DIVERSE ESCHERICHIA COLI PATHOTYPES
Escherichia coli are largely commensal bacteria residing in the mucus layer of the mammalian colon. However, several strains have virulence attributes that give them the capacity to cause diarrheal, urogenic, or systemic illnesses. Pathogenic E coli have been categorized into several pathotypes, each causing illness with distinctive features, and 6 pathotypes, including enterohemorrhagic E coli (EHEC), enterotoxogenic E coli (ETEC), and enteroaggregative E coli (EAEC), are associated with intestinal disease.1 These may also be subdivided into serogroups and serotypes based on their lipopolysaccharide (O) or flagellar (H) antigens.
SHIGA TOXIN–PRODUCING E COLI, INCLUDING EHEC
Shiga toxin-producing E coli (STEC) are a diverse group of bacteria that produce 1 or more types of Shiga toxin (Stx).1,2 They comprise many serotypes, and virulence may differ between strains, with some having an estimated infectious dose in the range of 1 to 100 colony forming units.2 EHEC are a subset of STEC that carry the locus of enterocyte effacement (LEE) pathogenicity island (described later) and are associated with disease in humans. A subset of EHEC, EHEC 1, includes serotype O157:H7 and is clinically the most important group, responsible for ~73,000 cases annually in the United States3 and most STEC infections worldwide.4,5 Recently, there has been an increasing awareness that non-O157 STEC strains represent a significant and growing health threat.6
STX-INDUCED DISEASE
Individuals of both sexes and all ages can suffer severe EHEC-mediated disease, but children and women seem to be at a higher risk, and elderly people often develop neurologic disease and have a higher mortality.7,8 Hemorrhagic colitis is a serious local manifestation of Stx-mediated disease and can progress to gangrenous colitis, bowel perforation, as well as peritonitis or sepsis.8 In the United States, approximately 5% to 10% of reported O157:H7 infections result in hemolytic uremic syndrome (HUS),3,7 the triad of hemolytic anemia, thrombocytopenia, and renal failure.
HUS is characterized by a thrombotic microangiopathy that involves Stx-mediated dysregulation of the alternative complement pathway, damage to endothelium, and consumption of platelets.9 The kidney is the most frequently affected organ, and HUS is a leading cause of renal failure in children, with a mortality of 3% to 5%.10 The vasculopathy can be seen in extrarenal sites as well, including the mesenteric bed, lung, heart, and pancreas. Central nervous system involvement is also present in a subset of cases, and neurologic sequelae may be ominous and associated with significant rates of mortality.7 Histologic lesions of HUS consist of endothelial damage and platelet-fibrin thrombi, frequently in the glomeruli of the kidney. Ultrastructural evaluation of kidney biopsies in patients with HUS shows glomerular endothelial swelling and loss of fenestrations, as well as separation of the endothelial cell from the basement membrane by an intervening accumulation of electron lucent debris. The endothelial damage precedes the development of the classic clinical triad of oliguric or anuric acute kidney injury, microangiopathic hemolytic anemia, and decreased platelet count.11 Long-term chronic sequelae including chronic kidney disease, arterial hypertension, neurologic impairment, and diabetes mellitus have been reported to occur in 20% of patients with childhood HUS.8
The spectrum of tissue damage in HUS is likely caused by tissue distribution of the Stx receptor globotriaosyl ceramide (Gb3).12 Human glomerular epithelial cells, proximal tubular cells, and renal microvascular endothelial cells produce Gb3 and are sensitive to Stx.13–15 Similarly, microvascular and neural tissue of the central nervous system are Gb3-positive, providing a plausible explanation for the neurologic manifestations of STEC-mediated disease.16
CLINICAL COURSE OF EHEC O157:H7 INFECTION
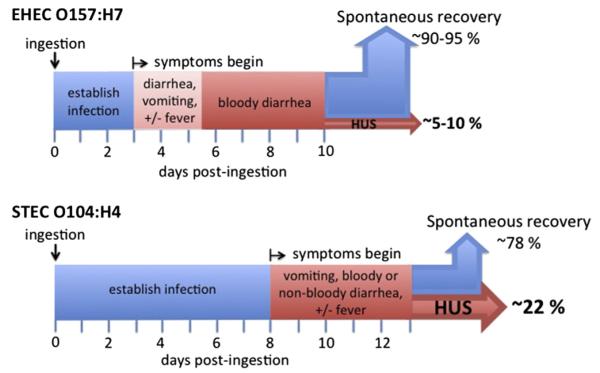
On EHEC O157:H7 ingestion, diarrhea typically begins after just a few days (although the range may span 2–12 days), and after 1 to 3 days the diarrhea becomes bloody in 80% to 90% of patients (Fig. 1).11,17,18 Approximately a week after the onset of diarrhea, most patients begin to show signs of improvement, but 5% to 10% develop severe systemic disease, such as HUS. Given the seriousness of HUS, bloody diarrhea after 1 to 3 days of nonbloody diarrhea, especially in children, warrants concern for infection with EHEC.19
Fig. 1.
The course of disease of EHEC O157:H7 infection differs from that of STEC O104:H4. Note the longer median incubation time before symptom onset for the STEC O104:H4 outbreak strain compared with EHEC O157:H7. (Data from Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005;365(9464):1073–86; and Frank C, Werber D, Cramer JP, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 2011;365(19):1771–80.)
DIAGNOSIS OF EHEC O157:H7
In the United States, EHEC O157:H7, which can be identified on sorbitol MacConkey (SMAC) agar, is the only STEC for which screening is common (although not uniformly routine20). The SMAC agar assay does not specifically detect non-O157 STEC serogroups,3 so measurement of stool-associated Stx by enzyme immune assay (EIA) is often advised to identify these infections.3,21 However, Stx in stool may be at nondetectable concentrations, thereby requiring enrichment steps. Furthermore, EIAs can deliver false-positive results or detect STEC that are unlikely to cause HUS. Both SMAC and EIA detection methods require significant time, potentially delaying appropriate patient management, and therefore molecular diagnostics such as polymerase chain reaction are a potentially time-saving alternative.22 However, no molecular diagnostics for STEC have been approved by the US Food and Drug Administration.3
VIRULENCE FEATURES OF EHEC
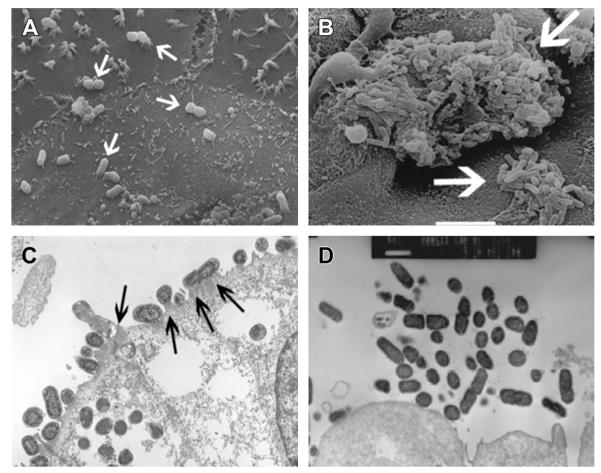
A key virulence feature of EHEC O157:H7 is its ability to form attaching and effacing (AE) lesions on the intestinal epithelium. These lesions are characterized by the intimate attachment of bacteria to the host cell, the effacement of epithelial microvilli, and formation of actin pedestallike structures beneath bound bacteria (Fig. 2) on the surface of epithelial cells. The formation of AE lesions depends on the LEE, a pathogenicity island that encodes proteins required for attachment, several effector proteins that act in the host cell cytoplasm, and a type 3 secretion system that mediates the injection of these effectors into the host cell cytoplasm.23
Fig. 2.
