Abstract
Hematological malignancies rarely affect the breast, and the majority of those that do are lymphomas. In this review, we describe the clinical aspects and multimodal imaging findings of breast lymphoma. We also illustrate the key clinical and radiological findings that allow it to be distinguished from various other malignant and benign diseases of the breast. Breast lymphoma manifests as a breast mass, a change in the subcutaneous tissue or the skin, or enlargement of the associated lymph node on radiological examination. Radiological findings associated with other breast malignancies, such as calcifications, spiculations, or architectural distortions are extremely rare. Skin and subcutaneous changes frequently accompany T-cell lymphoma. Multimodal breast imaging characteristics may aid in the diagnosis of breast lymphoma.
Keywords: Breast, Computed tomography, Lymphoma, Magnetic resonance imaging, Ultrasonography
INTRODUCTION
Hematological malignancies include lymphoid, myeloid, and histiocytic/dendritic neoplasms as defined in the most recent World Health Organization classification, updated in 2008 [1]. Lymphoma is the most common hematological malignancy affecting the breast. However, breast lymphoma accounts for only approximately 0.04% to 0.7% of all breast cancer cases [2,3], and this rarity may be related to the fact that the breast contains very little lymphoid tissue [4,5]. In these cases, it is often difficult to determine the relationship between the breast lesion and the hematological malignancy. Although several previous studies have addressed the imaging features of breast lymphoma, these findings are generally nonspecific and are typically presented as a list that was insufficient to guide clinical practice. As a result, clinicians may misinterpret the imaging findings associated with this lesion and consequently miss its significance. However, unlike a breast carcinoma, a mass of lymphoma cells in the breast does not require excision. To avoid unnecessary treatment, clinicians and radiologists need to know the characteristic imaging features of breast lymphoma.
The purpose of this pictorial review is to illustrate the clinical aspects and multimodal imaging findings of lymphoma affecting the breast. We also described the key radiological and clinical findings that allow it to be distinguished from other breast malignancies and inflammations. We identified articles that concerned breast lymphoma, were published in English between 1983 and 2012, and were indexed on PubMed. In addition, we evaluated the radiological, pathological, and clinical findings of 10 patients who had lymphoma affecting the breast and who underwent multimodal breast imaging studies in our institute between January 2003 and March 2012. The multimodal imaging consisted of mammography, ultrasonography (US), dynamic low-dose computed tomography (CT), and dynamic magnetic resonance imaging (MRI). The protocol for breast low-dose CT differs from that of conventional chest CT, as the former is performed in a prone position to spread the whole breast parenchyma and lower radiation dose settings (120 kVP and 50 mA) are used. The American College of Radiology recommends an average glandular dose of ≤3 mGy for standard screening mammography, and thus, the effective radiation dose should be 6 mGy or less for routine, two-view mammography [6]. When measured radiation doses administered to a CT phantom at 120 kVp and 50 mA were compared with the recommended dose for mammography, a CTDI100 of ≤6 mGy was obtained (2.01-4.08 mGy) [7]. We have already published both the phantom and patient results of the breast CT scan using this protocol and demonstrated satisfactory image quality and radiation doses [7-9]. The radiological findings were evaluated using a modified version of the Breast Imaging Reporting and Data System lexicon [10]. Because this lexicon does not include breast CT, we described CT findings using MRI descriptions, and the density of a breast lesion on a CT scan was compared with that of the pectoralis muscles. Thus, for example, if a lesion was of identical density to the pectoralis muscle, it was described as "isodense."
CLINICAL ASPECTS
Epidemiology
Breast lymphoma may occur as either a primary or a secondary lesion. Wiseman and Liao [11] proposed that, for a diagnosis of primary breast lymphoma, the breast should be the site of the first or major manifestation of the lymphoma and that there should be no evidence of lymphoma elsewhere, except at the ipsilateral axillary node. Primary breast lymphoma accounts for 0.85% to 2.2% of all extranodal malignant lymphomas, and secondary breast lymphoma is more common [12]. The age distribution of reported cases is wide (16-93 years at the time of diagnosis) and the reported median age ranges from 55 to 65 years [13,14], which is similar to the median age of extramammary lymphoma patients [15,16]. Of the 10 cases we described, six (60%) were primary breast lymphomas and the remaining four were secondary lymphomas (Table 1). The median age of the patients was 49.5 years (range, 21-65 years) and all of the patients were women.
Table 1.
