic cells, T cells, B cells, co-stimulatory molecules, cytokines, kinases, etc. There is evidence that current biologics might induce immune tolerance in addition to their intended mechanism of action. For example, Abatacept (CTLA4-lg) prevents T cell activation by mimicking the action of naturally occurring CTLA4 expressed on the surface of activated T cells and thereby interrupting the interaction of T cell co-stimulatory signal.(70) This action renders the T cell functionally anergic or tolerant. Based on promising animal studies several trials of immune tolerance induction with oral collagen in RA patients have been conceived and conducted over the years. However, no clear clinical efficacy has emerged from these trials.(69) Hypothesizing that induction of oral tolerance requires extended exposure of the intestinal immune system to the specific antigens Kim and colleagues administered PLGA nanoparticles entrapping collagen type II (CII) or CII peptides to mice and investigated the tolerogenic effect of PLGA-CII nanoparticles on the development of CIA.(71, 72) They found that PLGA-CII nanoparticles significantly suppressed CIA and TNF-α expression in treated animals, suggesting that the slow sustained release of collagen from PLGA may provide a suitable delivery system for oral tolerance induction. However, unlike the CIA model in which the etiologic antigen (CII) is well defined, specific antigen(s) in RA remains elusive. In addition PLGA nanoparticles themselves have immunodulatory properties that could affect host immune response, thus potentially limiting their use. Newer specific targets, such as heat shock proteins (HSPs),(73) are currently being defined and these could eventually be incorporated into an optimized and safe delivery system for immune tolerance induction.
Conclusion
While the development of biologics has revolutionized the treatment of RA, a significant portion of patients remains or becomes refractory to current therapeutic interventions. In addition, few patients attain and remain in remission without continued immunosuppressive therapy. Nanosystems promise specific and localized delivery of drugs while minimizing the quantity of drug used, thus limiting potential off-target unwanted effects (Table 2). These systems may allow the prolonged use of NSAIDs and GCs in high-risk patient populations such as the young and the elderly. The versatility of the newer nanoplatforms also prompts investigators to reconsider formerly established drugs that were considered too toxic or insoluble for systemic use. Nanotechnology-based gene therapy represents an alternative that may circumvent the oncogenic concerns pertaining to viral-based gene transfer methods. Targeted anti-angiogenic nanotherapy, as single or combination therapy, has been proven effective in animal models and awaits clinical trials. More recently, there is considerable interest in the development of inhibitors for the specific targeting of signaling pathways that drive inflammation [Janus kinase (JAK), spleen tyrosine kinase (Syk), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways].(74, 75) The main concern regarding the oral administration of these agents remain generalized suppression of multiple physiologic functions. Nanoparticle-based targeted delivery systems therefore represent an ideal therapeutic approach to evaluate these signaling molecule inhibitors in the future, as they can potentially limit generalized immunosuppression. Lastly, this review focuses on therapeutic approaches for RA; however, the same nanocarrier-based targeting concepts may be adapted to other human disorders that are driven by similar pathways of inflammation.
Table 2.
