Abstract
Purpose
We investigated the relationship between BRCA mutations, pathological findings, and magnetic resonance imaging (MRI) features in patients with breast cancer at risk for the mutation.
Methods
Genetic testing for BRCA mutations was performed in 275 breast cancer patients with at least one risk factor for the mutation. Using the breast imaging reporting and data system MR lexicon, morphological and kinetic features were reviewed on MRI scans of 230 tumors in 209 patients. The relationship between BRCA mutations, pathologic findings, and MRI data was examined, and disease recurrence was estimated.
Results
BRCA mutations were detected in 48 patients (23.0%), of which 21 (10.0%) were in BRCA1, and 25 (12.0%) in BRCA2. Additionally, two patients (1.0%) had mutations in both genes. Cancers in patients with BRCA1 mutations more frequently showed a higher nuclear grade (p=0.0041), and triple-negative (TN) phenotype (p<0.0001). On MRI scans, the cancers were seen as mass-type in 182 out of 230 lesions (79.1%), and nonmass type in 48 cases (20.9%). Among the features indentified by MRI, rim enhancement was significantly associated with molecular subtypes based on immunohistochemistry (p<0.0001), and nuclear grade (p=0.0387) in multiple logistic regression analysis. Rim enhancement on MRI, along with advanced pathologic N stage, was associated with increased disease recurrence (p=0.0023) based on multivariate analysis. However, the proportion of mass and nonmass tumors, and the distribution of morphological shape, margin, internal enhancement, and kinetic features assessed by MRI were not different according to BRCA mutation status.
Conclusion
BRCA1 mutations were associated with aggressive pathological characteristics, and the TN phenotype. Rim enhancement was frequently seen on MRI scans of high-grade cancers and in the TN phenotype. And it was a significant predictor of disease recurrence. However, a direct association with BRCA mutations was not observed.
Keywords: BRCA1 genes, Breast neoplasms, Magnetic resonance imaging, Recurrence
INTRODUCTION
The BRCA1 and BRCA2 genes are involved in DNA repair and recombination, cell-cycle control, and transcription [1]. Mutations in these genes are associated with the development of breast and ovarian cancer [2,3]. In addition, BRCA mutations may predispose patients to other primary cancers, including those of the stomach, pancreas, biliary tract, and prostate [4]. BRCA1 mutation carriers are more likely to have triple-negative breast cancers, which do not express estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) [5,6]. The triple-negative phenotype, which serves as a substitute for basal-like breast cancer, is associated with a younger age of onset, higher nuclear grade, poorer histological grade, early development of distant metastasis, and decreased survival [5-7]. BRCA1 mutation carriers with triple-negative breast cancer also tend to have tumors with a higher nuclear grade [5].
Magnetic resonance imaging (MRI) has been widely used for screening women at increased risk for breast cancer [8]. The sensitivity of MRI for detecting breast tumors ranges from 77% to 91%, which is higher than mammography (33%-40%), in women at high familial risk for breast cancer [9,10]. Triple-negative breast cancer is associated with a round or oval shape, smooth mass margins, and rim enhancement on MRI [11]. In addition, rim enhancement on MRI is a well-known predictor of higher tumor grade [12]. Because BRCA1 mutations are associated with a triple-negative phenotype and aggressive pathological characteristics, the features identified by MRI in the tumors of patients with BRCA1 mutations may match those described above. However, whether BRCA mutations can be predicted based on MRI features remains unclear and controversial [13-15]. The tumors of BRCA mutation carriers are associated with round shape, sharp margins, and rim enhancement on MRI [13], while fibroadenoma-like masses were found on MRI scans in 23% of invasive cancers in women at familial risk [14]. A review of the United Kingdom magnetic resonance imaging in breast screening (MARIBS) trial data shows that most of the cancers in BRCA1- and BRCA2-mutation carriers are poorly defined, with irregular or spiculated margins and ring-like or heterogeneous enhancement patterns. In addition, no significant differences in the MRI features were noted between the genetic subtypes [15].
The studies described above were limited to analyzing the relationships between genetic, molecular, and histological subtypes, and imaging features separately. To directly evaluate the relationships between BRCA mutations and MRI features, we performed genetic testing for BRCA mutations in breast cancer patients with risk factors for carrying the mutations, and reviewed their MRI and pathological features. We then analyzed the relationships between the genetic subtypes, features on MRI, and the pathological characteristics. Finally, the relationships of these factors with recurrence of breast cancer were assessed.
METHODS
Patients
Genetic testing was carried out in 275 patients who underwent curative surgery for breast cancer between November 2005 and May 2012, and who carried at least one of the following risk factors for BRCA mutations: a reported family history of breast or ovarian cancer, <40 years of age at diagnosis, bilateral breast cancer, or male gender. After obtaining informed consent, genetic testing for the BRCA1/2 mutations was performed using direct sequencing. Genomic DNA was extracted and purified from peripheral blood leukocytes. After amplification of the whole exons and intrinsic flanking sequences of the BRCA1 and BRCA2 genes by polymerase chain reaction, sequences were compared with reference sequences using Sequencher software (Gene Codes Corp., Ann Arbor, USA). Searches for genetic mutations were limited to known deleterious mutations such as frame-shift or nonsense mutations, and variants of unknown significance were excluded from analysis. The Institutional Review Board of Samsung Medical Center approved this study (2013-07-011).