Patients characteristics
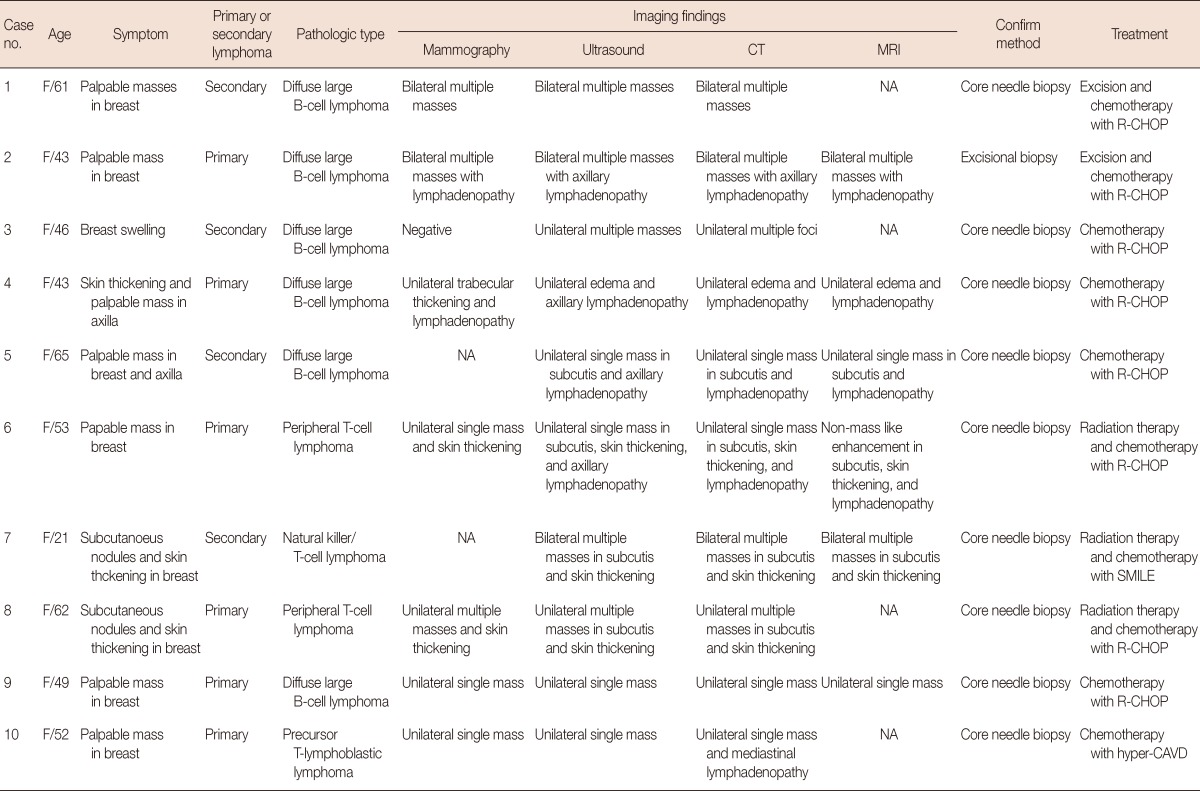
CT=computed tomography; MRI=magnetic resonance imaging; F=female; NA=not available; R-CHOP=rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; SMILE=dexamethasone, methotrexate, l-asparaginase, and etoposide; Hyper CAVD=hyperfractionated chemotherapy in small dose with cycloposphamide, vincristine, doxorubicin, and dexamethasone.
Clinical findings
The most common symptom of breast lymphoma is a painless, palpable mass. Nipple retraction or discharge and skin change can also occur, but are rare [12,17]. Most B-cell lymphomas of the breast are present as palpable masses, whereas skin changes, edema, and local pain are more commonly associated with T-cell lymphoma [18]. Ipsilateral axillary lymphadenopathy has been reported in 13% to 50% of cases [19]. In our study, all patients had clinical symptoms. These were painless palpable masses in the breast and/or axilla in seven cases (70%), tender subcutaneous nodules in two cases (20%), and breast swelling in one case (10%) (Table 1). Three patients (30%) complained of skin thickening. Both patients (100%) with subcutaneous nodules and two of the three patients (67%) with skin thickening were found to have T-cell lymphoma on pathological examination. Therefore, skin or subcutaneous changes were more common in T-cell lymphoma, which is consistent with the findings of the previous study [18].
Pathophysiology
Most breast lymphomas are of the B-cell type. In a recent study by Surov et al. [12], 94% of breast lymphomas were found to be of the B-cell type and only 6% were of the T-cell type. However, some forms of T-cell lymphoma are more common in Asia than in Western countries [20], most notably peripheral T-cell, nasal T-cell, and natural killer (NK)/T-cell lymphomas. In the breast, T-cell lymphoma occurs only rarely and is reported on a case-by-case basis [21-24]. Included in these reports are lymphoblastic lymphoma, peripheral T-cell lymphoma, multilobated T-cell lymphoma, NK/T-cell lymphoma, cutaneous T-cell lymphoma, and anaplastic large cell lymphoma.
Of growing concern is a possible association between anaplastic large cell lymphoma and breast implants [22,23,25-28]. Previously regarded as chemically inert, silicone has been shown to induce inflammatory T-cell reactions [29]. If implants are causally related to lymphoma, the incidence of T-cell lymphoma in the breast may soon increase. In our study, six patients (60%) had diffuse large B-cell lymphomas and the remaining four (40%) had various T-cell lymphomas: two cases of peripheral T-cell lymphoma and a single case each of NK/T-cell lymphoma and precursor T-lymphoblastic lymphoma (Table 1). We included lymphoma patients who had breast involvement in this study and excluded patients who had only lymph node enlargement, and as a result, T-cell lymphoma manifesting as breast parenchymal or subcutaneous nodules might have been more common in study.
Treatment and prognosis
The optimal treatment for B-cell lymphoma is a multiagent regimen consisting of anthracycline-based chemotherapy with rituximab, possibly also combined with radiation. In patients with an indolent lymphoma such as lymphoplasmacytic lymphoma, mucosa-associated lymphoid tissue lymphoma, low-grade follicular lymphoma, or small lymphocytic lymphoma, radiation therapy is potentially curative. Central nervous system relapse occurs more frequently (3%-27%) in primary breast lymphoma cases than in those involving extramammary lymphoma, and thus, cranial radiation therapy or intrathecal chemotherapy may be performed prophylactically [15]. Treatment for T-cell lymphoma varies widely because there are so many different types. Standard lymphoma therapies including chemotherapy, radiation, bone marrow transplantation, and surgery may be effective in some cases, and ultraviolet light therapy or electron beam radiation is effective for T-cell lymphoma with skin involvement. With appropriate management, a high rate of locoregional control can be achieved for primary breast lymphoma, although there is often systemic relapse. The 5-year overall survival for diffuse large B-cell lymphoma in the breast has been reported to range from 60% to 65% [30-32]. Of the 10 cases included in our study, two patients underwent excision with chemotherapy, three received radiation therapy with chemotherapy, and the remaining five received only chemotherapy (Table 1). Radiation therapy was administered in 75% of the T-cell lymphoma cases. Seven of our 10 patients (70%) had complete remission after treatment, two patients (20%) underwent salvage treatment due to tumor recurrence after complete remission, and the remaining patient (10%) achieved a partial remission.
MULTIMODAL BREAST IMAGING FINDINGS
Lymphoma affecting the breast manifests as a breast mass, a change in the subcutaneous tissue or the skin, or enlargement of the associated lymph node on radiological examination.