Characteristics of nanocarriers cited in references
| Carrier systems | Mean size nm |
Drugs/Agents | Delivery/Target | Refs |
|---|---|---|---|---|
| Liposomes | 120–160 | Clodronate | Macrophages | (17) |
| Lipid microspheres | 150 | Indomethacin | EPR/Macrophages | (23, 24, 25) |
| Nanocapsules | 240 | Indomethacin | EPR | (26) |
| PAMAM dendrimers | < 100 | Flurbiprofen | EPR | (28) |
| PAMAM dendrimers | < 100 | Indomethacin | Folate receptor* | (29) |
| Core/shell NPa | 430 | Diclofenac | Magnetic field | (30) |
| Liposomes | 100 | Prednisolone | EPR | (31, 32) |
| PLGA/PLA copolymers | 45-115 | Betamethasone | EPR/Macrophages | (33, 34) |
| Cyclodextrin polymers | 27 | Methylprednisolone | EPR/Macrophages | (35) |
| Iron oxide particles | 1000 | Dexamethasone | Magnetic field | (37, 38) |
| 10,000 | Synovial fibroblasts | |||
| Lipoplexes | 700 | siRNA against | Macrophages | (46, 47) |
| TNF, IL-1, IL-6, IL-18 | ||||
| Chitosan NP | 350–450 | siRNA against TNF | Macrophages | (49, 50) |
| Lipoplexes | 700 | siRNA against cPLA2α | Splenic CD11b+ cells | (51) |
| Chitosan NP | <200 | IL-1ra cDNA | Folate receptor* | (52) |
| Nanogold | 13 | Nanogold | VGEF | (59) |
| SSMb | 13 | Camptothecin | VIP* | (61) |
| PFCc NP | 250 | Fumagillin | αvβ33 integrin* | (63, 64) |
| PLGANP | 300 | Collagen | Lymphocytes | (71, 72) |
Active targeting
NP: nanoparticles
SSM: sterically stabilized micelles
PFC: perfluorocarbon
References
- 1.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheum. 2008;26:S35–S61. [PubMed] [Google Scholar]
- 2.McInnes IB, O'Dell JR. State-of-the-art: rheumatoid arthritis. Ann Rheum Dis. 2010;69:1898–1906. doi: 10.1136/ard.2010.134684. [DOI] [PubMed] [Google Scholar]
- 3.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 4.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 5.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–5055. [PubMed] [Google Scholar]
- 7.Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2009;4:CD007848. doi: 10.1002/14651858.CD007848.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2:CD008794. doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarner IH, Muller-Ladner U, Gay S. Emerging targets of biologic therapies for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2007;3:336–345. doi: 10.1038/ncprheum0506. [DOI] [PubMed] [Google Scholar]
- 10.Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci. 2008;11:81s–110s. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- 11.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 12.Salliot C, Finckh A, Katchamart W, Lu Y, Sun Y, et al. Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysis. Ann Rheum Dis. 2011;70:266–271. doi: 10.1136/ard.2010.132134. [DOI] [PubMed] [Google Scholar]
- 13.Hoes JN, Jacobs JW, Buttgereit F, Bijlsma JW. Current view of glucocorticoid co-therapy with DMARDs in rheumatoid arthritis. Nat Rev Rheumatol. 2010;6:693–702. doi: 10.1038/nrrheum.2010.179. [DOI] [PubMed] [Google Scholar]
- 14.Hetland ML, Stengaard-Pedersen K, Junker P, Lottenburger T, Hansen I, et al. Aggressive combination therapy with intra-articular glucocorticoid injections and conventional disease-modifying anti-rheumatic drugs in early rheumatoid arthritis: second-year clinical and radiographic results from the CIMESTRA study. Ann Rheum Dis. 2008;67:815–822. doi: 10.1136/ard.2007.076307. [DOI] [PubMed] [Google Scholar]