After excluding patients who had an MRI at an outside hospital (n=8), no MRI (n=35), or who had no residual cancer after previous excision (n=23), the number of eligible patients with available MRI data was 209. In addition, 21 patients had synchronous or metachronous bilateral breast cancer during the study period, and so a total of 230 cancers were retrospectively reviewed. Medical records, including operative and pathological reports, were also reviewed.
MR imaging
MR imaging was performed in the prone position using a 1.5-T system (Sigma; General Electric Medical Systems, Milwaukee, USA), or a 3.0-T system (Achieva; Philips Medical Systems, Best, The Netherlands) scanner with a dedicated surface breast coil. A fat-suppressed, axial fast spin, echo T2-weighted sequence (repetition time ms/echo time ms, 4,000/120), and fat-suppressed unilateral sagittal or bilateral axial dynamic images with a gradient echo sequence were obtained. Imaging on the 1.5-T scanner covered a single breast with a minimum repetition time/echo time of 17.3/1.3, a flip angle of 60°, and a section thickness of 2 mm with no gap. Imaging on the 3.0-T scanner covered both breasts with a minimum repetition time/echo time of 8.7/4.3 for the dynamic images, a flip angle of 20°, and a section thickness of 1.5 mm with no gap. A 0.1 mmol/kg bolus of gadobutrol (Gadovist; Schering AG, Berlin, Germany), followed by a 10-mL saline flush, was injected for dynamic contrast enhancement. Standard and reverse subtraction images were obtained by subtracting the precontrast images and the last postcontrast image from the early postcontrast images on a pixel-by-pixel basis.
Interpretation of MR imaging and histopathological features
Two radiologists, who were blinded to the clinicopathologic data, reviewed the MRI scans. In patients who received neoadjuvant chemotherapy, the MRIs taken at initial diagnosis were used for the study. The lesion type was classified as mass or nonmass enhancement, based on the American College of Radiology Breast imaging reporting and data system (BI-RADS) MR lexicon [16]. The shape, margin, enhancement pattern, and kinetics at the delayed phase were then evaluated for mass-type lesions. For non-mass-like-lesions, the distribution and internal enhancement were evaluated. The kinetics at the delayed phase was defined as persistent, plateau, or washout, as defined in the BI-RADS MR lexicon. If there was a discrepancy in the assessment of the MRI, a consensus was given by the two radiologists.
An experienced pathologist was responsible for histopathological diagnoses. Nuclear grade, multiplicity, tumor stage, and the status of immunohistochemical (IHC) staining for estrogen receptor (ER), progesterone receptor (PR), and HER2 were recorded, and positive immunoreactivity for ER and PR was defined by Allred scores, ranging from 3 to 8 [17]. Positive HER2 status was determined using IHC 3+ staining, or amplification using fluorescence in situ hybridization. Molecular subtypes were classified into three groups: luminal (ER or PR positive), triple-negative (ER, PR, and HER2 negative), or HER2 overexpressing (ER and PR negative, and HER2 positive).
Statistical analysis
To analyze the relationships between BRCA mutations, clinicopathological status, and MRI findings, chi-square or Fisher exact tests were used. Patients with both BRCA1 and BRCA2 germline mutations were classified as having a BRCA1 mutation, because the histopathological features of double heterozygosity tend to be primarily influenced by BRCA1 mutation [18]. A multiple logistic regression analysis was applied to identify factors associated with morphological and enhancement features on MRI. Disease recurrence was defined as recurrence of breast cancer at any site (including local, regional, or distant), and contralateral breast cancer or second primary cancers were not considered. The recurrence rate was estimated using the Kaplan-Meier test, and was compared by log-rank test. The Cox proportional hazards regression analysis model was used to analyze the prognostic factors affecting disease recurrence. SAS software (SAS 9.1.3; SAS Institute Inc., Cary, USA) was used for statistical analysis.
RESULTS
The median age of the 209 eligible patients was 40 years (ranging from 28 to 52 years), and BRCA mutations were detected in 48 of the patients (23.0%). BRCA1 and BRCA2 mutations were detected in 21 (10.0%) and 25 (12.0%) patients, respectively, and 2 patients (1.0%) had mutations in both genes. A higher frequency of family history and bilateral breast cancer was observed in patients with BRCA mutations (p=0.0141 and p=0.0039, respectively) (Table 1). Invasive ductal carcinoma and ductal carcinoma in situ accounted for 82.2% and 10.9% of cancers, respectively, and the histopathological characteristics of the tumors in each genetic subgroup are presented in Table 2. Patients with BRCA mutations underwent mastectomy more frequently, while BRCA1 mutation carriers had a significantly higher number of cancers with high nuclear grade (p=0.0041) and the triple-negative phenotype (p< 0.0001).