Breast mass
On mammography, breast lymphoma appears as a solitary, noncalcified, circumscribed, or indistinctly delineated, oval or round mass that can vary in density (Figures 1-3) [33,34]. Calcifications, spiculations, and architectural distortion are distinctively absent [17,33,34]. For B-cell lymphoma, diffuse or ill-defined increases in breast density representing irregular infiltrating processes may be observed. Mammography is limited in its capacity to detect lymphomas that present as a diffuse infiltration or a small mass (Figure 4). For these lesions, which may be associated with skin thickening or breast edema, US is often more informative (Figures 4, 5), as breast lymphoma usually appears as a hypoechoic solid mass with circumscribed or indistinct margins (Figures 1-4) [28,29,33-36]. Heterogeneous echo patterns, hypoechogenicity, and hyperechogenicity are also seen frequently in breast lymphoma, and were present in 23% of cases in a study by Yang et al. (Figures 6-8) [13]. Posterior acoustic enhancement is another common feature, as is an echogenic rim or onion peel-like rim surrounding the mass that may represent lymphedema (Figure 2). The value of MRI of breast lymphoma is not firmly established, but inhomogeneous enhancement is seen in most cases on T1-weighted images after the administration of contrast media (Figure 2) [12,37,38]. Kinetic analysis on MRI shows that most breast lymphomas exhibit a rapid initial enhancement and plateau or a washout delayed phase enhancement (Figure 2) [12,13]. On CT, most lymphomas present as a circumscribed round or oval mass with various degrees of enhancement (Figures 2-4) [12].
Figure 1.
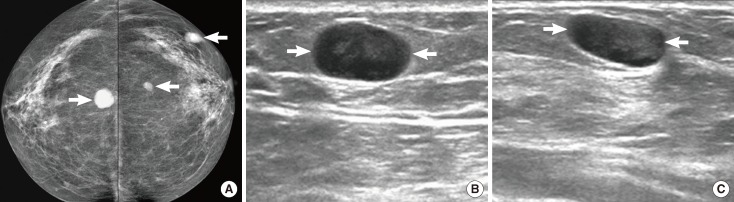
A 61-year-old woman with diffuse large B-cell lymphoma. (A) Both craniocaudal mammograms show multiple circumscribed oval or round masses (arrows) in both breasts. On (B) right and (C) left breast ultrasonography, the masses (arrows) are circumscribed and very low echoic, mimicking cysts.
Figure 3.
A 46-year-old woman with diffuse large B-cell lymphoma. (A) Both craniocaudal and mediolateral oblique mammograms are negative. (B) Ultrasonography scans of the right breast show multiple circumscribed oval hypoechoic masses (arrows). (C) Enhanced breast computed tomography (CT) image shows enlargement of right breast with multiple small enhancing foci (arrowheads). (D) Enhanced breast CT image obtained after chemotherapy shows the disappearance of enhancing foci in the right breast.
Figure 4.
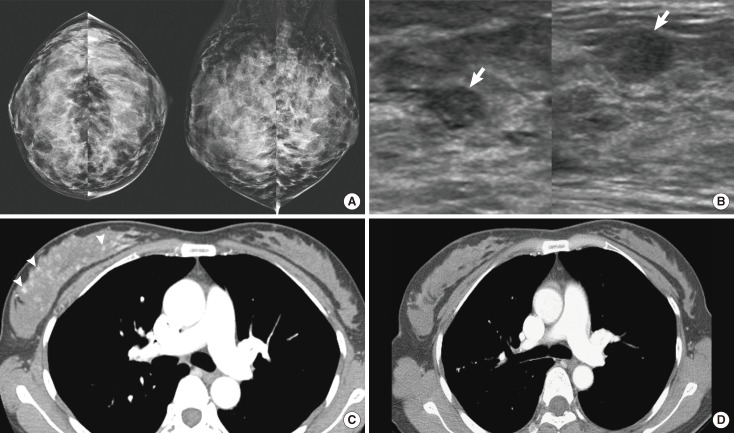
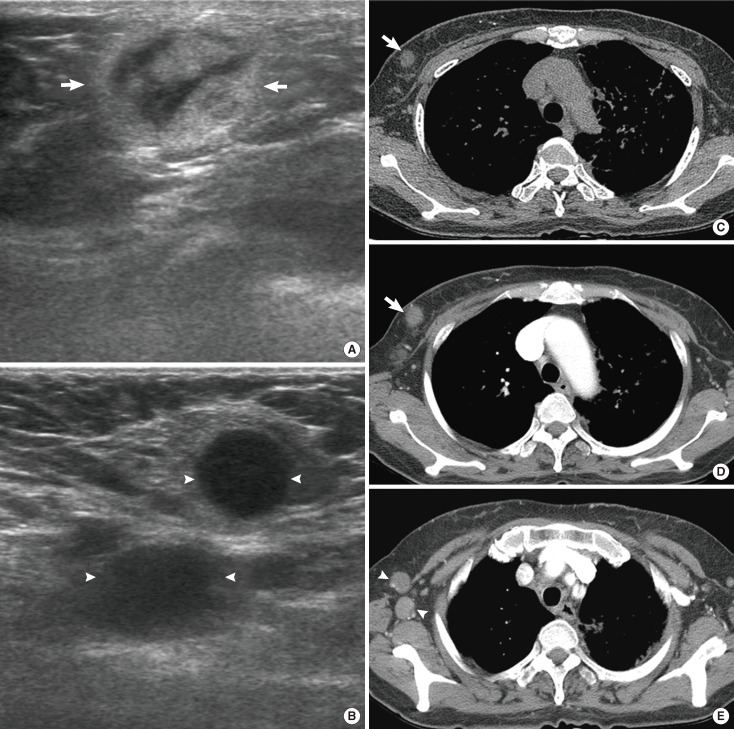
A 52-year-old woman with precursor T-lymphoblastic lymphoma. (A) Right mediolateral oblique mammogram shows a circumscribed and partially obscured marginated round hyperdense mass (arrows). (B) Ultrasonography scan of the right breast demonstrates a circumscribed oval hypoechoic mass (arrows). (C) Enhanced computed tomography scan demonstrates an oval isodense mass (arrow) in right breast and mediastinal widening (arrowheads) due to lymphadenopathy.