- 15.van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 16.Pennanen N, Lapinjoki S, Urtti A, Monkkonen J. Effect of liposomal and free bisphosphonates on the IL-1 beta, IL-6 and TNF alpha secretion from RAW 264 cells in vitro. Pharm Res. 1995;12:916–922. doi: 10.1023/a:1016281608773. [DOI] [PubMed] [Google Scholar]
- 17.Ceponis A, Waris E, Monkkonen J, Laasonen L, Hyttinen M, et al. Effects of low-dose, noncytotoxic, intraarticular liposomal clodronate on development of erosions and proteoglycan loss in established antigen-induced arthritis in rabbits. Arthritis Rheum. 2001;44:1908–1916. doi: 10.1002/1529-0131(200108)44:8<1908::AID-ART329>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.van Lent PL, van den Bersselaar L, van den Hoek AE, van de Ende M, Dijkstra CD, et al. Reversible depletion of synovial lining cells after intra-articular treatment with liposome-encapsulated dichloromethylene diphosphonate. Rheum Int. 1993;13:21–30. doi: 10.1007/BF00290330. [DOI] [PubMed] [Google Scholar]
- 19.Patrignani P, Tacconelli S, Sciulli MG, Capone ML. New insights into COX-2 biology and inhibition. Brain Res. 2005;48:352–359. doi: 10.1016/j.brainresrev.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 21.Amer M, Bead VR, Bathon J, Blumenthal RS, Edwards DN. Use of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease: a cautionary tale. Cardiol Rev. 2010;18:204–212. doi: 10.1097/CRD.0b013e3181ce1521. [DOI] [PubMed] [Google Scholar]
- 22.Khan Z, Khan N, Tiwari RP, Sah NK, Prasad GB, Bisen PS. Biology of Cox-2: An Application in Cancer Therapeutics. Curr Drug Targets. 2011;12:1082–1093. doi: 10.2174/138945011795677764. [DOI] [PubMed] [Google Scholar]
- 23.Srinath P, Vyas SP, Diwan PV. Preparation and pharmacodynamic evaluation of liposomes of indomethacin. Drug Dev Ind Pharm. 2000;26:313–321. doi: 10.1081/ddc-100100359. [DOI] [PubMed] [Google Scholar]
- 24.Srinath P, Chary MG, Vyas SP, Diwan PV. Long-circulating liposomes of indomethacin in arthritic rats--a biodisposition study. Pharm Acta Helv. 2000;74:399–404. doi: 10.1016/s0031-6865(00)00023-6. [DOI] [PubMed] [Google Scholar]
- 25.Palakurthi S, Vyas SP, Diwan PV. Biodisposition of PEG-coated lipid microspheres of indomethacin in arthritic rats. Int J Pharm. 2005;290:55–62. doi: 10.1016/j.ijpharm.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Fahmy TM, Fong PM, Park J, Constable T, Saltzman WM. Nanosystems for simultaneous imaging and drug delivery to T cells. AAPS J. 2007;9:E171–E180. doi: 10.1208/aapsj0902019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernardi A, Zilberstein AC, Jager E, Campos MM, Morrone FB, et al. Effects of indomethacin-loaded nanocapsules in experimental models of inflammation in rats. Br J Pharm. 2009;158:1104–1111. doi: 10.1111/j.1476-5381.2009.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asthana A, Chauhan AS, Diwan PV, Jain NK. Poly(amidoamine) (PAMAM) dendritic nanostructures for controlled site-specific delivery of acidic anti-inflammatory active ingredient. AAPS PharmSciTech. 2005;6:E536–E542. doi: 10.1208/pt060367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandrasekar D, Sistla R, Ahmad FJ, Khar RK, Diwan PV. Folate coupled poly(ethyleneglycol) conjugates of anionic poly(amidoamine) dendrimer for inflammatory tissue specific drug delivery. J Biomed Mater Res A. 2007;82:92–103. doi: 10.1002/jbm.a.31122. [DOI] [PubMed] [Google Scholar]
- 30.Arias JL, Lopez-Viota M, Lopez-Viota J, Delgado AV. Development of iron/ethylcellulose (core/shell) nanoparticles loaded with diclofenac sodium for arthritis treatment. Int J Pharm. 2009;382:270–276. doi: 10.1016/j.ijpharm.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Metselaar JM, Wauben MH, Wagenaar-Hilbers JP, Boerman OC, Storm G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 2003;48:2059–2066. doi: 10.1002/art.11140. [DOI] [PubMed] [Google Scholar]