Table 1.
Distribution of risk factors according to BRCA mutation status in 209 patients with breast cancer*
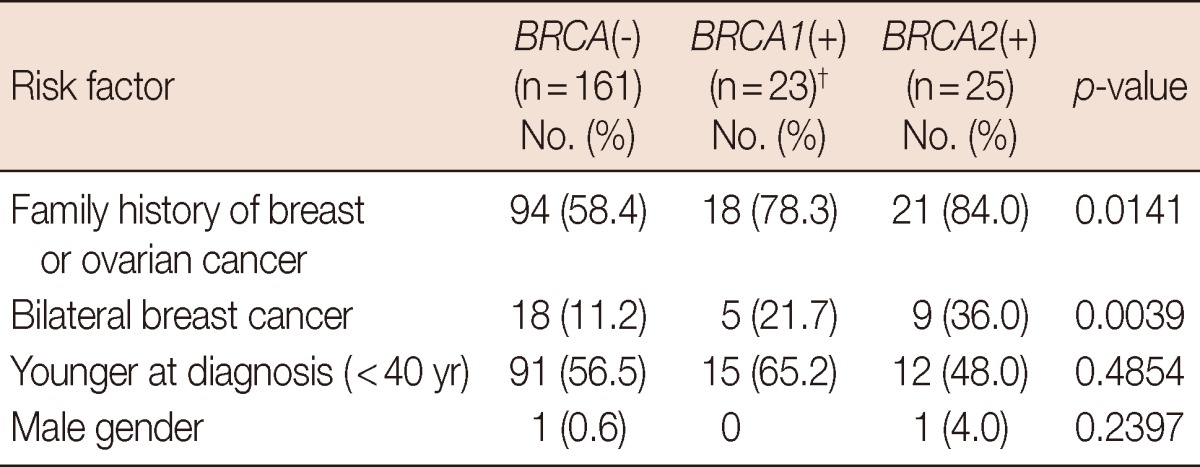
*Including 72 patients with multiple risk factors; †Including the two patients with both BRCA1 and BRCA2 mutations.
Table 2.
Histopathological findings of 230 breast cancers in 209 patients
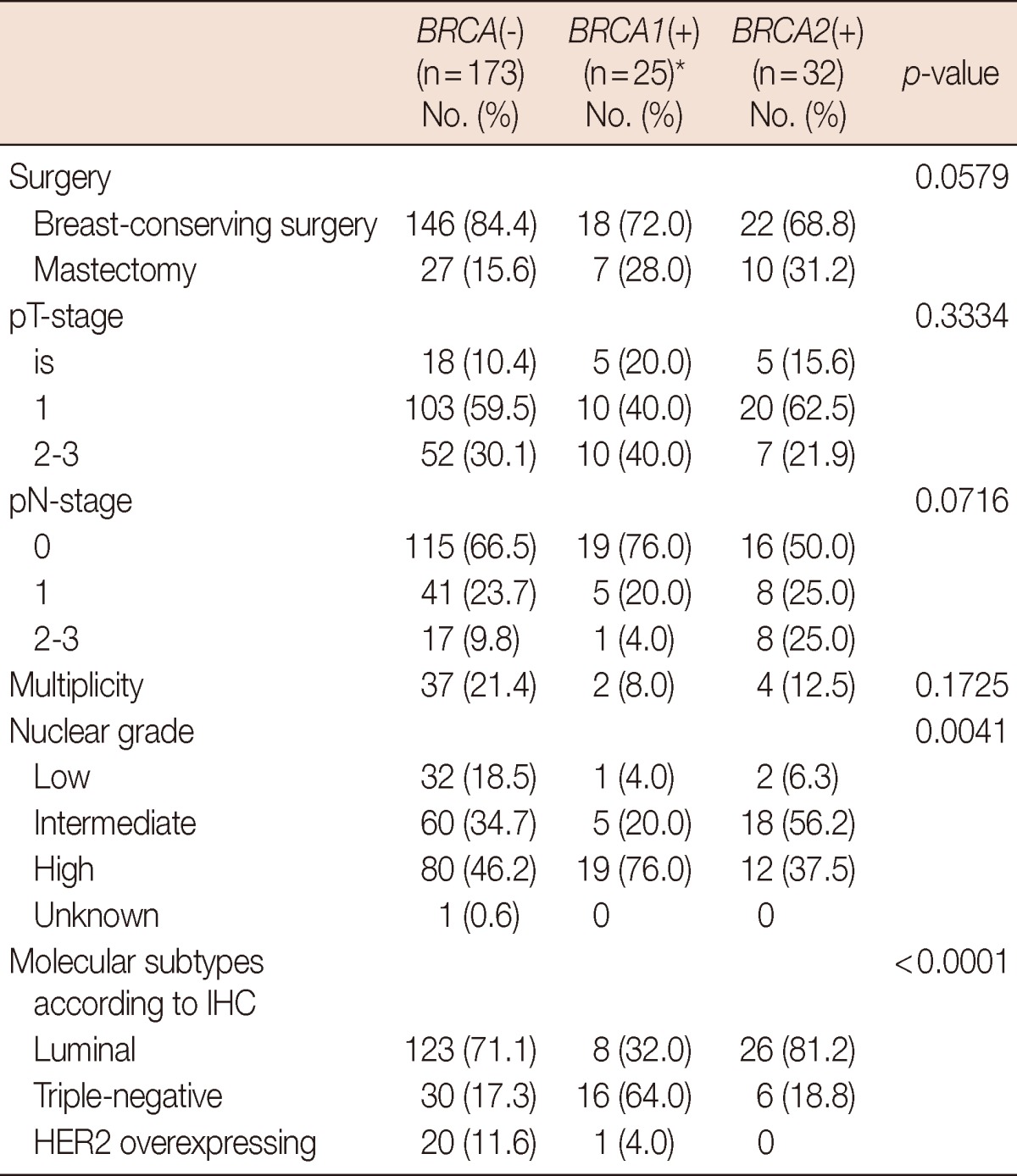
IHC=immunohistochemistry; HER2=human epidermal growth factor receptor 2.
