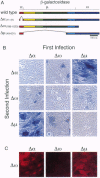
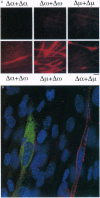
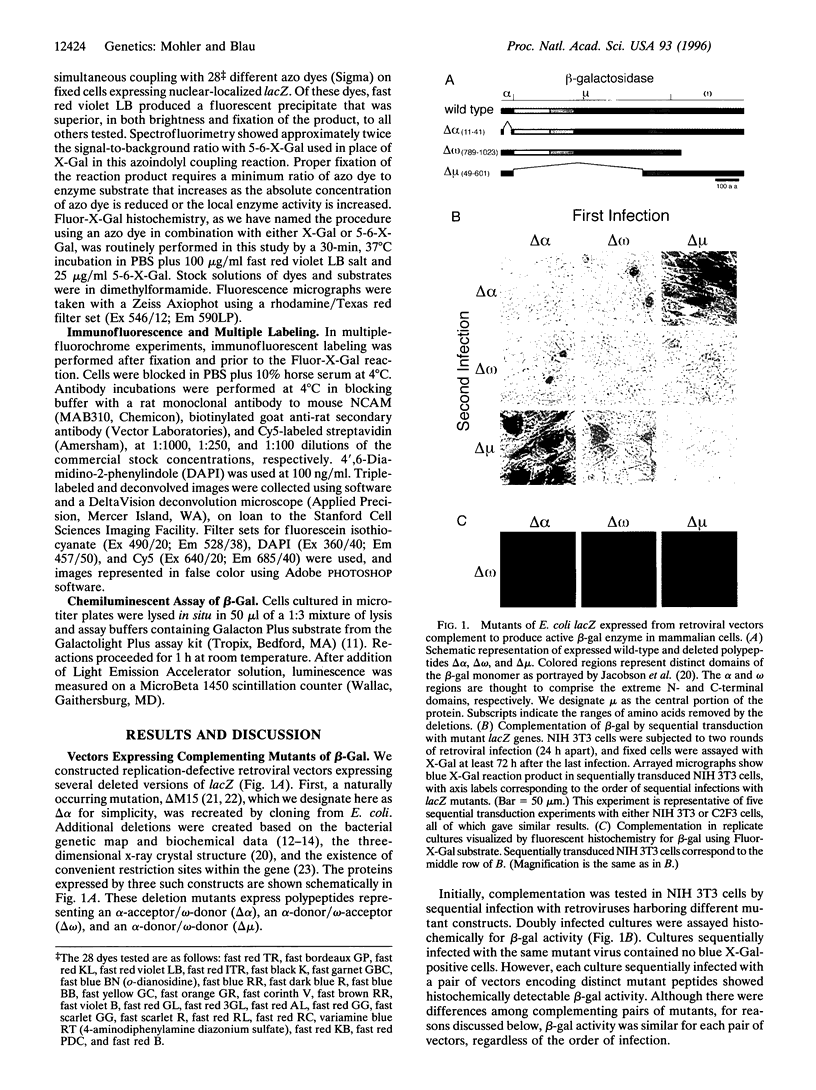
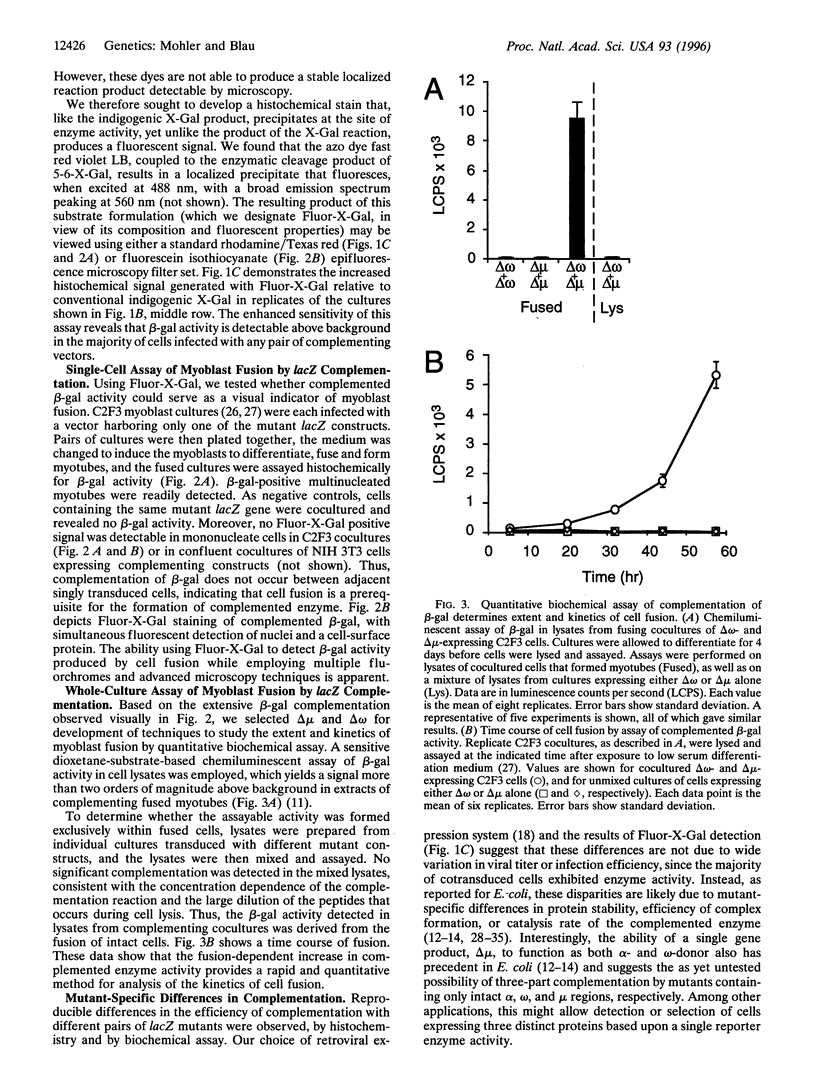
Abstract
Complementing reporter genes provide biological indicators of coincident expression of proteins in cells. We have adapted intracistronic complementation of the Escherichia coli lacZ gene for use in mammalian cells. Enzymatic activity detectable by quantitative biochemical assay, flow cytometry, or microscopy is produced upon convergent expression of two distinct mutant lacZ peptides within single cells, or upon fusion of cells expressing such mutants. A novel fluorescent substrate for beta-galactosidase (Fluor-X-Gal) increases detection and permits simultaneous microscopic visualization of other fluorescent markers. The enzymatic complementation described here should facilitate studies of cell fusion, cell lineage, and signal transduction, by producing activity only when two proteins are expressed at the same time and place in intact cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Hidaka K., Siminovitch L. Expression of bacterial beta-galactosidase in animal cells. Mol Cell Biol. 1982 Dec;2(12):1628–1632. doi: 10.1128/mcb.2.12.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKWITH J. R. A DELETION ANALYSIS OF THE LAC OPERATOR REGION IN ESCHERICHIA COLI. J Mol Biol. 1964 Mar;8:427–430. doi: 10.1016/s0022-2836(64)80206-0. [DOI] [PubMed] [Google Scholar]
- Bronstein I., Edwards B., Voyta J. C. 1,2-dioxetanes: novel chemiluminescent enzyme substrates. Applications to immunoassays. J Biolumin Chemilumin. 1989 Jul;4(1):99–111. doi: 10.1002/bio.1170040116. [DOI] [PubMed] [Google Scholar]
- Celada F., Ullmann A., Monod J. An immunological study of complementary fragments of beta-galactosidase. Biochemistry. 1974 Dec 31;13(27):5543–5547. doi: 10.1021/bi00724a014. [DOI] [PubMed] [Google Scholar]
- Fire A., Harrison S. W., Dixon D. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene. 1990 Sep 14;93(2):189–198. doi: 10.1016/0378-1119(90)90224-f. [DOI] [PubMed] [Google Scholar]