- 32.van den Hoven JM, Hofkens W, Wauben MH, Wagenaar-Hilbers JP, Beijnen JH, et al. Optimizing the therapeutic index of liposomal glucocorticoids in experimental arthritis. Int J Pharm. 2011 doi: 10.1016/j.ijpharm.2011.03.025. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Ishihara T, Kubota T, Choi T, Higaki M. Treatment of experimental arthritis with stealth-type polymeric nanoparticles encapsulating betamethasone phosphate. J Pharmacol Exp Ther. 2009;329:412–417. doi: 10.1124/jpet.108.150276. [DOI] [PubMed] [Google Scholar]
- 34.Ishihara T, Takahashi M, Higaki M, Mizushima Y, Mizushima T. Preparation and characterization of a nanoparticulate formulation composed of PEG-PLA and PLA as anti-inflammatory agents. Int J Pharm. 2010;385:170–175. doi: 10.1016/j.ijpharm.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Hwang J, Rodgers K, Oliver JC, Schluep T. Alpha-methylprednisolone conjugated cyclodextrin polymer-based nanoparticles for rheumatoid arthritis therapy. Int J Nanomedicine. 2008;3:359–371. doi: 10.2147/ijn.s3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derendorf H, Mollmann H, Gruner A, Haack D, Gyselby G. Pharmacokinetics and pharmacodynamics of glucocorticoid suspensions after intra-articular administration. Clin Pharmacol Ther. 1986;39:313–317. doi: 10.1038/clpt.1986.45. [DOI] [PubMed] [Google Scholar]
- 37.Butoescu N, Seemayer CA, Foti M, Jordan O, Doelker E. Dexamethasone-containing PLGA superparamagnetic microparticles as carriers for the local treatment of arthritis. Biomaterials. 2009;30:1772–1780. doi: 10.1016/j.biomaterials.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Butoescu N, Seemayer CA, Palmer G, Guerne PA, Gabay C, et al. Magnetically retainable microparticles for drug delivery to the joint: efficacy studies in an antigen-induced arthritis model in mice. Arthritis Res Ther. 2009;11:R72. doi: 10.1186/ar2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 40.Jorgensen C, Apparailly F. Prospects for gene therapy in inflammatory arthritis. Best Pract Res Clin Rheumatol. 2010;24:541–552. doi: 10.1016/j.berh.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Putnam D. Polymers for gene delivery across length scales. Nat Mater. 2006;5:439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 42.Lundin KE, Simonson OE, Moreno PM, Zaghloul EM, Oprea II, et al. Nanotechnology approaches for gene transfer. Genetica. 2009;137:47–56. doi: 10.1007/s10709-009-9372-0. [DOI] [PubMed] [Google Scholar]
- 43.Pathak A, Patnaik S, Gupta KC. Recent trends in non-viral vector-mediated gene delivery. Biotechnol J. 2009;4:1559–1572. doi: 10.1002/biot.200900161. [DOI] [PubMed] [Google Scholar]
- 44.Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2011 doi: 10.1016/j.nano.2011.05.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Schiffelers RM, Xu J, Storm G, Woodle MC, Scaria PV. Effects of treatment with small interfering RNA on joint inflammation in mice with collagen-induced arthritis. Arthritis Rheum. 2005;52:1314–1318. doi: 10.1002/art.20975. [DOI] [PubMed] [Google Scholar]
- 46.Khoury M, Louis-Plence P, Escriou V, Noel D, Largeau C, et al. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor alpha in experimental arthritis. Arthritis Rheum. 2006;54:1867–1877. doi: 10.1002/art.21876. [DOI] [PubMed] [Google Scholar]