*Including three tumors of the two patients with both BRCA1 and BRCA2 mutations.
On the MRI scans, cancers were identified as mass type in 182 of the 230 lesions (79.1%), and nonmass type in 48 cases (20.9%). Mass-type lesions were identified in 79.2%, 76.0%, and 81.3% of BRCA-negative, BRCA1-positive, and BRCA2-positive cases, respectively (p=0.8888). The morphological and kinetic features of mass-type lesions on the MRI data are detailed in Table 3. We found no difference in morphological shape, margin, internal enhancement, or kinetic features when comparing the different genetic subtypes. Rim enhancement was observed in 36 cancers (Figure 1), and accounted for 31.6% and 23.1% of cancers harboring the BRCA1- and BRCA2-mutations, respectively. The prevalence of rim enhancement was not different from cancers in patients without BRCA mutations (17.5%, p=0.4884). Regardless of genetic subtype, most cancers showed irregular shape and margins, heterogeneous enhancement, and washout patterns at the delayed phase. Among the 36 cancers with rim enhancement, high nuclear grade and triple-negative phenotype was observed in 29 (80.6%) and 23 (63.9%) tumors, respectively. Using multiple logistic regression analysis, rim enhancement was associated with molecular subtype (p<0.0001) and nuclear grade (p=0.0387), but not genetic subtype (p=0.1189) (Table 4).
Table 3.
The morphological and kinetic features of mass-type lesions on magnetic resonance imaging (n=182)
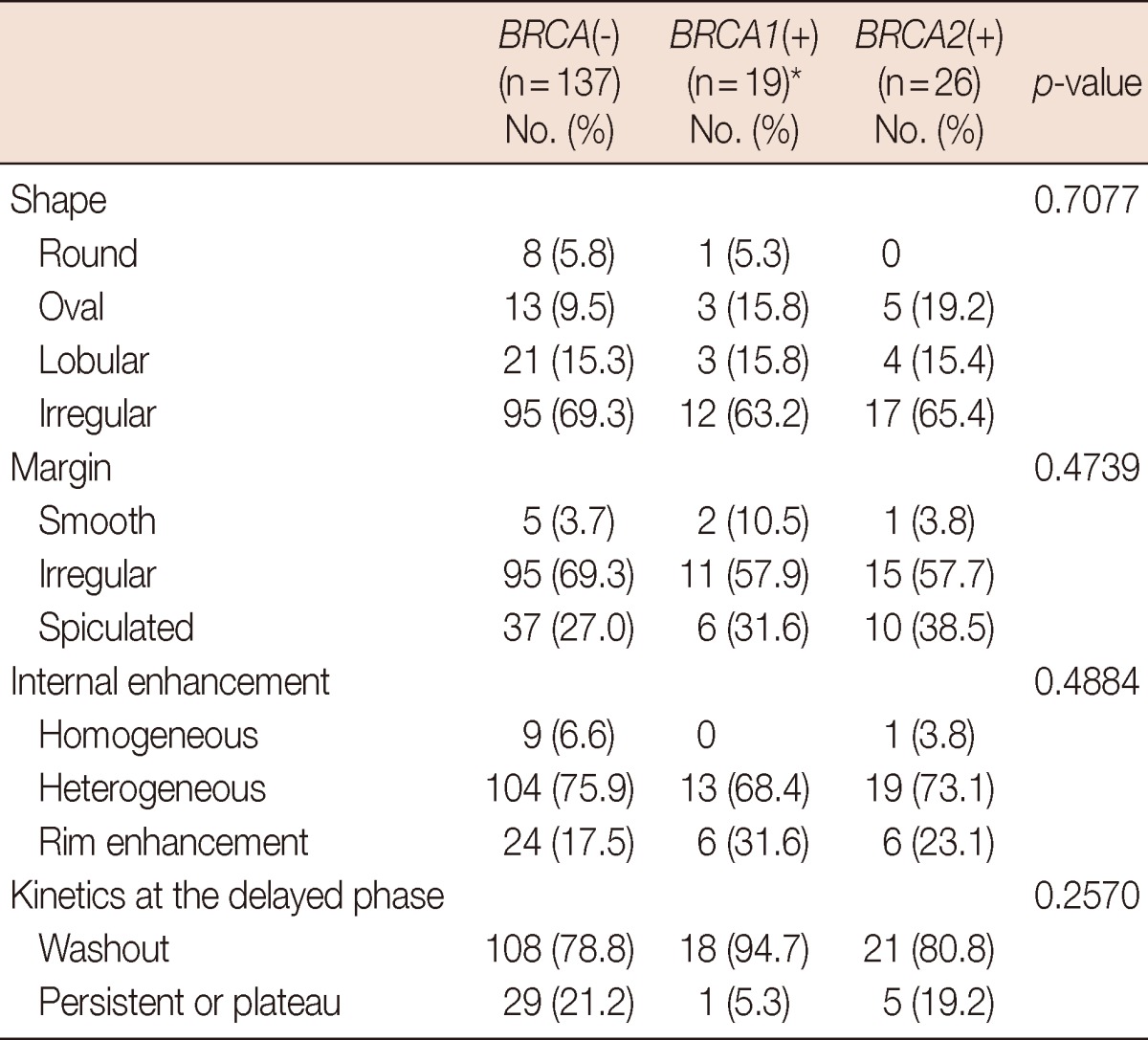
*Including two tumors of the two patients with both BRCA1 and BRCA2 mutations.
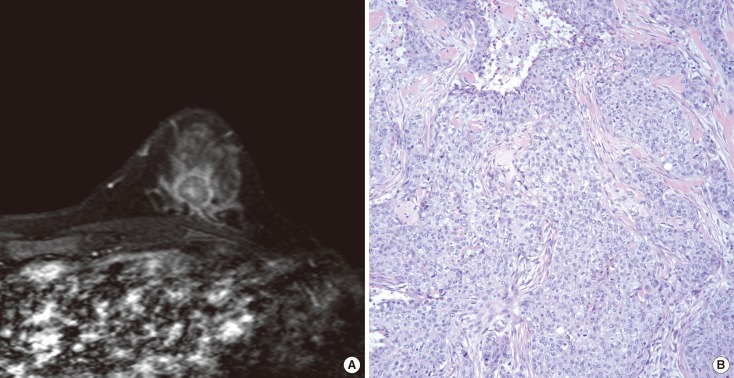
Figure 1.