EHEC and EAEC interact with host cells in distinct fashions. (A) CaCo-2a cells infected with EHEC derivative strain TUV-93104 and scanning electron microscopy (SEM) showed cell-attached EHEC (arrows). (B) Cultured human intestinal explants were infected with EAEC and SEM showed EAEC aggregates (arrows). (C) Gnotobiotic piglets were infected with TUV-93 and transmission electron microscopy (TEM) showed pedestals beneath intimately attached EHEC (arrows). (D) Polarized T84 intestinal epithelial cells were infected with EAEC strain 042 and TEM indicated attached bacterial aggregates and effacement of the apical brush border. ([B, D] Adapted from Nataro JP, Hicks S, Phillips AD, et al. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun 1996;64(11):4761–8, with permission; and Nataro JP, Steiner T, Guerrant RL. Enteroaggregative Escherichia coli. Emerg Infect Dis 1998;4(2):251–61; [C] Courtesy of A. Donohue-Rolfe and S. Tzipori.)
PROPERTIES OF STX
Although HUS as a clinical syndrome can occur outside bacterial infection (so-called atypical HUS), EHEC, by virtue of its production of Stxs, is responsible for most HUS cases.24–26 Based on protein sequence and serotype, Stxs are grouped into 2 major types (Stx1 or Stx2),27 and for reasons that are not clear, EHEC O157:H7 strains that produce only Stx2 are associated with a higher risk for HUS.2 Stxs are potent cytotoxins consisting of a single enzymatically active A-subunit noncovalently associated with 5 B-subunits.2 The Stx B-subunits bind a host cell surface glycosphingolipid receptor termed Gb3. After receptor binding, the toxin is endocytosed and by a process termed retrotranslocation moves from the early endosome through the Golgi to the endoplasmic reticulum, where the A-subunit is translocated to the cytoplasm. The A-subunit depurinates a specific adenine residue of the 28S ribosomal RNA subunit,28,29 resulting in the inhibition of protein synthesis and activation of proinflammatory and proapoptotic pathways.30–32
TREATMENT OF EHEC O157:H7 INFECTION
Data from EHEC O157:H7 outbreaks and experimental models suggest Stx upregulation on treatment with ciprofloxacin,33–38 and antimicrobials, particularly fluoroquinolones, are generally withheld because of the concern that such therapy may precipitate HUS.8,11,39 A potential explanation for the apparent increased risk of HUS after antibiotic treatment as observed in some studies is that activation of the SOS stress response by certain antibiotics such as fluoroquinolones can induce the lysogenic phage encoding Stx, resulting in the production and release of toxin.40–42 In addition, released phage may infect other susceptible E coli present in the gut, further amplifying Stx production.43 However, the response to some antibiotics may be strain dependent,44,45 and some studies suggest that antibiotic treatment is not associated with a risk of HUS46 or might reduce the risk of HUS.47
Given that no therapy has been conclusively shown to prevent the onset of HUS or reduce renal damage once HUS has occurred, treatment of EHEC-mediated disease is generally limited to supportive measures.8,11,24,48 Treatments used for other forms of diarrhea or for diseases similar to HUS, such as thrombotic thrombocytopenia purpura (TTP) or atypical HUS, are either contraindicated for treating STEC-associated disease or have limited or conflicting evidence supporting efficacy. For example, the use of antimotility agents in patients with STEC infection has been associated with a greater risk of HUS and neurologic manifestations, or a sustained duration of bloody diarrhea in patients who do not have HUS.46,49,50 The efficacy or safety of treatments such as plasma exchange, the use of glucocorticoids, and recently eculizumab (Soliris), which is used to treat atypical HUS, is undetermined.51,52 In contrast, the supportive therapy for volume expansion beginning within the first 4 days after presentation of EHEC O157:H7-mediated diarrhea is associated with protection from oligoanuria, emphasizing the importance of early detection and hospitalization of patients with EHEC infection.53,54
EAEC
A second E coli pathotype is EAEC (also known as EAggEC), which was first described in the mid-1980s.55,56 EAEC is a major cause of travelers’ diarrhea,57 persistent diarrhea amongst patients positive for the human immunodeficiency virus58,59 and malnourished children,60,61 acute diarrhea in adults and children in the United States,62 and an agent of food-borne outbreaks.63,64 EAEC can also persist subclinically.65 A characteristic attribute of EAEC is its ability to form biofilms on abiotic surfaces and its corresponding aggregative adherence (AA) to mammalian cells, which has been described as resulting in a stacked-brick appearance.66 EAEC encompass diverse serotypes, but notably with respect to the recent emergence of a new non-O157 STEC strain (see later discussion) include strains of serotype O104:H4.67 Thus, clinical and phylogenetic features support the conclusion that EAEC represent a distinct but highly heterogeneous E coli pathotype.68
PATHOGENESIS OF EAEC
EAEC causes tissue damage, including local inflammation, on colonization of the intestinal mucosa (reviewed in Ref.68). Inflammation may be a result of the exuberant colonization of the mucosal surface, but EAEC also encodes toxins that can directly damage host cells (Table 1). Although few data are available concerning the segment(s) of the human intestine that are colonized by EAEC, infection of organ cultures/human intestinal biopsy cultures suggests that EAEC can adhere to the small and large bowel mucosa, although the relative specificity for each of these intestinal segments may differ between strains.68,69 In gnotobiotic piglets, EAEC form a thick mucus gellike matrix containing stacked-brick bacterial aggregates on the epithelium of the distal small intestine and cecum, with concomitant hyperemia and diarrhea.70 In intestinal loop models, EAEC strains induce villus shortening and hemorrhagic necrosis of the villus tips, edema, and submucosal mononuclear infiltration.71
Table 1.
Virulence factors of EAEC
| EAEC Virulence Factors | Clinical Attributes and
Biological Characteristics |
2011 Outbreak Straina |
|---|---|---|
| Adhesins and Colonization Factors | ||
| AAF | Contributes to the characteristic
AA phenotype and facilitates adherence, pithelial barrier disruption, and inflammation. AAF I, II, III, and IV are plasmid encoded73–75,105,106 |
+ (AAF/I) |
| Other non-AAF adhesins (eg, Hda) |
Contributes to the characteristic
stackedbrick phenotype107 |
|
| Dispersin (aap) | Promotes penetration of intestinal
mucus and may promote colonization of the epithelium108,109 |
+ |
| Enterotoxins and Hemolysins | ||
| Enteroaggregative
heat-stabile toxin 1 (EAST1, astA) |
Similar to the heat-stabile enterotoxin
of ETEC79 |
|
| Shigella enterotoxin-1 (ShET-1) | Enterotoxin that induces secretion110 | |
| Hemolysin E (hlyE) | A pore-forming hemolysin; the role
in pathogenesis has not been determined, and it is present in pathogenic and nonpathogenic EAEC111 |
|
| Member of SPATE (Serine Protease | Autotransporters of Enterobacteriaceae) | |
| Pet (plasmid encoded toxin) | Enterotoxin with protease and
cytoskeletal altering activities112,113 |
|
| Pic (protein involved
in colonization) |
Modulates immune responses and
induces the secretion and degradation of mucin; may contribute to the mucus-rich biofilm that is characteristic of EAEC mucosal colonization76,114 |
+ |
| SigA | The Shigella flexneri homolog
alters the cytoskeleton in intestinal epithelial cells, similar to Pet77 |
+ |
| SepA | Associated with illness, and the
Shigella flexneri homolog has been shown to contribute to intestinal inflammation and mucosal atrophy78,115 |
+ |
| Transcription Factor | ||
| Transcriptional regulator
AggR (aggR) |
Global regulator of EAEC virulence
genes, including the AAF operons and aap; common to most EAEC; not absolutely required for virulence80,116 |
+ |
| Other | ||
| Flagellin | Highly conserved bacterial
protein required for motility; interaction with basolaterally expressed toll-like receptor 5 on intestinal epithelial cells results in induction of the neutrophil chemoattractant interleukin-8117,118 |
+ |
Abbreviation: AAF, AA fimbriae.