- 47.Khoury M, Escriou V, Courties G, Galy A, Yao R, et al. Efficient suppression of murine arthritis by combined anticytokine small interfering RNA lipoplexes. Arthritis Rheum. 2008;58:2356–2367. doi: 10.1002/art.23660. [DOI] [PubMed] [Google Scholar]
- 48.Dash PR, Read ML, Fisher KD, Howard KA, Wolfert M, et al. Decreased binding to proteins and cells of polymeric gene delivery vectors surface modified with a multivalent hydrophilic polymer and retargeting through attachment of transferrin. J Biol Chem. 2000;275:3793–3802. doi: 10.1074/jbc.275.6.3793. [DOI] [PubMed] [Google Scholar]
- 49.Howard KA, Dash PR, Read ML, Ward K, Tomkins LM, et al. Influence of hydrophilicity of cationic polymers on the biophysical properties of polyelectrolyte complexes formed by self-assembly with DNA. Biochim Biophys Acta. 2000;1475:245–255. doi: 10.1016/s0304-4165(00)00076-3. [DOI] [PubMed] [Google Scholar]
- 50.Howard KA, Paludan SR, Behlke MA, Besenbacher F, Deleuran B, Kjems J. Chitosan/siRNA nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol Ther. 2009;17:162–168. doi: 10.1038/mt.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Courties G, Baron M, Presumey J, Escriou V, van Lent P, et al. Cytosolic phospholipase A2alpha gene silencing in the myeloid lineage alters development of Th1 responses and reduces disease severity in collagen-induced arthritis. Arthritis Rheum. 2011;63:681–690. doi: 10.1002/art.30174. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes JC, Wang H, Jreyssaty C, Benderdour M, Lavigne P, et al. Bone-protective effects of nonviral gene therapy with folate-chitosan DNA nanoparticle containing interleukin-1 receptor antagonist gene in rats with adjuvant-induced arthritis. Mol Ther. 2008;16:1243–1251. doi: 10.1038/mt.2008.99. [DOI] [PubMed] [Google Scholar]
- 53.Wittmann J, Jack HM. microRNAs in rheumatoid arthritis: midget RNAs with a giant impact. Ann Rheum Dis. 2011;70(Suppl 1):i92–i96. doi: 10.1136/ard.2010.140152. [DOI] [PubMed] [Google Scholar]
- 54.Nagata Y, Nakasa T, Mochizuki Y, Ishikawa M, Miyaki S, et al. Induction of apoptosis in the synovium of mice with autoantibody-mediated arthritis by the intraarticular injection of double-stranded MicroRNA-15a. Arthritis Rheum. 2009;60:2677–2683. doi: 10.1002/art.24762. [DOI] [PubMed] [Google Scholar]
- 55.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–166. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 56.Szekanecz Z, Besenyei T, Szentpetery A, Koch AE. Angiogenesis and vasculogenesis in rheumatoid arthritis. Curr Opin Rheumatol. 2010;22:299–306. doi: 10.1097/BOR.0b013e328337c95a. [DOI] [PubMed] [Google Scholar]
- 57.Clavel G, Valvason C, Yamaoka K, Lemeiter D, Laroche L, et al. Relationship between angiogenesis and inflammation in experimental arthritis. Eur Cytokine Netw. 2006;17:202–210. [PubMed] [Google Scholar]
- 58.Westra J, Molema G, Kallenberg CG. Hypoxia-inducible factor-1 as regulator of angiogenesis in rheumatoid a rthritis-therapeutic implications. Curr Med Chem. 2010;17:254–263. doi: 10.2174/092986710790149783. [DOI] [PubMed] [Google Scholar]
- 59.Nagashima M, Wauke K, Hirano D, Ishigami S, Aono H, et al. Effects of combinations of anti-rheumatic drugs on the production of vascular endothelial growth factor and basic fibroblast growth factor in cultured synoviocytes and patients with rheumatoid arthritis. Rheumatology (Oxford) 2000;39:1255–1262. doi: 10.1093/rheumatology/39.11.1255. [DOI] [PubMed] [Google Scholar]