Images representing rim enhancement. (A) An axial contrast-enhanced T1-weighted magnetic resonance imaging scan a 38-year-old patient with BRCA1 mutation demonstrated rim enhancement. A 1.8-cm mass with irregular shape and spiculated margin was observed in the left lower center breast. (B) On the haematoxylin and eosin stained slides (×200), the tumor was a 2.1-cm poorly differentiated invasive ductal carcinoma with high nuclear grade. Immunohistochemistry results were all negative for estrogen receptor and progesterone receptor, and human epidermal growth factor receptor 2.
Table 4.
Multiple logistic regression analysis for mass enhancement on magnetic resonance imaging
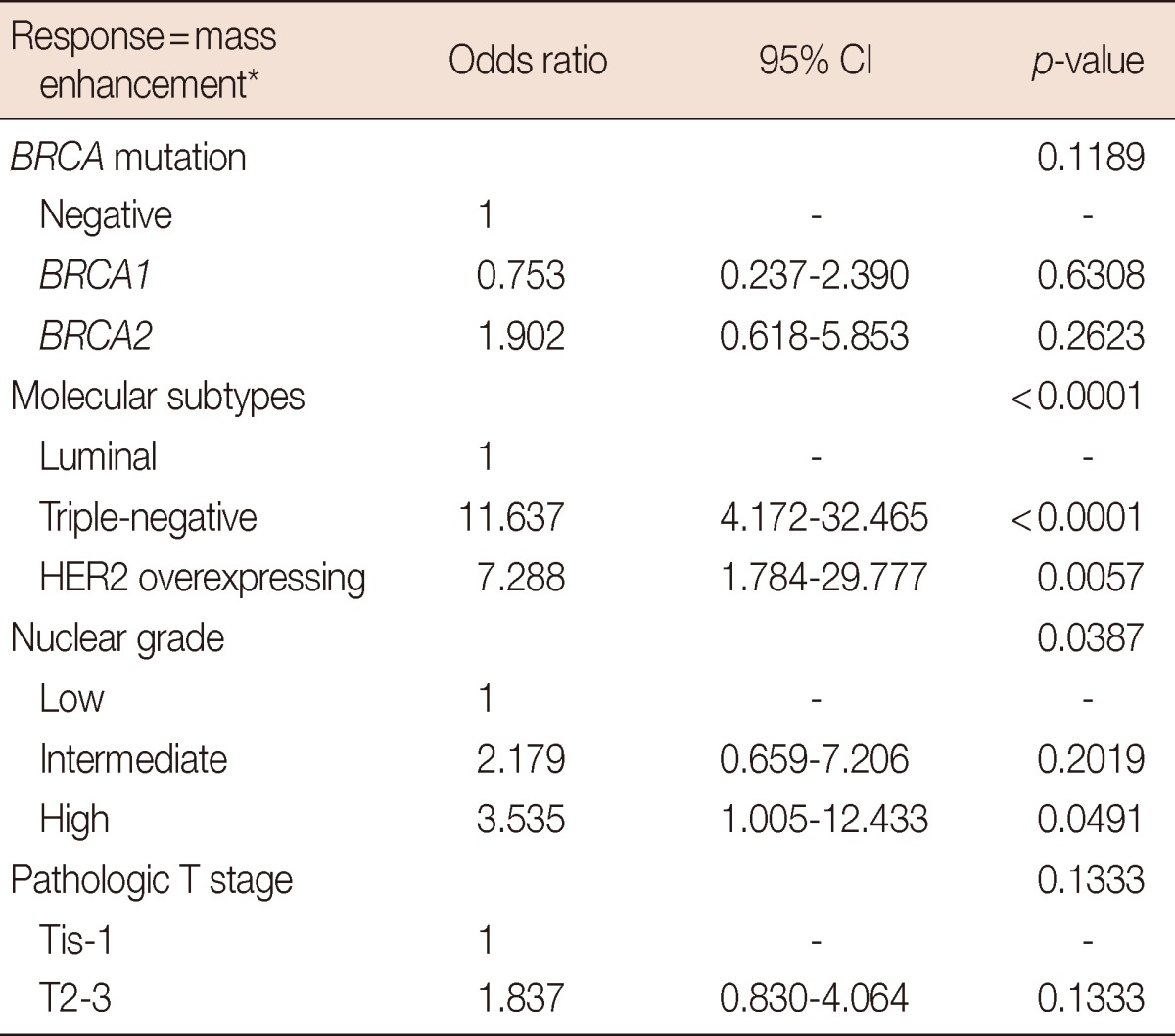
*Homogeneous vs. heterogeneous vs. rim enhancement.
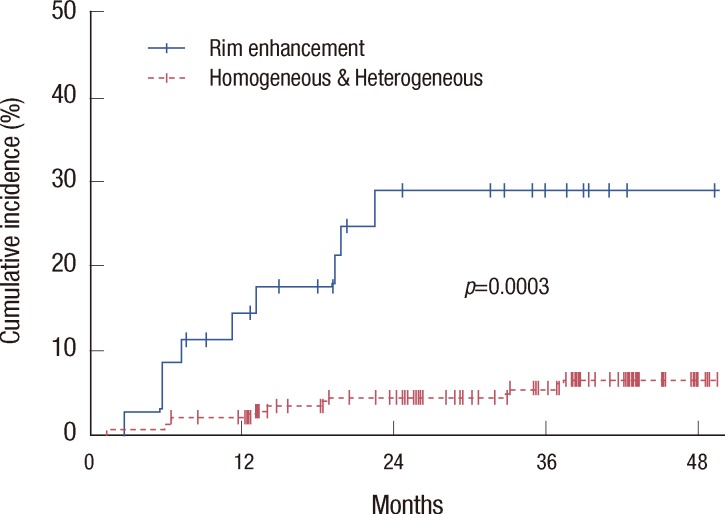
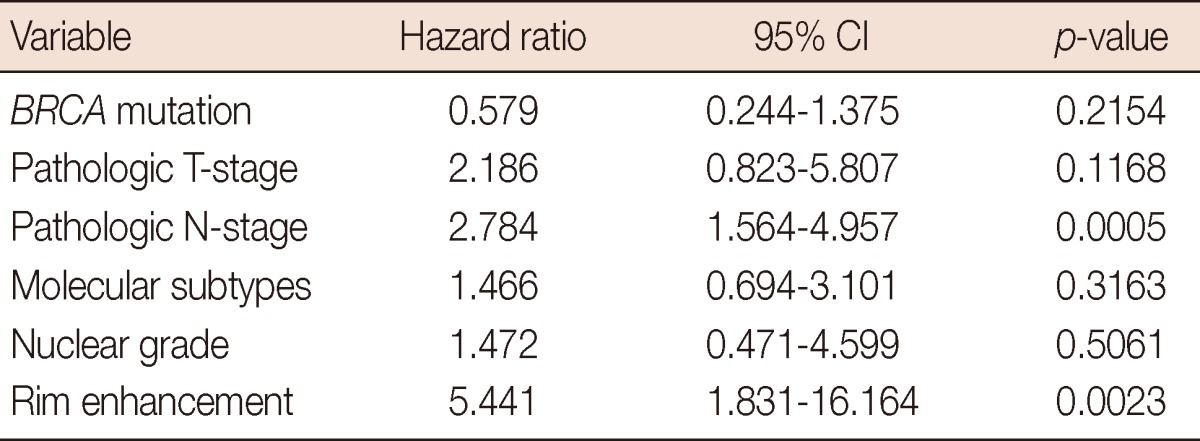
The median duration of follow-up, calculated from the date of surgery, was 38.0 months (ranging from 2.1 to 84.3 months). During the follow-up period, 17 patients (8.1%) experienced disease recurrence, with a median recurrence time of 12.8 months (ranging from 1.3 to 37.5 months). The median time to recurrence of 9 patients with rim enhancement on MRI was 11.3 months (ranging from 2.8 to 22.5 months). The 2-year rate of disease-recurrence for all patients was 7.0%, which was increased in patients with the BRCA1 mutation (17.4%, p=0.0542), the triple-negative phenotype (22.6%, p< 0.0001), rim enhancement on MRI scans (28.9%, p=0.0003) (Figure 2), high nuclear grade (10.6%, p=0.0500), pathologic T2-3 disease (12.6%, p=0.0299), and pathologic N2-3 disease (12.6%, p=0.0164). Using multivariate analysis, advanced pathological N-stage and rim