Figure 5.
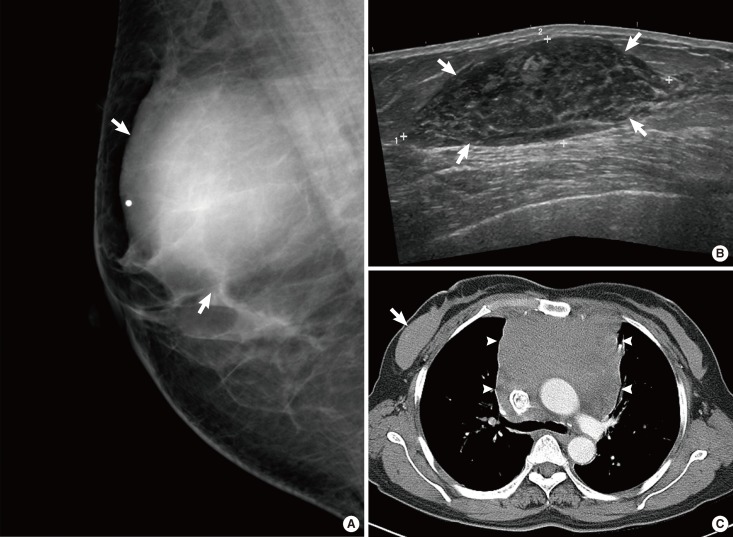
A 43-year-old woman with diffuse large B-cell lymphoma. (A) Left mediolateral oblique mammogram shows axillary lymphadenopathy (arrows) and trabecular thickening (arrowheads). (B) Ultrasonography (US) scans of the left breast show skin thickening (arrows) and dilated dermal lymphatics (arrowheads). (C, D) US scans of the left axilla show enlarged lymph nodes (arrows). One node is indistinct and irregular in shape (C) and the other is circumscribed and ovoid (D). The nodes have cortical thickening. (E) Enhanced breast computed tomography image shows multiple-rim enhancing lymph nodes (arrows) in left axilla.
Figure 6.
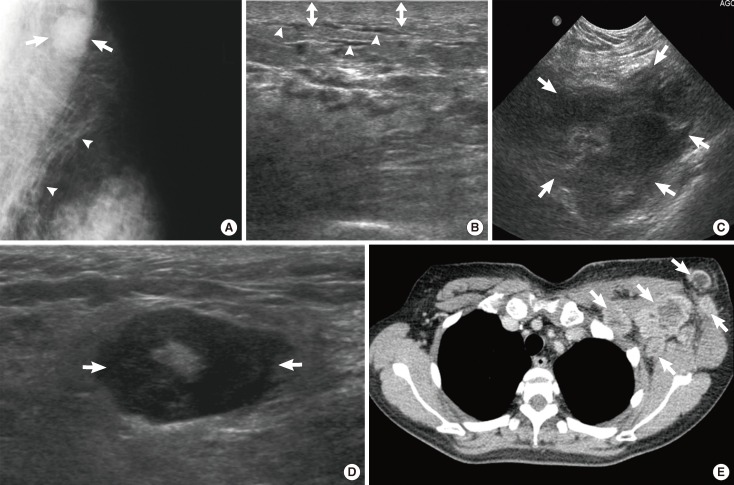
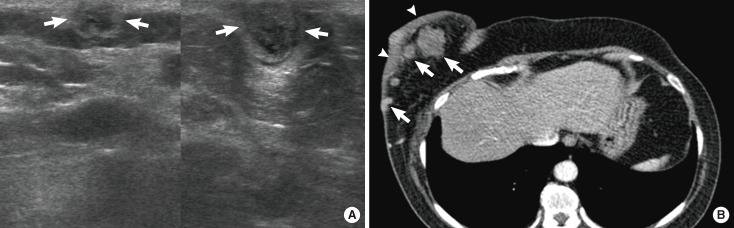
A 65-year-old woman with diffuse large B-cell lymphoma. (A) Ultrasonography (US) scan of the right breast shows an indistinct oval hyperechoic mass (arrows) in the subcutaneous fat layer. (B) US scan of the right axilla shows enlarged lymph nodes with loss of internal fatty hila (arrowheads). (C) Pre-enhanced and (D) enhanced breast computed tomography (CT) images demonstrate an indistinct oval isodense mass (arrows) in right breast. The mass in right breast reveals 39 Hounsfield unit (HU) on pre-enhanced image and 45 HU on enhanced images. (E) Multiple enlarged axillary lymph nodes (arrowheads) are seen on enhanced CT scan.
Figure 8.
A 62-year-old woman with peripheral T-cell lymphoma. (A) Ultrasonography (US) scans of the right breast show multiple oval or round masses (arrows) in skin and subcutaneous fat layers. (B) Enhanced computed tomography scan of right breast shows multiple isodense or hyperdense masses (arrows) with skin thickening (arrowheads).
Figure 2.