+ indicates that the German STEC O104:H4 outbreak strain encodes the virulence factor.
VIRULENCE FEATURES OF EAEC
Given the signs and symptoms and intestinal pathology of EAEC infection, much of the effort to understand the pathogenesis of EAEC infection has centered on virulence factors that promote AA, mucosal damage, inflammation, or fluid secretion. The EAEC strain 042 and a few other strains have served as models for diarrheal EAEC in many studies, and 1 caveat to our understanding of EAEC is that a small group of strains may not reflect the full heterogeneity of this pathotype. The documented or putative EAEC virulence factors are summarized in Table 1.
AA fimbriae (AAF), as well as proteins that promote proper localization of fimbriae on the bacterial surface, facilitate adherence to the human intestinal mucosa and formation of a thick biofilm within the mucus layer covering the epithelium, thus promoting persistent mucosal colonization.72 This process may also trigger host inflammatory responses73,74 and disrupt epithelial barrier function.75 The serine protease autotransporters of Enterobacteriaceae (SPATEs), which are commonly found in EAEC as well as other diarrheagenic E coli, modulate immune responses,76 alter the intestinal epithelial cytoskeleton,77 and in some studies, are strongly associated with clinical illness.78 The putative virulence factor EAEC heat-stabile enterotoxin 1 shares amino acid similarity with the heat-stabile enterotoxin of ETEC and shows enterotoxic activity in vitro.79 AggR is a transcriptional regulator that controls several genes, including those associated with AAF and at least 2 pathogenicity islands.80,81
TREATMENT OF EAEC
EAEC-associated diarrhea lasted a mean of 17 days in an early cohort study, indicating that this pathogen can cause a persistent infection that might lead to malnutrition in children, and providing a potential rationale for antibiotic-mediated eradication or nutritional supplementation.82,83 Early eradication of EAEC using antibiotics may also prevent person-to-person transmission, particularly during outbreaks. Treatment of EAEC can be limited by the ubiquitous presence of antibiotic resistance genes. Ninety percent of diarrheal EAEC isolates were found to be resistant or partially resistant to several antibiotics, including β-lactams, chloramphenicol, streptomycin, kanamycin, tetracycline, sulfamethoxazole, and trimethoprim.84 Resistance to carbapenems and quinolones was absent or rare among the isolates analyzed. Ciprofloxacin resistance has been noted rarely, and the drug has been used successfully to treat EAEC infection.83 In general, fluoroquinolones, amoxicillin/clavulanic acid, azithromycin, rifaximin, and nalidixic acid may be effective treatments for EAEC.85–87
GERMAN STEC O104:H4 OUTBREAK OF 2011, ASSOCIATED WITH A HIGH RATE OF HUS
Between early May and late July 2011, a cluster of STEC outbreaks took place in Europe, resulting in 4075 infections, 908 cases of HUS, and more than 50 deaths, 34 of which were associated with HUS.88,89 This episode represents the largest recorded outbreak of HUS. Although persons from 16 countries were affected, cases in Germany represented more than 95% of the reported infections. The causative agent in this outbreak was STEC O104:H4, which was traced to contaminated fenugreek sprouts.90 The O104:H4 serotype has been associated with non–Stx-producing EAEC isolates67 as well as with rare STEC-mediated disease in humans,91–95 with only 5 reported cases in the last 12 years. The nature of the German outbreak differed from previous EHEC O157 outbreaks in several other ways. First, the incidence of bloody diarrhea or HUS was higher in adults than in children,89 in contrast to the more commonly observed increased risk of serious disease among children and the elderly. Although it is hard to assess to what extent the different epidemiologic features reflect the differences in the mode of acquisition, they may reflect (unidentified) differences in fundamental aspects of pathogenesis. Consistent with the latter suggestion, the median time of incubation before the development of symptoms (8 days) was greater than the 3-day to 4-day incubation period typically reported for EHEC O157:H7 (see Fig. 1).89 In addition, the percentage of HUS cases among infected individuals (22%) was higher than the 5% to 10% rate, typically reported for HUS from large EHEC O157:H7 outbreaks, suggesting that the outbreak strain was particularly virulent.3,7
PATHOGENESIS OF STEC O104:H4
Although the German outbreak is too recent to permit extensive exploration of the pathogenesis of STEC O104:H4, it is clear that this strain causes a disease distinct from that caused by EHEC O157:H7. For example, whereas EHEC O157:H7 forms AE lesions on the epithelial surface (see Fig. 2), the O104:H4 strain forms aggregates closely associated with the mucus layer in germ-free mice96 or on monkey colonic explants (Fig. 3). Germ-free mice infected with EHEC O157:H7 developed acute renal tubular necrosis (ATN) 5 days after infection, whereas animals infected with STEC O104:H4 did not develop ATN until 13 to 15 days after infection, consistent with the longer incubation period for human disease (see Fig. 1).89,96 Ampicilin-treated mice infected with STEC O104:H4 lost weight, developed ATN, and died, whereas the disease in mice infected with Stx2-negative O104:H4 strains was less severe. These findings were recapitulated in a rabbit model, emphasizing a role of Stx2 in the virulence of STEC O104:H4.97
Fig. 3.
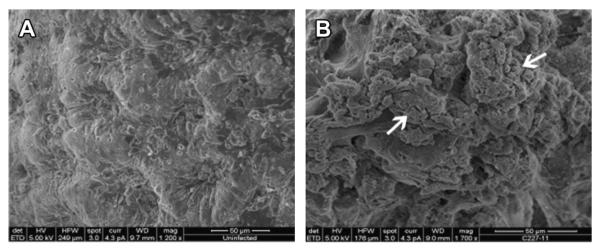
STEC O104:H4 strain C227-11 forms aggregates on colonic mucosa. Uninfected monkey colonic explants (A) or those infected with the German outbreak strain C227-11 (B) were subjected to SEM. Arrow indicates bacterial aggregates. (Courtesy of N. Boisen and J. Nataro, University of Virginia School of Medicine, VA. Processed at the Core Imaging Facility at the University of Maryland, Baltimore, MD.)
STEC O104:H4, A HYBRID PATHOGEN
Consistent with its unique features, the STEC O104:H4 strain responsible for the German outbreak differs in 2 main aspects from most other clinically important STEC strains. First, it lacks the LEE pathogenicity island that encodes the type III translocation system. Second, the STEC O104:H4 outbreak strain encodes many virulence factors commonly produced by EAEC, including AAF (specifically AAF/I), SPATE proteases, and the AggR global regulator (Fig. 4).67,98
Fig. 4.
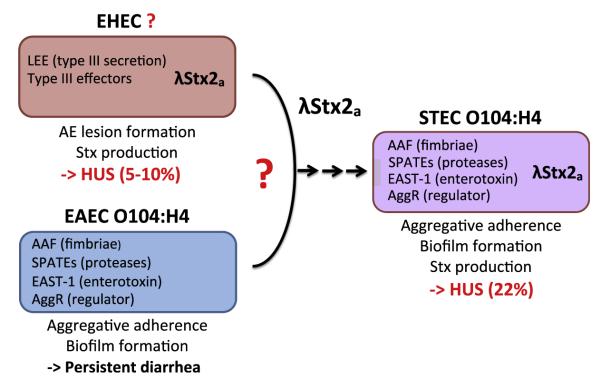
Possible derivation of the 2011 German outbreak strain STEC O104:H4. EHEC and EAEC encode distinct sets of virulence factors and are associated with different modes of pathogenesis. EAEC O104:H4 may have acquired the lambdoid Stx2a phage from a hypothetical EHEC donor to generate STEC O104:H4. This strain, which encodes a combination of EAEC and EHEC virulence factors, is associated with an increased rate of HUS. (Data from Rasko DA, Webster DR, Sahl JW, et al. Origins of the E. coli strain causing an outbreak of hemolyticuremic syndrome in Germany. N Engl J Med 2011;365(8):709–17; and Brzuszkiewicz E, Thurmer A, Schuldes J, et al. Genome sequence analyses of two isolates from the recent Escherichia coli outbreak in Germany reveal the emergence of a new pathotype: enteroaggregative-haemorrhagic Escherichia coli (EAHEC). Arch Microbiol 2011;193(12):883–91.)