enhancement were significantly associated with disease recurrence (Table 5). The hazard ratio was 2.784 (95% confidence interval [CI], 1.564-4.957; p=0.0005), and 5.441 (95% CI, 1.831-16.164; p=0.0023) for pathologic N-stage, and rim enhancement, respectively.
Figure 2.
Cumulative incidence of disease recurrence according to mass enhancement on magnetic resonance imaging. Rim enhancement was significantly associated with increased disease recurrence (p=0.0003).
Table 5.
Cox proportional hazards multivariate model for breast cancer recurrence
DISCUSSION
In this study, BRCA mutations were detected in 48 of the 209 eligible patients (23.0%) who underwent curative surgery for breast cancer, with risk factors for carrying BRCA mutations. The prevalence of BRCA mutations was comparable to recently reported studies [19,20]. The relationships between genetic subtypes, MRI, and pathological findings were examined. BRCA1 mutation was associated with high nuclear grade (76.0%, p=0.0041) and triple-negative phenotype (64.0%, p< 0.0001) (Table 2). However, there was no difference in morphological shape, margin, internal enhancement, or kinetic features between the genetic subtypes (Table 3). Although rim enhancement was more frequently observed in BRCA1-mutation carriers (31.6%) than in the other genetic subtypes (17.5% and 23.1% for BRCA-negative and BRCA2-mutation carriers, respectively), these differences were not statistically significant (p=0.4884). Using multiple logistic regression analysis, rim enhancement was associated with molecular subtype (p< 0.0001) and nuclear grade (p=0.0387), but not genetic subtype (p=0.1189).