- 60.Klimiuk PA, Sierakowski S, Domyslawska I, Fiedorczyk M, Chwiecko J. Reduction of soluble adhesion molecules (sICAM-1, sVCAM-1, and sE-selectin) and vascular endothelial growth factor levels in serum of rheumatoid arthritis patients following multiple intravenous infusions of infliximab. Arch Immunol Ther Exp (Warsz) 2004;52:36–42. [PubMed] [Google Scholar]
- 61.Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, et al. Antiangiogenic properties of gold nanoparticles. Clin Cancer Res. 2005;11:3530–3534. doi: 10.1158/1078-0432.CCR-04-2482. [DOI] [PubMed] [Google Scholar]
- 62.Merchant B. Gold, the noble metal and the paradoxes of its toxicology. Biologicals. 1998;26:49–59. doi: 10.1006/biol.1997.0123. [DOI] [PubMed] [Google Scholar]
- 63.Tsai CY, Shiau AL, Chen SY, Chen YH, Cheng PC, et al. Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum. 2007;56:544–554. doi: 10.1002/art.22401. [DOI] [PubMed] [Google Scholar]
- 64.Jackson JK, Higo T, Hunter WL, Burt HM. Topoisomerase inhibitors as anti-arthritic agents. Inflamm Res. 2008;57:126–134. doi: 10.1007/s00011-007-7163-6. [DOI] [PubMed] [Google Scholar]
- 65.Koo OM, Rubinstein I, Onyuksel H. Actively targeted low-dose camptothecin as a safe, long-acting, disease-modifying nanomedicine for rheumatoid arthritis. Pharm Res. 2011;28:776–787. doi: 10.1007/s11095-010-0330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lainer-Carr D, Brahn E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat Clin Pract Rheumatol. 2007;3:434–442. doi: 10.1038/ncprheum0559. [DOI] [PubMed] [Google Scholar]
- 67.Zhou HF, Chan HW, Wickline SA, Lanza GM, Pham CT. Alphavbeta3-targeted nanotherapy suppresses inflammatory arthritis in mice. FASEB J. 2009;23:2978–2985. doi: 10.1096/fj.09-129874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou HF, Hu G, Wickline SA, Lanza GM, Pham CT. Synergistic effect of antiangiogenic nanotherapy combined with methotrexate in the treatment of experimental inflammatory arthritis. Nanomedicine (London) 2010;5:1065–1074. doi: 10.2217/nnm.10.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albani S, Koffeman EC, Prakken B. Induction of immune tolerance in the treatment of rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:272–281. doi: 10.1038/nrrheum.2011.36. [DOI] [PubMed] [Google Scholar]
- 70.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 71.Kim W-U, Lee WK, Ryoo JW, Kim SH, Kim J, et al. Suppression of collagen-induced arthritis by single administration of poly(lactic-co-glycolic acid) nanoparticles entrapping type II collagen: a novel treatment strategy for induction of oral tolerance. Arthritis Rheum. 2002;46:1109–1120. doi: 10.1002/art.10198. [DOI] [PubMed] [Google Scholar]
- 72.Lee WK, Park JY, Jung S, Yang CW, Kim W-U, et al. Preparation and characterization of biodegradable nanoparticles entrapping immunodominant peptide conjugated with PEG for oral tolerance induction. J Control Release. 2005;105:77–88. doi: 10.1016/j.jconrel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Pockley AG. Heat shock proteins in health and disease: therapeutic targets or therapeutic agents? Expert Rev Mol Med. 2001;3:1–21. doi: 10.1017/S1462399401003556. [DOI] [PubMed] [Google Scholar]
- 74.Bamborough P, Morse MA, Ray KP. Targeting IKKbeta for the treatment of rheumatoid arthritis. Drug News Perspect. 2010;23:483–490. doi: 10.1358/dnp.2010.23.8.1447844. [DOI] [PubMed] [Google Scholar]
- 75.Cohen S, Fleischmann R. Kinase inhibitors: a new approach to rheumatoid arthritis treatment. Curr Opin Rheum. 2010;22:330–335. doi: 10.1097/BOR.0b013e3283378e6f. [DOI] [PubMed] [Google Scholar]