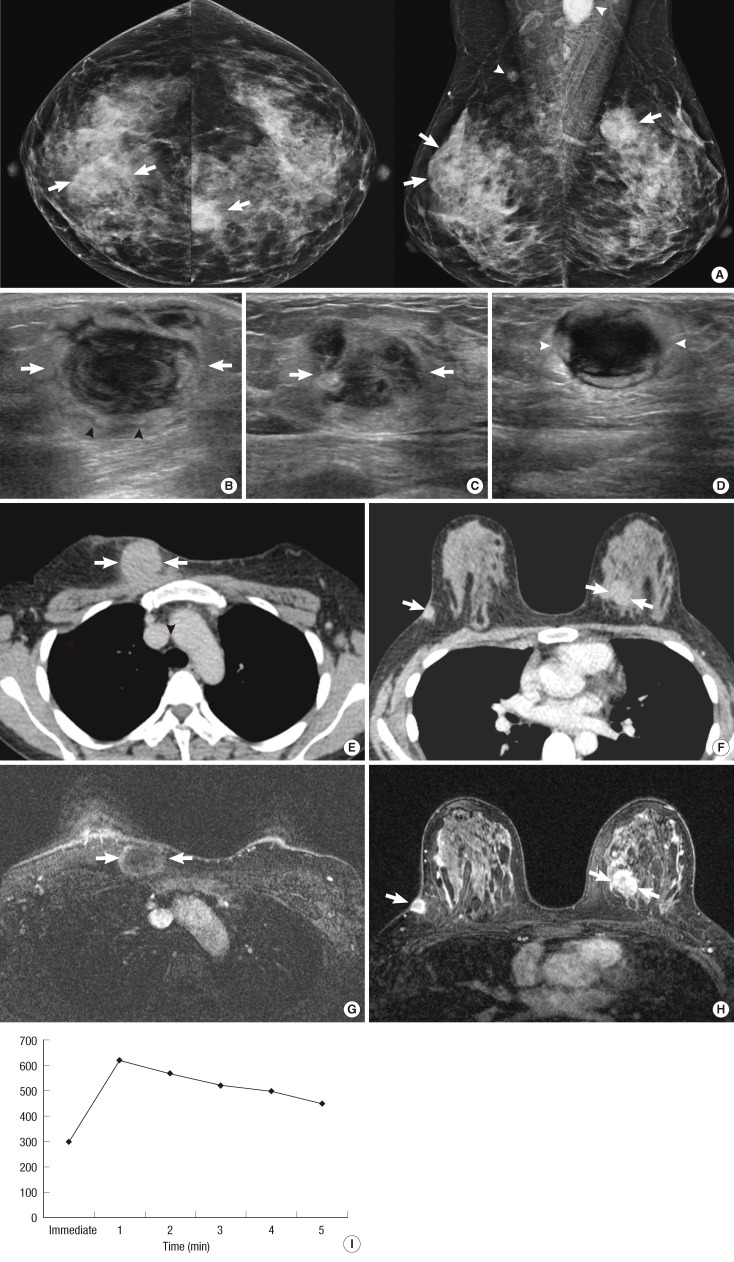
A 43-year-old woman with diffuse large B-cell lymphoma. (A) Both craniocaudal and mediolateral oblique mammograms demonstrate bilateral indistinct oval or round masses (arrows) and axillary lymph node enlargements (white arrowheads). (B) Ultrasonography (US) scan of the right breast shows an indistinct oval hypoechoic mass (arrows) with onion peel-like rims (black arrowheads). (C) US scan of the left breast shows an indistinct irregular hypoechoic mass (arrows). (D) US scan of the left axilla demonstrates an enlarged lymph node (white arrowheads). The node has indistinct margins and is very hypoechoic. (E, F) Enhanced breast computed tomography images show bilateral masses (arrows) with homogeneous enhancement. (G, H) Enhanced T1-weighted transverse magnetic resonance images show bilateral masses (arrows) with peripheral rim enhancement. (I) A time-enhancement curve obtained from the mass in left breast reveals rapid initial and washout delayed phase enhancement.
Subcutaneous or skin change
US, CT, and MRI facilitate the evaluation of diffuse infiltrative lesions, tiny nodules, or superficial lesions involving the skin or subcutis in breast tissue (Figures 3-8). Radiological reports of T-cell lymphoma in the breast are extremely rare compared to those of B-cell lymphoma, although T-cell lymphoma, especially subcutaneous panniculitis-like T-cell or peripheral T-cell lymphoma, preferentially infiltrate the subcutaneous tissues (Figures 6-8) [21,39,40]. US shows indistinct and irregular hyperechoic masses in the subcutis, and the hyperechoic areas may contain internal tubular branching hypoechogenicity (Figures 6, 7) [40,41]. Hyperechogenicity in breast lymphoma probably reflects the cellularity of these tumors [42]. On MRI, irregular masses with a rim or heterogeneous enhancement in the subcutis may be present. No characteristic CT findings of breast T-cell lymphoma have been reported to date, although in our study, we observed circumscribed or indistinct isodense masses with skin thickening on dynamic CT scans (Figures 6-8).
Figure 7.
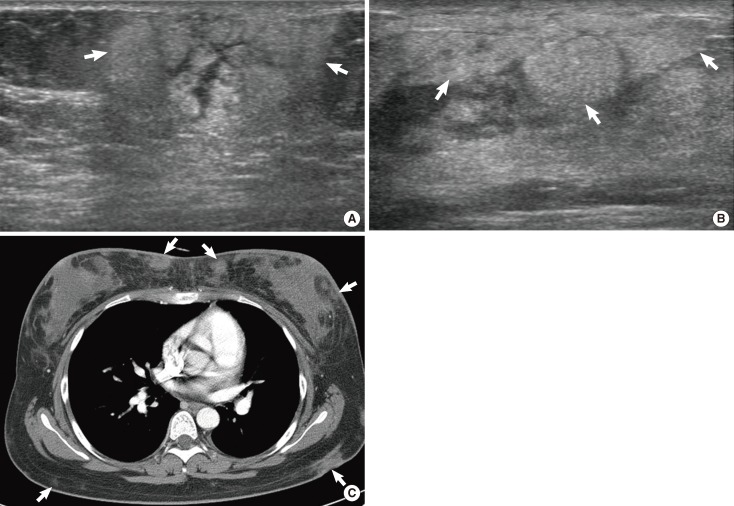
A 21-year-old woman with NK/T-cell lymphoma. (A) Ultrasonography (US) scan of the right breast shows an indistinct oval hyperechoic mass (arrows) with internal tubular hypoechogenicities and thickening of overlying skin. (B) US scan of the left breast shows increased echotexture of subcutaneous fat layer (arrows). (C) Enhanced breast computed tomography scan shows multiple indistinct isodense masses (arrows) in both breasts and back, and diffuse skin thickening in both breasts.