Most of the cancers demonstrated irregular shape, irregular or spiculated margin, and heterogeneous or rim enhancement on MRI (Table 3), findings that were consistent with the MARIBS trial data [15]. There was no significant difference in the morphological and enhancement features of cancers from patients with BRCA1 and BRCA2 mutations. However, these observations contradict a previously published Dutch study [13], where cancers in patients with BRCA mutations more frequently exhibited a round shape (59.3%), sharp margin (74.1%), and rim enhancement (25.9%) when compared with the control group. A type-III washout curve, which is strongly suggestive of a malignant lesion, was seen in 81.5% of cancers with a BRCA-mutation. In the present study, round or oval shape, smooth margin, and washout at the delayed phase were observed in 20.0%, 6.7%, and 86.7% of cancers in BRCA-mutation carriers, respectively. Our results are consistent with the Dutch study in terms of malignant features such as washout at the delayed phase and rim enhancement, but our results differ with respect to benign morphological features such as round shape or sharp margin. However, benign morphological features were still more prevalent than nonselected sporadic breast cancer in the present study, which has been reported to be less than 2% [21]. The inclusion of patients with risk factors for BRCA mutations in the present study could give an explanation for the prevalent benign morphological features on MRI. MRI features could differ between BRCA mutation carriers and sporadic breast cancer cases from the general population, but evaluation of this relationship was limited in the present study due to the inclusion criteria.