In contrast to EAEC strains, STEC O104:H4 encodes Stx2, consistent with the ability to induce HUS. As mentioned earlier, EHEC O157:H7 strains expressing Stx2 alone (rather than Stx1 alone or both Stx1 and Stx2) are associated with a greater risk of HUS.2 Genomic sequencing showed that, similar to EHEC, the German STEC O104:H4 strain encodes Stx2a within a lysogenized lambdoid bacteriophage.67 A phylogenetic comparison of outbreak isolates with several EAEC O104:H4 strains showed that only the outbreak isolates are lysogenic for the Stx2a phage.67 In addition, the outbreak strain carries a plasmid that encodes an extended-spectrum β-lactamase CTX-M-15, a β-lactamase that is uncommon amongst other O104:H4 isolates.67 These observations support the hypothesis that the recent acquisition of the phage-encoded virulence factor Stx2a, as well as an antibiotic resistance determinant, has given rise to the exceptionally virulent STEC O104:H4 German outbreak strain (see Fig. 4).
TREATMENT OF STEC O104:H4 INFECTION
The enormity of the 2011 STEC O104:H4 outbreak in Germany resulted in many patients undergoing different treatments throughout the country, and allowed for a multi-center case-controlled study concerning the efficacy of different strategies to treat STEC O104:H4-associated HUS.52 Treatments reported include the use of antibiotics, therapeutic plasma exchange (TPE), TPE with glucocorticoids, immunoadsorption, and the use of the alternative complement pathway inhibitor eculizumab (summarized in Table 2).
Table 2.
Studies of treatment efficacy for STEC O104:H4 infection
| Treatment | Notes/Results | Reference(s) |
|---|---|---|
| Meropenam/ciprofloxacin (intensive care unit: + rifaximin)a |
A significant decrease in mortality, duration
of STEC excretion in stools (ie, 8 days shorter), and incidence of seizures in treated patients, who presented with HUS before antibiotic treatment |
52 |
| Azithromycinb | Used for meningococcal prophylaxis in
patients with HUS being treated with eculizumab. Treatment was associated with a decrease in the frequency of long-term O104:H4 carriage |
101 |
| Various antibiotics | Study of 24 patients, of whom 7 were
treated with various antibiotics, including ciprofloxacin. 57% of antibiotic-treated patients compared with 88% of controls developed HUS. |
101 |
| TPE | No benefit among 251 patients with HUS
who underwent TPE vs 47 patients not given TPE, but who also had milder disease |
52 |
| TPE | 5 patients with HUS with progressive
neurologic dysfunction who underwent TPE recovered. Justification of TPE has been questioned |
120,121 |
| Prednisone + TPE | No benefit detected in patients pretreated
with prednisone before TPE vs TPE alone |
52 |
| Immunoadsorption | 12 patients with HUS who developed
neurologic signs a median of 8 days after enteritis onset were treated with multiple courses of immunoadsorption. All patients survived and 10 recovered completely. The rationale for treatment was that the late onset of neurologic symptoms indicated an autoimmune response, but autoantibodies were not immunologically validated |
102 |
| Eculizumab | One report of 3 children with HUS
who underwent TPE and eculizumab treatment who were reported to have improved dramatically |
103 |
| Eculizumab | No benefit was conferred to 67 adult
HUS patients who were treated with eculizumab and TPE vs a control group of patients with HUS with similar disease severity who were treated with TPE but not eculizumab |
52 |
Some patients who were admitted to the intensive care unit were also treated with rifaximin.
Whether or not to treat diarrheal infections with antimicrobials during the German STEC O104:H4 outbreak strain was not straightforward. This strain was shown to be resistant to all penicillins and cephalosporins, consistent with the presence of a β-lactamase–producing plasmid.95,98 Although susceptible to fluoroquinolones, aminoglycosides, and carbapenems, this strain is also resistant to trimethoprim-sulfamethoxazole.98 Second, as has been described earlier for EHEC O157:H7 infection, there was significant concern that antibiotic treatment of patients infected with the STEC O104:H4 outbreak strain would increase the risk of HUS. As a result, during the 2011 outbreak, the German Society for Infectious Disease recommended that fluoroquinolones, aminoglycosides, cotrimoxazole, and fosfomycin not be used to treat patients with STEC infection.99
Nevertheless, not all studies indicate that antibiotic treatment of EHEC O157:H7 infection is associated with an increased risk of HUS.46,47,100 In addition, given that EAEC can cause a persistent infection, the concern that chronic infection by an Stx-producing E coli might lead to HUS and neurologic dysfunction motivated treatment of some individuals. Consistent with the effectiveness of antibiotic treatment of non– Stx-EAEC infection, azithromycin therapy in persons with HUS appeared to significantly reduce rates of bacterial colonization, seizure, and mortality during the STEC O104:H4 outbreak (see Table 2).101 In addition, a case-controlled study addressing ciprofloxacin use suggested that treatment of patients with HUS reduced long-term intestinal carriage and seizure frequency (see Table 2).52 This finding pertained to treatment during but not before HUS. Although the current data are inconclusive as to the risk or benefit of antibiotic treatment of STEC O104:H4 infection, it is tempting to speculate that antibiotics incapable of inducing Stx phage could be beneficial.
TPE is a cornerstone of therapy for TTP,102 which, like HUS, is a thrombotic microangiopathy. Nevertheless, rather than a manifestation of toxemia, TTP seems in many cases to be caused by a self-reactive antibody to the metalloproteinase ADAMTS13, resulting in lower rates of cleavage of von Willebrand factor multimers and a subsequent procoagulant state. TPE decreases levels of the pathogenic antibody in TTP. Stx-mediated HUS does not seem to respond to this treatment,9 a finding that could be caused by the short half-life of Stx in circulation or to irreversible endothelial injury that may occur before clinical manifestations and initiation of apheresis.52 Nevertheless, depletion of the immunoglobulin fraction followed by intravenous immunoglobulin repletion was suggested to improve short-term neurologic status in a small cohort of STEC O104:H4–infected individuals who developed HUS.102
Eculizumab, a novel monoclonal antibody directed against the C5 complement component, is a therapeutic option in atypical (non–Stx-associated) HUS, in which dysfunctional complement regulatory proteins result in unchecked activation of the alternative complement pathway. Coinciding with the early weeks of the 2011 outbreak, eculizumab had been reported to be effective in decreasing neurologic impairment in the days after infusion in 3 children with severe EHEC-Stx HUS.103 However, trials of eculizumab in affected adults appeared to show no short-term benefit.51
SUMMARY
Because STEC strains vary greatly in their capacity to cause human disease, virulence determinants in addition to the simple production of Stx are likely to function as key factors in the ability of a given STEC strain to induce serious systemic disease. In recent years, there has been an increased awareness of the clinical importance of non-O157 STEC. Genomic analysis suggests that the particularly virulent German STEC strain, one that caused HUS at an increased rate and in a population not typically associated with HUS, is a hybrid E coli strain of serotype O104:H4. This strain encodes several virulence factors associated with EAEC and forms aggregates on the intestinal mucosa similarly to EAEC, but has acquired an Stx2a-producing phage. The EAEC-like features of STEC O104:H4 may have contributed to the high rate of HUS and the unique epidemiology witnessed during the 2011 STEC O104:H4 outbreak, indicating that the dynamic evolution of pathogens can give rise to highly virulent strains. Given that specific therapies to treat or prevent HUS are not yet clearly defined, the early and specific detection of both O157 and non-O157 STEC is critical to ensure the best possible prognosis for an infected individual.