Lymphadenopathy
Routine breast imaging does not usually help to distinguish between primary and secondary lymphoma, although bilateral axillary lymphadenopathy or breast edema could indicate a secondary lymphoma. US, CT, and MRI are all more useful techniques than mammography for the evaluation of enlarging lymph nodes (Figures 4-6), and CT may be useful for the evaluation of intrathoracic and extrathoracic lymphadenopathy and involvement of the chest wall or lung in lymphoma patients (Figure 4).
DIFFERENTIAL DIAGNOSIS FROM OTHER BREAST MALIGNANCIES AND INFLAMMATORY BREAST DISEASES
It is difficult to distinguish breast lymphoma from various benign or malignant breast diseases on the basis of clinical and radiological findings. However, it is important to distinguish between breast lymphoma and other malignant diseases (for example, invasive breast carcinoma), inflammatory breast carcinoma, or metastasis, so that the appropriate treatment can be selected. The diagnostic strategy for lymphoma affecting the breast is determined by the presence or absence of known systemic lymphoma. If a patient is known to have systemic lymphoma, changes in the breasts must raise the possibility of a lymphomatous involvement [43]. However, in cases where lymphoma is not otherwise suspected, lymphoma of the breast is usually only suggested as one possibility in the differential diagnosis.
The most common symptom of breast malignancy is a painless palpable lump. Local pain, edema, or subcutaneous or skin nodules are frequent in lymphoma, especially the T-cell type, whereas nipple retraction or discharge is extremely rare [12,17,18]. With respect to radiological findings, the characteristic features of more common breast carcinomas, including calcifications, spiculations, or architectural distortion, are distinctively absent in lymphoma [17,33,34]. Inflammatory breast carcinoma manifests as breast edema with a mass on breast imaging that is usually of irregular shape and has indistinct margins or is spiculated, characteristics that are somewhat different from those of breast lymphoma. Metastasis to the breast manifests as a localized lesion or diffuse infiltration [43]. The former presents as a circumscribed oval or round mass and the latter appears as breast edema with dilated dermal lymphatics on breast imaging. These are very similar findings to those of lymphoma affecting the breast. There are currently no reliable criteria for distinguishing breast lymphoma from metastasis, although a history of a known metastasis or lymphoma in another organ is important in the diagnosis of a breast lesion. The findings of our study revealed that because a subcutaneous breast mass or nodule on radiological examination is rare in invasive breast carcinoma, inflammatory breast carcinoma, or metastasis, a change in the subcutis might aid in the diagnosis of breast lymphoma. Although there are some indicative clinical and radiological features of breast lymphoma, imaging-guided core needle biopsy or excisional biopsy should be performed for confirmative diagnosis. If a patient has a skin or subcutaneous lesion, a full thickness excisional skin biopsy should be performed in order to differentiate breast lymphoma from diffuse infiltrative metastasis and inflammatory carcinoma.
Differentiating breast lymphoma from inflammatory diseases is challenging, especially in patients with a painful breast lump, erythema, or skin thickening [44,45]. Inflammatory breast diseases include infectious mastitis, abscess, or idiopathic granulomatous lobular mastitis. Mammography and US often reveal unilateral breast edema, possibly with accompanying masses, in both breast lymphoma and inflammatory diseases. However, on breast US, the echo pattern of masses and associated findings differ between lymphoma and inflammation. In the latter, the masses are complicated cysts that have movable echoes or sedimentations, complex cysts, which have cystic and solid contents or are hypoechoic [43]. Dilated ducts or fistulous tracts to the skin are also often associated with inflammatory disease [43]. Conversely, a complex cyst is extremely rare and dilated ducts or fistulas are never associated with breast lymphoma. If a breast lesion is located in the subcutis, breast lymphoma needs to be distinguished from panniculitis or fat necrosis. Subcutaneous panniculitis, like T-cell lymphoma of the breast, is indistinguishable from inflammatory panniculitis and fat necrosis because of their clinical and pathological similarities [21,40], as both present as solitary or multiple subcutaneous nodules. Fat lobules in the subcutaneous layer are surrounded by neoplastic T-cells in cases of subcutaneous panniculitis like T-cell lymphoma, and inflammatory cells and some atypical cells are present in inflammatory panniculitis and fat necrosis on pathological examination, resulting in radiologically similar features. Clinically, inflammatory panniculitis spontaneously regresses without treatment within 1 to 4 weeks and frequently occurs in the lower extremities [46]. For the diagnosis of fat necrosis, it is important know the history of breast surgery, trauma, and biopsy [47].