In agreement our observations, rim enhancement is known to be a significant independent predictor of high grade, larger size, lymph node status, and triple-negative breast cancer [11,12]. Active angiogenesis in the periphery of the tumor and subsequent central necrosis have been suggested as underlying mechanisms for rim enhancement on MRI [12]. In addition to pathological and molecular correlations, rim enhancement was associated with increased disease recurrence, and was found to be an independent prognostic factor affecting disease recurrence using multivariate analysis. Because early recurrence of the disease is a clinical characteristic of triple-negative breast cancer, which is frequently observed in patients with BRCA1 mutations, rim enhancement on MRI could be used as a prognostic indicator in patients with breast cancer at risk for BRCA mutations. For example, the patient illustrated in Figure 1 experienced local recurrence during adjuvant chemotherapy. The cancer was high grade and triple-negative phenotype, and demonstrated rim enhancement on MRI. Early or up-front application of adjuvant radiotherapy should be considered in patients with aggressive cancer characteristics such as this.
Using MRI as a screening tool enables the earlier detection of breast cancer in women with familial risk for breast cancer or with BRCA mutations [9,10]. Nevertheless, mammography shows an acceptable level of detecting ductal carcinoma in situ, and shows a higher specificity than MRI in the general population [9,10]. For the diagnosis of familial breast cancer with or without BRCA mutations, the complementary use and integrated interpretation of several imaging modalities are essential. Further studies with additional imaging systems, including mammography and ultrasonography, need to be conducted in order to elucidate the relationship between the genetic and molecular subtypes, and the imaging features from several modalities. In addition, comparison with sporadic breast cancer cases from the general population is important and so future studies may need broader inclusion criteria. And longer follow-up is needed to confirm the accuracy of the prognostic implications of rim enhancement on MRI.
In summary, BRCA1 mutations were associated with the triple-negative phenotype and high-grade cancers. These aggressive characteristics were associated with rim enhancement on MRI, which is a significant predictor of increased disease recurrence. However, there was no direct relationship between BRCA mutation and MRI features. If rim enhancement is observed on MRI, its association with aggressive phenotypes should be taken into consideration, leading to individualized treatment approaches and closer follow-up.
Footnotes
This study was partly supported by Samsung Biomedical Research Institute grant, #SBRI C-B1-132-1, which contributed to genetic testing for BRCA mutations.
The authors declare that they have no competing interests.
References
- 1.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 2.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 3.Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 4.Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed. 2005;7:60. [PMC free article] [PubMed] [Google Scholar]
- 5.Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282–4288. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- 7.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 8.Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, König R, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010;28:1450–1457. doi: 10.1200/JCO.2009.23.0839. [DOI] [PubMed] [Google Scholar]
- 9.Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 10.Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005;365:1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 11.Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology. 2009;250:638–647. doi: 10.1148/radiol.2503081054. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Cho N, Kim SJ, Cha JH, Cho KS, Ko ES, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol. 2008;9:10–18. doi: 10.3348/kjr.2008.9.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veltman J, Mann R, Kok T, Obdeijn IM, Hoogerbrugge N, Blickman JG, et al. Breast tumor characteristics of BRCA1 and BRCA2 gene mutation carriers on MRI. Eur Radiol. 2008;18:931–938. doi: 10.1007/s00330-008-0851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrading S, Kuhl CK. Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology. 2008;246:58–70. doi: 10.1148/radiol.2461062173. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert FJ, Warren RM, Kwan-Lim G, Thompson DJ, Eeles RA, Evans DG, et al. Cancers in BRCA1 and BRCA2 carriers and in women at high risk for breast cancer: MR imaging and mammographic features. Radiology. 2009;252:358–368. doi: 10.1148/radiol.2522081032. [DOI] [PubMed] [Google Scholar]
- 16.American College of Radiology, BI-RADS Committee. ACR BI-RADS Breast Imaging and Reporting Data System: Breast Imaging Atlas. 4th ed. Reston: American College of Radiology; 2003. [Google Scholar]
- 17.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 18.Noh JM, Choi DH, Nam SJ, Lee JE, Kim JW, Kim SW, et al. Characteristics of double heterozygosity for BRCA1 and BRCA2 germline mutations in Korean breast cancer patients. Breast Cancer Res Treat. 2012;131:217–222. doi: 10.1007/s10549-011-1718-5. [DOI] [PubMed] [Google Scholar]
- 19.Son BH, Ahn SH, Kim SW, Kang E, Park SK, Lee MH, et al. Prevalence of BRCA1 and BRCA2 mutations in non-familial breast cancer patients with high risks in Korea: the Korean Hereditary Breast Cancer (KOHBRA) Study. Breast Cancer Res Treat. 2012;133:1143–1152. doi: 10.1007/s10549-012-2001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han SA, Kim SW, Kang E, Park SK, Ahn SH, Lee MH, et al. The prevalence of BRCA mutations among familial breast cancer patients in Korea: results of the Korean Hereditary Breast Cancer study. Fam Cancer. 2013;12:75–81. doi: 10.1007/s10689-012-9578-7. [DOI] [PubMed] [Google Scholar]
- 21.Sickles EA. Nonpalpable, circumscribed, noncalcified solid breast masses: likelihood of malignancy based on lesion size and age of patient. Radiology. 1994;192:439–442. doi: 10.1148/radiology.192.2.8029411. [DOI] [PubMed] [Google Scholar]