KEY POINTS.
Pathogenic Escherichia coli are genetically diverse and encompass a broad variety of pathotypes, such as Shiga toxin–producing Escherichia coli (STEC) or enteroaggregative E coli (EAEC), which cause distinct clinical syndromes.
STEC is a major food-borne pathogen worldwide and can cause hemolytic uremic syndrome (HUS), the triad of anemia, thrombocytopenia, and renal failure.
The STEC most commonly associated with disease is E coli serotype O157:H7, but there has been increasing awareness of the threat posed by non-O157 STEC strains.
A major outbreak of STEC disease in Germany in 2011 was associated with an unusually high rate of HUS, with more than 900 cases, making it the single most severe recorded outbreak of STEC.
The German outbreak strain, STEC O104:H4, is genetically similar to EAEC O104:H4, but is lysogenized by a lambdoid phage that encodes Shiga toxin.
STEC O104:H4, likely derived in part by the acquisition of a Shiga toxin–encoding phage by an EAEC strain, represents an emerging food-borne pathogen with enhanced capacity to cause severe illness.
ACKNOWLEDGMENTS
We would like to give special thanks to Nadia Boisen and James Nataro for providing the scanning electron microscope images in Fig. 3 and to S. Tzipori and A. Donohue-Rolfe for providing the transmission electron microscope images in Fig. 2.
Disclosure: Support of our work was made through grants from the National Institute of Health, Bethesda, MA: AI088336-02 (D.M.J.), DK56754 and DK33506 (B.A.M.), and AI46454 (J.M.L.); the Carlsberg Foundation Post-Doctoral Scholarship (E.J.B.); and the Charlton Grant Research Program, Tufts University (D.M.J.).
REFERENCES
- 1.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–40. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 2.Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11(3):450–79. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould LH, Bopp C, Strockbine N, et al. Recommendations for diagnosis of Shiga toxin–producing Escherichia coli infections by clinical laboratories. MMWR Recomm Rep. 2009;58(RR-12):1–14. [PubMed] [Google Scholar]
- 4.Whittam TS. Evolution of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. In: Kaper JB, O’Brien AD, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology; Washington, DC: 1998. pp. 195–212. [Google Scholar]
- 5.Karmali MA, Gannon V, Sargeant JM. Verocytotoxin-producing Escherichia coli (VTEC) Vet Microbiol. 2010;140(3–4):360–70. doi: 10.1016/j.vetmic.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Johnson KE, Thorpe CM, Sears CL. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin Infect Dis. 2006;43(12):1587–95. doi: 10.1086/509573. [DOI] [PubMed] [Google Scholar]
- 7.Gould LH, Demma L, Jones TF, et al. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000-2006. Clin Infect Dis. 2009;49(10):1480–5. doi: 10.1086/644621. [DOI] [PubMed] [Google Scholar]
- 8.Bitzan M, Schaefer F, Reymond D. Treatment of typical (enteropathic) hemolytic uremic syndrome. Semin Thromb Hemost. 2010;36(6):594–610. doi: 10.1055/s-0030-1262881. [DOI] [PubMed] [Google Scholar]
- 9.Trachtman H, Austin C, Lewinski M, et al. Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat Rev Nephrol. 2012;8(11):658–69. doi: 10.1038/nrneph.2012.196. [DOI] [PubMed] [Google Scholar]
- 10.Scheiring J, Andreoli SP, Zimmerhackl LB. Treatment and outcome of Shigatoxin-associated hemolytic uremic syndrome (HUS) Pediatr Nephrol. 2008;23(10):1749–60. doi: 10.1007/s00467-008-0935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365(9464):1073–86. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 12.Obrig TG. Escherichia coli Shiga toxin mechanisms of action in renal disease. Toxins (Basel) 2010;2(12):2769–94. doi: 10.3390/toxins2122769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes AK, Stricklett PK, Schmid D, et al. Cytotoxic effect of Shiga toxin-1 on human glomerular epithelial cells. Kidney Int. 2000;57(6):2350–9. doi: 10.1046/j.1523-1755.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- 14.Hughes AK, Stricklett PK, Kohan DE. Cytotoxic effect of Shiga toxin-1 on human proximal tubule cells. Kidney Int. 1998;54(2):426–37. doi: 10.1046/j.1523-1755.1998.00015.x. [DOI] [PubMed] [Google Scholar]
- 15.Obrig TG, Louise CB, Lingwood CA, et al. Endothelial heterogeneity in Shiga toxin receptors and responses. J Biol Chem. 1993;268(21):15484–8. [PubMed] [Google Scholar]
- 16.Obata F, Tohyama K, Bonev AD, et al. Shiga toxin 2 affects the central nervous system through receptor globotriaosylceramide localized to neurons. J Infect Dis. 2008;198(9):1398–406. doi: 10.1086/591911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appleman SS, Ascher D, Park C. Clinical spectrum of Shiga toxin-producing Escherichia coli (STEC) in adults and children. Clin Pediatr (Phila) 2009;48(1):99–102. doi: 10.1177/0009922808321901. [DOI] [PubMed] [Google Scholar]
- 18.Tarr PI. Shiga toxin-associated hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: distinct mechanisms of pathogenesis. Kidney Int Suppl. 2009;(112):S29–32. doi: 10.1038/ki.2008.615. [DOI] [PubMed] [Google Scholar]
- 19.Holtz LR, Neill MA, Tarr PI. Acute bloody diarrhea: a medical emergency for patients of all ages. Gastroenterology. 2009;136(6):1887–98. doi: 10.1053/j.gastro.2009.02.059. [DOI] [PubMed] [Google Scholar]
- 20.Tarr PI, Neill MA. Escherichia coli O157:H7. Gastroenterol Clin North Am. 2001;30(3):735–51. doi: 10.1016/s0889-8553(05)70208-9. [DOI] [PubMed] [Google Scholar]
- 21.Klein EJ, Stapp JR, Clausen CR, et al. Shiga toxin-producing Escherichia coli in children with diarrhea: a prospective point-of-care study. J Pediatr. 2002;141(2):172–7. doi: 10.1067/mpd.2002.125908. [DOI] [PubMed] [Google Scholar]
- 22.Gerritzen A, Wittke JW, von Ahsen N, et al. Direct faecal PCR for diagnosis of Shiga-toxin-producing Escherichia coli. Lancet Infect Dis. 2012;12(2):102. doi: 10.1016/S1473-3099(11)70369-3. [DOI] [PubMed] [Google Scholar]
- 23.Melton-Celsa A, Mohawk K, Teel L, et al. Pathogenesis of Shiga-toxin producing Escherichia coli. Curr Top Microbiol Immunol. 2012;357:67–103. doi: 10.1007/82_2011_176. [DOI] [PubMed] [Google Scholar]
- 24.Loirat C, Saland J, Bitzan M. Management of hemolytic uremic syndrome. Presse Med. 2012;41(3 Pt 2):e115–35. doi: 10.1016/j.lpm.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Karmali MA, Petric M, Lim C, et al. Escherichia coli cytotoxin, haemolyticuraemic syndrome, and haemorrhagic colitis. Lancet. 1983;2(8362):1299–300. doi: 10.1016/s0140-6736(83)91167-4. [DOI] [PubMed] [Google Scholar]
- 26.Thorpe CM, Ritchie JM, Acheson DW. Enterohemorrhagic and other Shiga toxin-producing Escherichia coli. In: Donnenberg MS, editor. Escherichia coli: virulence mechanisms of a versatile pathogen. Elsevier; San Diego (CA): 2002. pp. 119–54. [Google Scholar]