CONCLUSION
Here we have described the clinical aspects and multimodal imaging findings of lymphoma affecting the breast. Breast lymphoma manifests as a breast mass, changes in the subcutaneous tissue or the skin, or enlargement of the associated lymph node on radiological examination. We also have illustrated the key clinical and radiological findings that can distinguish breast lymphoma from various breast malignancies and benign diseases. In breast lymphoma, usual malignant radiological features such as calcifications, spiculations, or architectural distortions are extremely rare, and skin and subcutaneous changes frequently accompany T-cell lymphoma. Multimodal breast imaging characteristics may aid the diagnosis of breast lymphoma.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Swerdlow SH International Agency for Research on Cancer. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC Press; 2008. [Google Scholar]
- 2.Tavassoli FA, Devilee P International Agency for Research on Cancer. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press; 2003. pp. 9–112. [Google Scholar]
- 3.Giardini R, Piccolo C, Rilke F. Primary non-Hodgkin's lymphomas of the female breast. Cancer. 1992;69:725–735. doi: 10.1002/1097-0142(19920201)69:3<725::aid-cncr2820690320>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Dao AH, Adkins RB, Jr, Glick AD. Malignant lymphoma of the breast: a review of 13 cases. Am Surg. 1992;58:792–796. [PubMed] [Google Scholar]
- 5.Brogi E, Harris NL. Lymphomas of the breast: pathology and clinical behavior. Semin Oncol. 1999;26:357–364. [PubMed] [Google Scholar]
- 6.Mainiero MB, Lourenco A, Mahoney MC, Newell MS, Bailey L, Barke LD, et al. ACR Appropriateness Criteria Breast Cancer Screening. Reston: American College of Radiology; 2006. pp. 217–225. [Google Scholar]
- 7.Lee WJ, Seo BK, Cho PK, Yie A, Cho KR, Woo OH, et al. The clinical use of low-dose multidetector row computed tomography for breast cancer patients in the prone position. J Breast Cancer. 2010;13:357–365. [Google Scholar]
- 8.Yi A, Seo BK, Cho PK, Pisano ED, Lee KY, Je BK, et al. Optimal multidetector row CT parameters for evaluations of the breast: a phantom and specimen study. Acad Radiol. 2010;17:744–751. doi: 10.1016/j.acra.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Seo BK, Pisano ED, Cho KR, Cho PK, Lee JY, Kim SJ. Low-dose multidetector dynamic CT in the breast: preliminary study. Clin Imaging. 2005;29:172–178. doi: 10.1016/j.clinimag.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 10.American College of Radiology, BI-RADS Committee. ACR BI-RADS Breast Imaging and Reporting Data System: Breast Imaging Atlas. 4th ed. Reston: American College of Radiology; 2003. [Google Scholar]
- 11.Wiseman C, Liao KT. Primary lymphoma of the breast. Cancer. 1972;29:1705–1712. doi: 10.1002/1097-0142(197206)29:6<1705::aid-cncr2820290640>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Surov A, Holzhausen HJ, Wienke A, Schmidt J, Thomssen C, Arnold D, et al. Primary and secondary breast lymphoma: prevalence, clinical signs and radiological features. Br J Radiol. 2012;85:e195–e205. doi: 10.1259/bjr/78413721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang WT, Lane DL, Le-Petross HT, Abruzzo LV, Macapinlac HA. Breast lymphoma: imaging findings of 32 tumors in 27 patients. Radiology. 2007;245:692–702. doi: 10.1148/radiol.2452061726. [DOI] [PubMed] [Google Scholar]
- 14.Arber DA, Simpson JF, Weiss LM, Rappaport H. Non-Hodgkin's lymphoma involving the breast. Am J Surg Pathol. 1994;18:288–295. doi: 10.1097/00000478-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Caon J, Wai ES, Hart J, Alexander C, Truong PT, Sehn LH, et al. Treatment and outcomes of primary breast lymphoma. Clin Breast Cancer. 2012;12:412–419. doi: 10.1016/j.clbc.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Avilés A, Delgado S, Nambo MJ, Neri N, Murillo E, Cleto S. Primary breast lymphoma: results of a controlled clinical trial. Oncology. 2005;69:256–260. doi: 10.1159/000088333. [DOI] [PubMed] [Google Scholar]
- 17.Domchek SM, Hecht JL, Fleming MD, Pinkus GS, Canellos GP. Lymphomas of the breast: primary and secondary involvement. Cancer. 2002;94:6–13. doi: 10.1002/cncr.10163. [DOI] [PubMed] [Google Scholar]
- 18.Gualco G, Chioato L, Harrington WJ, Jr, Weiss LM, Bacchi CE. Primary and secondary T-cell lymphomas of the breast: clinico-pathologic features of 11 cases. Appl Immunohistochem Mol Morphol. 2009;17:301–306. doi: 10.1097/PAI.0b013e318195286d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talwalkar SS, Miranda RN, Valbuena JR, Routbort MJ, Martin AW, Medeiros LJ. Lymphomas involving the breast: a study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol. 2008;32:1299–1309. doi: 10.1097/PAS.0b013e318165eb50. [DOI] [PubMed] [Google Scholar]
- 20.Portlock C, Vose MJ, Cheson BD. T-cell lymphomas. Lymphoma Research Foundation; 2008. [Accessed July 24th, 2013]. http://www.lymphoma.org/atf/cf/%7B0363CDD6-51B5-427B-BE48-E6AF871ACEC9%7D/T-CELL%20LYMPHOMAS.PDF. [Google Scholar]
- 21.Uematsu T, Kasami M. 3T-MRI, elastography, digital mammography, and FDG-PET CT findings of subcutaneous panniculitis-like T-cell lymphoma (SPTCL) of the breast. Jpn J Radiol. 2012;30:766–771. doi: 10.1007/s11604-012-0112-5. [DOI] [PubMed] [Google Scholar]
- 22.Aladily TN, Medeiros LJ, Amin MB, Haideri N, Ye D, Azevedo SJ, et al. Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Surg Pathol. 2012;36:1000–1008. doi: 10.1097/PAS.0b013e31825749b1. [DOI] [PubMed] [Google Scholar]
- 23.Sahoo S, Rosen PP, Feddersen RM, Viswanatha DS, Clark DA, Chadburn A. Anaplastic large cell lymphoma arising in a silicone breast implant capsule: a case report and review of the literature. Arch Pathol Lab Med. 2003;127:e115–e118. doi: 10.5858/2003-127-e115-ALCLAI. [DOI] [PubMed] [Google Scholar]