- Gossrau R. Azoindoxylverfahren zum Hydrolasennachweis. II. Biochemische und histochemische Untersuchung der sauren beta-Galactosidase. Histochemistry. 1977 Mar 4;51(2-3):219–237. doi: 10.1007/BF00567226. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Hughes S. M., Blau H. M. Migration of myoblasts across basal lamina during skeletal muscle development. Nature. 1990 May 24;345(6273):350–353. doi: 10.1038/345350a0. [DOI] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella T. M., Nolan G. P. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996 Aug 1;7(12):1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- Langley K. E., Zabin I. beta-Galactosidase alpha complementation: properties of the complemented enzyme and mechanism of the complementation reaction. Biochemistry. 1976 Nov 2;15(22):4866–4875. doi: 10.1021/bi00667a018. [DOI] [PubMed] [Google Scholar]
- Lin S., Zabin I. Beta-galactosidase. Rates of synthesis and degradation of incomplete chains. J Biol Chem. 1972 Apr 10;247(7):2205–2211. [PubMed] [Google Scholar]
- Lis J. T., Simon J. A., Sutton C. A. New heat shock puffs and beta-galactosidase activity resulting from transformation of Drosophila with an hsp70-lacZ hybrid gene. Cell. 1983 Dec;35(2 Pt 1):403–410. doi: 10.1016/0092-8674(83)90173-3. [DOI] [PubMed] [Google Scholar]
- Mohler W. A., Charlton C. A., Blau H. M. Spectrophotometric quantitation of tissue culture cell number in any medium. Biotechniques. 1996 Aug;21(2):260-2, 264, 266. doi: 10.2144/96212st03. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Zipser D. Polypeptide products of nonsense mutations. I. Termination fragments from nonsense mutations in the Z gene of the lac operon of Escherichia coli. J Mol Biol. 1970 Jun 14;50(2):359–371. doi: 10.1016/0022-2836(70)90198-1. [DOI] [PubMed] [Google Scholar]
- Nielsen D. A., Chou J., MacKrell A. J., Casadaban M. J., Steiner D. F. Expression of a preproinsulin-beta-galactosidase gene fusion in mammalian cells. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5198–5202. doi: 10.1073/pnas.80.17.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlath G. K., Rich K., Webster S. G., Blau H. M. Localization of muscle gene products in nuclear domains. Nature. 1989 Feb 9;337(6207):570–573. doi: 10.1038/337570a0. [DOI] [PubMed] [Google Scholar]