- 27.Scheutz F, Teel LD, Beutin L, et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol. 2012;50(9):2951–63. doi: 10.1128/JCM.00860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obrig TG, Moran TP, Brown JE. The mode of action of Shiga toxin on peptide elongation of eukaryotic protein synthesis. Biochem J. 1987;244(2):287–94. doi: 10.1042/bj2440287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endo Y, Tsurugi K, Yutsudo T, et al. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988;171(1–2):45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- 30.Tesh VL. The induction of apoptosis by Shiga toxins and ricin. Curr Top Microbiol Immunol. 2012;357:137–78. doi: 10.1007/82_2011_155. [DOI] [PubMed] [Google Scholar]
- 31.Roche JK, Keepers TR, Gross LK, et al. CXCL1/KC and CXCL2/MIP-2 are critical effectors and potential targets for therapy of Escherichia coli O157:H7-associated renal inflammation. Am J Pathol. 2007;170(2):526–37. doi: 10.2353/ajpath.2007.060366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorpe CM, Smith WE, Hurley BP, et al. Shiga toxins induce, superinduce, and stabilize a variety of C-X-C chemokine mRNAs in intestinal epithelial cells, resulting in increased chemokine expression. Infect Immun. 2001;69(10):6140–7. doi: 10.1128/IAI.69.10.6140-6147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter AO, Borczyk AA, Carlson JA, et al. A severe outbreak of Escherichia coli O157:H7–associated hemorrhagic colitis in a nursing home. N Engl J Med. 1987;317(24):1496–500. doi: 10.1056/NEJM198712103172403. [DOI] [PubMed] [Google Scholar]
- 34.Riley LW, Remis RS, Helgerson SD, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308(12):681–5. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 35.Pavia AT, Nichols CR, Green DP, et al. Hemolytic-uremic syndrome during an outbreak of Escherichia coli O157:H7 infections in institutions for mentally retarded persons: clinical and epidemiologic observations. J Pediatr. 1990;116(4):544–51. doi: 10.1016/s0022-3476(05)81600-2. [DOI] [PubMed] [Google Scholar]
- 36.Ostroff SM, Kobayashi JM, Lewis JH. Infections with Escherichia coli O157:H7 in Washington State. The first year of statewide disease surveillance. JAMA. 1989;262(3):355–9. [PubMed] [Google Scholar]
- 37.Wong CS, Jelacic S, Habeeb RL, et al. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med. 2000;342(26):1930–6. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith KE, Wilker PR, Reiter PL, et al. Antibiotic treatment of Escherichia coli O157 infection and the risk of hemolytic uremic syndrome, Minnesota. Pediatr Infect Dis J. 2012;31(1):37–41. doi: 10.1097/INF.0b013e31823096a8. [DOI] [PubMed] [Google Scholar]
- 39.Seifert ME, Tarr PI. Therapy: azithromycin and decolonization after HUS. Nat Rev Nephrol. 2012;8(6):317–8. doi: 10.1038/nrneph.2012.87. [DOI] [PubMed] [Google Scholar]
- 40.Kimmitt PT, Harwood CR, Barer MR. Toxin gene expression by shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg Infect Dis. 2000;6(5):458–65. doi: 10.3201/eid0605.000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, McDaniel AD, Wolf LE, et al. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis. 2000;181(2):664–70. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]
- 42.Gamage SD, Patton AK, Strasser JE, et al. Commensal bacteria influence Escherichia coli O157:H7 persistence and Shiga toxin production in the mouse intestine. Infect Immun. 2006;74(3):1977–83. doi: 10.1128/IAI.74.3.1977-1983.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGannon CM, Fuller CA, Weiss AA. Different classes of antibiotics differentially influence Shiga toxin production. Antimicrob Agents Chemother. 2010;54(9):3790–8. doi: 10.1128/AAC.01783-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grif K, Dierich MP, Karch H, et al. Strain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1998;17(11):761–6. doi: 10.1007/s100960050181. [DOI] [PubMed] [Google Scholar]
- 45.Walterspiel JN, Ashkenazi S, Morrow AL, et al. Effect of subinhibitory concentrations of antibiotics on extracellular Shiga-like toxin I. Infection. 1992;20(1):25–9. doi: 10.1007/BF01704889. [DOI] [PubMed] [Google Scholar]
- 46.Bell BP, Griffin PM, Lozano P, et al. Predictors of hemolytic uremic syndrome in children during a large outbreak of Escherichia coli O157:H7 infections. Pediatrics. 1997;100(1):E12. doi: 10.1542/peds.100.1.e12. [DOI] [PubMed] [Google Scholar]
- 47.Ikeda K, Ida O, Kimoto K, et al. Effect of early fosfomycin treatment on prevention of hemolytic uremic syndrome accompanying Escherichia coli O157:H7 infection. Clin Nephrol. 1999;52(6):357–62. [PubMed] [Google Scholar]
- 48.Noris M, Remuzzi G. Hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16(4):1035–50. doi: 10.1681/ASN.2004100861. [DOI] [PubMed] [Google Scholar]
- 49.Cimolai N, Basalyga S, Mah DG, et al. A continuing assessment of risk factors for the development of Escherichia coli O157:H7-associated hemolytic uremic syndrome. Clin Nephrol. 1994;42(2):85–9. [PubMed] [Google Scholar]
- 50.Cimolai N, Morrison BJ, Carter JE. Risk factors for the central nervous system manifestations of gastroenteritis-associated hemolytic-uremic syndrome. Pediatrics. 1992;90(4):616–21. [PubMed] [Google Scholar]
- 51.Kielstein JT, Beutel G, Fleig S, et al. Best supportive care and therapeutic plasma exchange with or without eculizumab in Shiga-toxin-producing E. coli O104:H4 induced haemolytic-uraemic syndrome: an analysis of the German STEC-HUS registry. Nephrol Dial Transplant. 2012;27(10):3807–15. doi: 10.1093/ndt/gfs394. [DOI] [PubMed] [Google Scholar]
- 52.Menne J, Nitschke M, Stingele R, et al. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ. 2012;345:e4565. doi: 10.1136/bmj.e4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hickey CA, Beattie TJ, Cowieson J, et al. Early volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndrome. Arch Pediatr Adolesc Med. 2011;165(10):884–9. doi: 10.1001/archpediatrics.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ake JA, Jelacic S, Ciol MA, et al. Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics. 2005;115(6):e673–80. doi: 10.1542/peds.2004-2236. [DOI] [PubMed] [Google Scholar]
- 55.Scaletsky IC, Silva ML, Trabulsi LR. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45(2):534–6. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nataro JP, Kaper JB, Robins-Browne R, et al. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987;6(9):829–31. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Taylor DN, Bourgeois AL, Ericsson CD, et al. A randomized, double-blind, multi-center study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea. Am J Trop Med Hyg. 2006;74(6):1060–6. [PubMed] [Google Scholar]
- 58.Mossoro C, Glaziou P, Yassibanda S, et al. Chronic diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome associated with HEp-2 adherent Escherichia coli in adults infected with human immunodeficiency virus in Bangui, Central African Republic. J Clin Microbiol. 2002;40(8):3086–8. doi: 10.1128/JCM.40.8.3086-3088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wanke CA, Mayer H, Weber R, et al. Enteroaggregative Escherichia coli as a potential cause of diarrheal disease in adults infected with human immunodeficiency virus. J Infect Dis. 1998;178(1):185–90. doi: 10.1086/515595. [DOI] [PubMed] [Google Scholar]