- 24.Aguilera NS, Tavassoli FA, Chu WS, Abbondanzo SL. T-cell lymphoma presenting in the breast: a histologic, immunophenotypic and molecular genetic study of four cases. Mod Pathol. 2000;13:599–605. doi: 10.1038/modpathol.3880103. [DOI] [PubMed] [Google Scholar]
- 25.Jewell M, Spear SL, Largent J, Oefelein MG, Adams WP., Jr Anaplastic large T-cell lymphoma and breast implants: a review of the literature. Plast Reconstr Surg. 2011;128:651–661. doi: 10.1097/PRS.0b013e318221db81. [DOI] [PubMed] [Google Scholar]
- 26.Smith TJ, Ramsaroop R. Breast implant related anaplastic large cell lymphoma presenting as late onset peri-implant effusion. Breast. 2012;21:102–104. doi: 10.1016/j.breast.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PA, Lade S, Webster H, Ryan G, Prince HM. Effusion-associated anaplastic large cell lymphoma of the breast: time for it to be defined as a distinct clinico-pathological entity. Haematologica. 2010;95:1977–1979. doi: 10.3324/haematol.2010.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong D, Vasmel WL, de Boer JP, Verhave G, Barbé E, Casparie MK, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300:2030–2035. doi: 10.1001/jama.2008.585. [DOI] [PubMed] [Google Scholar]
- 29.Friis S, McLaughlin JK, Mellemkjaer L, Kjøller KH, Blot WJ, Boice JD, Jr, et al. Breast implants and cancer risk in Denmark. Int J Cancer. 1997;71:956–958. doi: 10.1002/(sici)1097-0215(19970611)71:6<956::aid-ijc8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 30.Jeanneret-Sozzi W, Taghian A, Epelbaum R, Poortmans P, Zwahlen D, Amsler B, et al. Primary breast lymphoma: patient profile, outcome and prognostic factors: a Multicentre Rare Cancer Network study. BMC Cancer. 2008;8:86. doi: 10.1186/1471-2407-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yhim HY, Kang HJ, Choi YH, Kim SJ, Kim WS, Chae YS, et al. Clinical outcomes and prognostic factors in patients with breast diffuse large B cell lymphoma: Consortium for Improving Survival of Lymphoma (CISL) study. BMC Cancer. 2010;10:321. doi: 10.1186/1471-2407-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan G, Martinelli G, Kuper-Hommel M, Tsang R, Pruneri G, Yuen K, et al. Primary diffuse large B-cell lymphoma of the breast: prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann Oncol. 2008;19:233–241. doi: 10.1093/annonc/mdm471. [DOI] [PubMed] [Google Scholar]
- 33.Lyou CY, Yang SK, Choe DH, Lee BH, Kim KH. Mammographic and sonographic findings of primary breast lymphoma. Clin Imaging. 2007;31:234–238. doi: 10.1016/j.clinimag.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 34.Irshad A, Ackerman SJ, Pope TL, Moses CK, Rumboldt T, Panzegrau B. Rare breast lesions: correlation of imaging and histologic features with WHO classification. Radiographics. 2008;28:1399–1414. doi: 10.1148/rg.285075743. [DOI] [PubMed] [Google Scholar]
- 35.Yang WT, Metreweli C. Sonography of nonmammary malignancies of the breast. AJR Am J Roentgenol. 1999;172:343–348. doi: 10.2214/ajr.172.2.9930779. [DOI] [PubMed] [Google Scholar]
- 36.Yang WT, Muttarak M, Ho LW. Nonmammary malignancies of the breast: ultrasound, CT, and MRI. Semin Ultrasound CT MR. 2000;21:375–394. doi: 10.1016/s0887-2171(00)90031-3. [DOI] [PubMed] [Google Scholar]
- 37.Mussurakis S, Carleton PJ, Turnbull LW. MR imaging of primary non-Hodgkin's breast lymphoma: a case report. Acta Radiol. 1997;38:104–107. doi: 10.1080/02841859709171251. [DOI] [PubMed] [Google Scholar]
- 38.Naganawa S, Endo T, Aoyama H, Ichihara S. MR imaging of the primary breast lymphoma: a case report. Breast Cancer. 1996;3:209–213. doi: 10.1007/BF02966986. [DOI] [PubMed] [Google Scholar]
- 39.Sy AN, Lam TP, Khoo US. Subcutaneous panniculitislike T-cell lymphoma appearing as a breast mass: a difficult and challenging case appearing at an unusual site. J Ultrasound Med. 2005;24:1453–1460. doi: 10.7863/jum.2005.24.10.1453. [DOI] [PubMed] [Google Scholar]
- 40.Lim HJ, Cho KR, Kim I, Hwang KW, Seo BK, Woo OH, et al. Primary peripheral T-cell lymphoma of the breast: radiologic and pathologic findings. J Breast Cancer. 2010;13:318–322. [Google Scholar]
- 41.Ko ES, Seol H, Shin JH, Ko EY. Primary anaplastic lymphoma kinase-negative anaplastic large-cell lymphoma of the breast in a male patient. Br J Radiol. 2012;85:e79–e82. doi: 10.1259/bjr/23296454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adrada B, Wu Y, Yang W. Hyperechoic lesions of the breast: radiologic-histopathologic correlation. AJR Am J Roentgenol. 2013;200:W518–W530. doi: 10.2214/AJR.12.9263. [DOI] [PubMed] [Google Scholar]
- 43.Heywang-Köbrunner SH, Dershaw DD, Schreer I. Diagnostic Breast Imaging: Mammography, Sonography, Magnetic Resonance Imaging, and Interventional Procedures. 2nd ed. New York: Thieme; 2001. pp. 236–251.pp. 325–338. [Google Scholar]
- 44.Sun LM, Huang EY, Meng FY, Chang NJ, Chung LM, Liang JA, et al. Primary breast lymphoma clinically mimicking acute mastitis: a case report. Tumori. 2011;97:233–235. doi: 10.1177/030089161109700218. [DOI] [PubMed] [Google Scholar]
- 45.Grubstein A, Givon-Madhala O, Morgenstern S, Cohen M. Extranodal primary B-cell non-Hodgkin lymphoma of the breast mimicking acute mastitis. J Clin Ultrasound. 2005;33:140–142. doi: 10.1002/jcu.20101. [DOI] [PubMed] [Google Scholar]
- 46.Pinho MC, Souza F, Endo E, Chala LF, Carvalho FM, de Barros N. Nonnecrotizing systemic granulomatous panniculitis involving the breast: imaging correlation of a breast cancer mimicker. AJR Am J Roentgenol. 2007;188:1573–1576. doi: 10.2214/AJR.05.0517. [DOI] [PubMed] [Google Scholar]
- 47.Taboada JL, Stephens TW, Krishnamurthy S, Brandt KR, Whitman GJ. The many faces of fat necrosis in the breast. AJR Am J Roentgenol. 2009;192:815–825. doi: 10.2214/AJR.08.1250. [DOI] [PubMed] [Google Scholar]