- Pear W. S., Nolan G. P., Scott M. L., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P. Nucleotide sequence of the classical lacZ deletion delta M15. Gene. 1992 Dec 1;122(1):231–232. doi: 10.1016/0378-1119(92)90056-u. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastinejad F., Blau H. M. Genetic complementation reveals a novel regulatory role for 3' untranslated regions in growth and differentiation. Cell. 1993 Mar 26;72(6):903–917. doi: 10.1016/0092-8674(93)90579-f. [DOI] [PubMed] [Google Scholar]
- Rivière I., Brose K., Mulligan R. C. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann A., Jacob F., Monod J. Characterization by in vitro complementation of a peptide corresponding to an operator-proximal segment of the beta-galactosidase structural gene of Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):339–343. doi: 10.1016/0022-2836(67)90341-5. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Jacob F., Monod J. On the subunit structure of wild-type versus complemented beta-galactosidase of Escherichia coli. J Mol Biol. 1968 Feb 28;32(1):1–13. doi: 10.1016/0022-2836(68)90140-x. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Perrin D., Jacob F., Monod J. Identification par complémentation in vitro et purification d'un segment peptidique de la beta-galatosidase d'escherichia coli. J Mol Biol. 1965 Jul;12(3):918–923. doi: 10.1016/s0022-2836(65)80338-2. [DOI] [PubMed] [Google Scholar]
- Villarejo M., Zamenhof P. J., Zabin I. Beta-galactosidase. In vivo -complementation. J Biol Chem. 1972 Apr 10;247(7):2212–2216. [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Zabin I. beta-Galactosidase alpha-complementation. A model of protein-protein interaction. Mol Cell Biochem. 1982 Nov 26;49(2):87–96. doi: 10.1007/BF00242487. [DOI] [PubMed] [Google Scholar]
- Zabin I. beta-galactosidase and the lactose operon. UCLA Forum Med Sci. 1979;(21):49–62. doi: 10.1016/b978-0-12-643150-6.50011-7. [DOI] [PubMed] [Google Scholar]