- 60.Wanke CA, Schorling JB, Barrett LJ, et al. Potential role of adherence traits of Escherichia coli in persistent diarrhea in an urban Brazilian slum. Pediatr Infect Dis J. 1991;10(10):746–51. doi: 10.1097/00006454-199110000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Bhatnagar S, Bhan MK, Singh KD, et al. Prognostic factors in hospitalized children with persistent diarrhea: implications for diet therapy. J Pediatr Gastroenterol Nutr. 1996;23(2):151–8. doi: 10.1097/00005176-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Nataro JP, Mai V, Johnson J, et al. Diarrheagenic Escherichia coli infection in Baltimore, Maryland, and New Haven, Connecticut. Clin Infect Dis. 2006;43(4):402–7. doi: 10.1086/505867. [DOI] [PubMed] [Google Scholar]
- 63.Itoh Y, Nagano I, Kunishima M, et al. Laboratory investigation of enteroaggregative Escherichia coli O untypeable:H10 associated with a massive outbreak of gastrointestinal illness. J Clin Microbiol. 1997;35(10):2546–50. doi: 10.1128/jcm.35.10.2546-2550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scavia G, Staffolani M, Fisichella S, et al. Enteroaggregative Escherichia coli associated with a foodborne outbreak of gastroenteritis. J Med Microbiol. 2008;57(Pt 9):1141–6. doi: 10.1099/jmm.0.2008/001362-0. [DOI] [PubMed] [Google Scholar]
- 65.Nataro JP, Steiner T, Guerrant RL. Enteroaggregative Escherichia coli. Emerg Infect Dis. 1998;4(2):251–61. doi: 10.3201/eid0402.980212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nataro JP, Steiner T. Enteroaggregative and diffusely adherent Escherichia coli. In: Donnenberg MS, editor. Escherichia coli: virulence mechanisms of a versatile pathogen. Elsevier; San Diego (CA): 2002. pp. 189–207. [Google Scholar]
- 67.Rasko DA, Webster DR, Sahl JW, et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med. 2011;365(8):709–17. doi: 10.1056/NEJMoa1106920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Estrada-Garcia T, Navarro-Garcia F. Enteroaggregative Escherichia coli pathotype: a genetically heterogeneous emerging foodborne enteropathogen. FEMS Immunol Med Microbiol. 2012;66(3):281–98. doi: 10.1111/j.1574-695X.2012.01008.x. [DOI] [PubMed] [Google Scholar]
- 69.Hicks S, Candy DC, Phillips AD. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun. 1996;64(11):4751–60. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tzipori S, Montanaro J, Robins-Browne RM, et al. Studies with enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect Immun. 1992;60(12):5302–6. doi: 10.1128/iai.60.12.5302-5306.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vial PA, Robins-Browne R, Lior H, et al. Characterization of enteroadherentaggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988;158(1):70–9. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- 72.Nataro JP. Enteroaggregative Escherichia coli pathogenesis. Curr Opin Gastroenterol. 2005;21(1):4–8. [PubMed] [Google Scholar]
- 73.Boll EJ, Struve C, Sander A, et al. The fimbriae of enteroaggregative Escherichia coli induce epithelial inflammation in vitro and in a human intestinal xenograft model. J Infect Dis. 2012;206(5):714–22. doi: 10.1093/infdis/jis417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harrington SM, Strauman MC, Abe CM, et al. Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cell Microbiol. 2005;7(11):1565–78. doi: 10.1111/j.1462-5822.2005.00588.x. [DOI] [PubMed] [Google Scholar]
- 75.Strauman MC, Harper JM, Harrington SM, et al. Enteroaggregative Escherichia coli disrupts epithelial cell tight junctions. Infect Immun. 2010;78(11):4958–64. doi: 10.1128/IAI.00580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruiz-Perez F, Wahid R, Faherty CS, et al. Serine protease autotransporters from Shigella flexneri and pathogenic Escherichia coli target a broad range of leukocyte glycoproteins. Proc Natl Acad Sci U S A. 2011;108(31):12881–6. doi: 10.1073/pnas.1101006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Hasani K, Navarro-Garcia F, Huerta J, et al. The immunogenic SigA enterotoxin of Shigella flexneri 2a binds to HEp-2 cells and induces fodrin redistribution in intoxicated epithelial cells. PLoS One. 2009;4(12):e8223. doi: 10.1371/journal.pone.0008223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boisen N, Scheutz F, Rasko DA, et al. Genomic characterization of enteroaggregative Escherichia coli from children in Mali. J Infect Dis. 2012;205(3):431–44. doi: 10.1093/infdis/jir757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savarino SJ, Fasano A, Watson J, et al. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc Natl Acad Sci U S A. 1993;90(7):3093–7. doi: 10.1073/pnas.90.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dudley EG, Thomson NR, Parkhill J, et al. Proteomic and microarray characterization of the AggR regulon identifies a pheU pathogenicity island in enteroaggregative Escherichia coli. Mol Microbiol. 2006;61(5):1267–82. doi: 10.1111/j.1365-2958.2006.05281.x. [DOI] [PubMed] [Google Scholar]
- 81.Nataro JP, Yikang D, Yingkang D, et al. AggR, a transcriptional activator of aggregative adherence fimbria I expression in enteroaggregative Escherichia coli. J Bacteriol. 1994;176(15):4691–9. doi: 10.1128/jb.176.15.4691-4699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhan MK, Raj P, Levine MM, et al. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989;159(6):1061–4. doi: 10.1093/infdis/159.6.1061. [DOI] [PubMed] [Google Scholar]
- 83.Okeke IN, Nataro JP. Enteroaggregative Escherichia coli. Lancet Infect Dis. 2001;1(5):304–13. doi: 10.1016/S1473-3099(01)00144-X. [DOI] [PubMed] [Google Scholar]
- 84.Yamamoto T, Echeverria P, Yokota T. Drug resistance and adherence to human intestines of enteroaggregative Escherichia coli. J Infect Dis. 1992;165(4):744–9. doi: 10.1093/infdis/165.4.744. [DOI] [PubMed] [Google Scholar]
- 85.Kaur P, Chakraborti A, Asea A. Enteroaggregative Escherichia coli: an emerging enteric food borne pathogen. Interdiscip Perspect Infect Dis. 2010;2010:254159. doi: 10.1155/2010/254159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glandt M, Adachi JA, Mathewson JJ, et al. Enteroaggregative Escherichia coli as a cause of traveler’s diarrhea: clinical response to ciprofloxacin. Clin Infect Dis. 1999;29(2):335–8. doi: 10.1086/520211. [DOI] [PubMed] [Google Scholar]
- 87.Infante RM, Ericsson CD, Jiang ZD, et al. Enteroaggregative Escherichia coli diarrhea in travelers: response to rifaximin therapy. Clin Gastroenterol Hepatol. 2004;2(2):135–8. doi: 10.1016/s1542-3565(03)00322-7. [DOI] [PubMed] [Google Scholar]
- 88.World Health Organization [Accessed June 22, 2013];Outbreaks of E. coli O104:H4 infection: update 30. 2011 Available at: http://www.euro.who.int/en/what-we-do/health-topics/emergencies/international-health-regulations/news/news/2011/07/outbreaks-of-e.-coli-o104h4-infection-update-30.
- 89.Frank C, Werber D, Cramer JP, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365(19):1771–80. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- 90.Buchholz U, Bernard H, Werber D, et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med. 2011;365(19):1763–70. doi: 10.1056/NEJMoa1106482. [DOI] [PubMed] [Google Scholar]
- 91.Scavia G, Morabito S, Tozzoli R, et al. Similarity of Shiga toxin-producing Escherichia coli O104:H4 strains from Italy and Germany. Emerg Infect Dis. 2011;17(10):1957–8. doi: 10.3201/eid1710.111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bae WK, Lee YK, Cho MS, et al. A case of hemolytic uremic syndrome caused by Escherichia coli O104:H4. Yonsei Med J. 2006;47(3):437–9. doi: 10.3349/ymj.2006.47.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monecke S, Mariani-Kurkdjian P, Bingen E, et al. Presence of enterohemorrhagic Escherichia coli ST678/O104:H4 in France prior to 2011. Appl Environ Microbiol. 2011;77(24):8784–6. doi: 10.1128/AEM.06524-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mellmann A, Bielaszewska M, Kock R, et al. Analysis of collection of hemolytic uremic syndrome-associated enterohemorrhagic Escherichia coli. Emerg Infect Dis. 2008;14(8):1287–90. doi: 10.3201/eid1408.071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mellmann A, Harmsen D, Cummings CA, et al. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One. 2011;6(7):e22751. doi: 10.1371/journal.pone.0022751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al Safadi R, Abu-Ali GS, Sloup RE, et al. Correlation between in vivo biofilm formation and virulence gene expression in Escherichia coli O104:H4. PLoS One. 2012;7(7):e41628. doi: 10.1371/journal.pone.0041628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zangari T, Melton-Celsa AR, Panda A, et al. Virulence of the Shiga toxin type 2-expressing Escherichia coli O104:H4 German outbreak isolate in two animal models. Infect Immun. 2013;81(5):1562–74. doi: 10.1128/IAI.01310-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bielaszewska M, Mellmann A, Zhang W, et al. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis. 2011;11(9):671–6. doi: 10.1016/S1473-3099(11)70165-7. [DOI] [PubMed] [Google Scholar]
- 99.DGI [Accessed June 22, 2013];EHEC infection and antibiotic therapy. 2011 Available at: http://www.dgi-net.de/images/stories/DGI-position_paper_EHECantibiotics_English_version_plus_references_20110604.pdf.
- 100.Neill MA. Treatment of disease due to Shiga toxin-producing Escherichia coli: infectious disease management. In: Kaper JB, Obrien AD, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press; Washington, DC: 1998. pp. 357–63. [Google Scholar]
- 101.Nitschke M, Sayk F, Hartel C, et al. Association between azithromycin therapy and duration of bacterial shedding among patients with Shiga toxin-producing enteroaggregative Escherichia coli O104:H4. JAMA. 2012;307(10):1046–52. doi: 10.1001/jama.2012.264. [DOI] [PubMed] [Google Scholar]
- 102.Greinacher A, Friesecke S, Abel P, et al. Treatment of severe neurological deficits with IgG depletion through immunoadsorption in patients with Escherichia coli O104:H4-associated haemolytic uraemic syndrome: a prospective trial. Lancet. 2011;378(9797):1166–73. doi: 10.1016/S0140-6736(11)61253-1. [DOI] [PubMed] [Google Scholar]
- 103.Lapeyraque AL, Malina M, Fremeaux-Bacchi V, et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med. 2011;364(26):2561–3. doi: 10.1056/NEJMc1100859. [DOI] [PubMed] [Google Scholar]
- 104.Campellone KG, Giese A, Tipper DJ, et al. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol Microbiol. 2002;43(5):1227–41. doi: 10.1046/j.1365-2958.2002.02817.x. [DOI] [PubMed] [Google Scholar]
- 105.Czeczulin JR, Balepur S, Hicks S, et al. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect Immun. 1997;65(10):4135–45. doi: 10.1128/iai.65.10.4135-4145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dudley EG, Abe C, Ghigo JM, et al. An IncI1 plasmid contributes to the adherence of the atypical enteroaggregative Escherichia coli strain C1096 to cultured cells and abiotic surfaces. Infect Immun. 2006;74(4):2102–14. doi: 10.1128/IAI.74.4.2102-2114.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boisen N, Struve C, Scheutz F, et al. New adhesin of enteroaggregative Escherichia coli related to the Afa/Dr/AAF family. Infect Immun. 2008;76(7):3281–92. doi: 10.1128/IAI.01646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Velarde JJ, Varney KM, Inman KG, et al. Solution structure of the novel dispersin protein of enteroaggregative Escherichia coli. Mol Microbiol. 2007;66(5):1123–35. doi: 10.1111/j.1365-2958.2007.05985.x. [DOI] [PubMed] [Google Scholar]
- 109.Sheikh J, Czeczulin JR, Harrington S, et al. A novel dispersin protein in enteroaggregative Escherichia coli. J Clin Invest. 2002;110(9):1329–37. doi: 10.1172/JCI16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fasano A, Noriega FR, Liao FM, et al. Effect of shigella enterotoxin 1 (ShET1) on rabbit intestine in vitro and in vivo. Gut. 1997;40(4):505–11. doi: 10.1136/gut.40.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Navarro-Garcia F, Elias WP. Autotransporters and virulence of enteroaggregative E. coli. Gut Microbes. 2011;2(1):13–24. doi: 10.4161/gmic.2.1.14933. [DOI] [PubMed] [Google Scholar]
- 112.Navarro-Garcia F, Sears C, Eslava C, et al. Cytoskeletal effects induced by pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect Immun. 1999;67(5):2184–92. doi: 10.1128/iai.67.5.2184-2192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eslava C, Navarro-Garcia F, Czeczulin JR, et al. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66(7):3155–63. doi: 10.1128/iai.66.7.3155-3163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Navarro-Garcia F, Gutierrez-Jimenez J, Garcia-Tovar C, et al. Pic, an autotransporter protein secreted by different pathogens in the Enterobacteriaceae family, is a potent mucus secretagogue. Infect Immun. 2010;78(10):4101–9. doi: 10.1128/IAI.00523-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Benjelloun-Touimi Z, Sansonetti PJ, Parsot C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol Microbiol. 1995;17(1):123–35. doi: 10.1111/j.1365-2958.1995.mmi_17010123.x. [DOI] [PubMed] [Google Scholar]
- 116.Morin N, Santiago AE, Ernst RK, et al. Characterization of the AggR regulon in enteroaggregative Escherichia coli. Infect Immun. 2013;81(1):122–32. doi: 10.1128/IAI.00676-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steiner TS, Nataro JP, Poteet-Smith CE, et al. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J Clin Invest. 2000;105(12):1769–77. doi: 10.1172/JCI8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gewirtz AT, Navas TA, Lyons S, et al. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167(4):1882–5. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 119.Geerdes-Fenge HF, Lobermann M, Nurnberg M, et al. Ciprofloxacin reduces the risk of hemolytic uremic syndrome in patients with Escherichia coli O104:H4-associated diarrhea. Infection. 2013;41:669–73. doi: 10.1007/s15010-012-0387-6. [DOI] [PubMed] [Google Scholar]
- 120.Colic E, Dieperink H, Titlestad K, et al. Management of an acute outbreak of diarrhoea-associated haemolytic uraemic syndrome with early plasma exchange in adults from southern Denmark: an observational study. Lancet. 2011;378(9796):1089–93. doi: 10.1016/S0140-6736(11)61145-8. [DOI] [PubMed] [Google Scholar]
- 121.Tarr PI, Sadler JE, Chandler WL, et al. Should all adult patients with diarrhoea-associated HUS receive plasma exchange? Lancet. 2012;379(9815):516. doi: 10.1016/S0140-6736(12)60225-6. [author reply: 7] [DOI] [PubMed] [Google Scholar]
- 122.Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–90. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seifert ME, Tarr PI. Azithromycin decolonization of STEC–a new risk emerges. Nat Rev Nephrol. 2012;8(7):429. doi: 10.1038/nrneph.2012.87-c1. [DOI] [PubMed] [Google Scholar]