Abstract
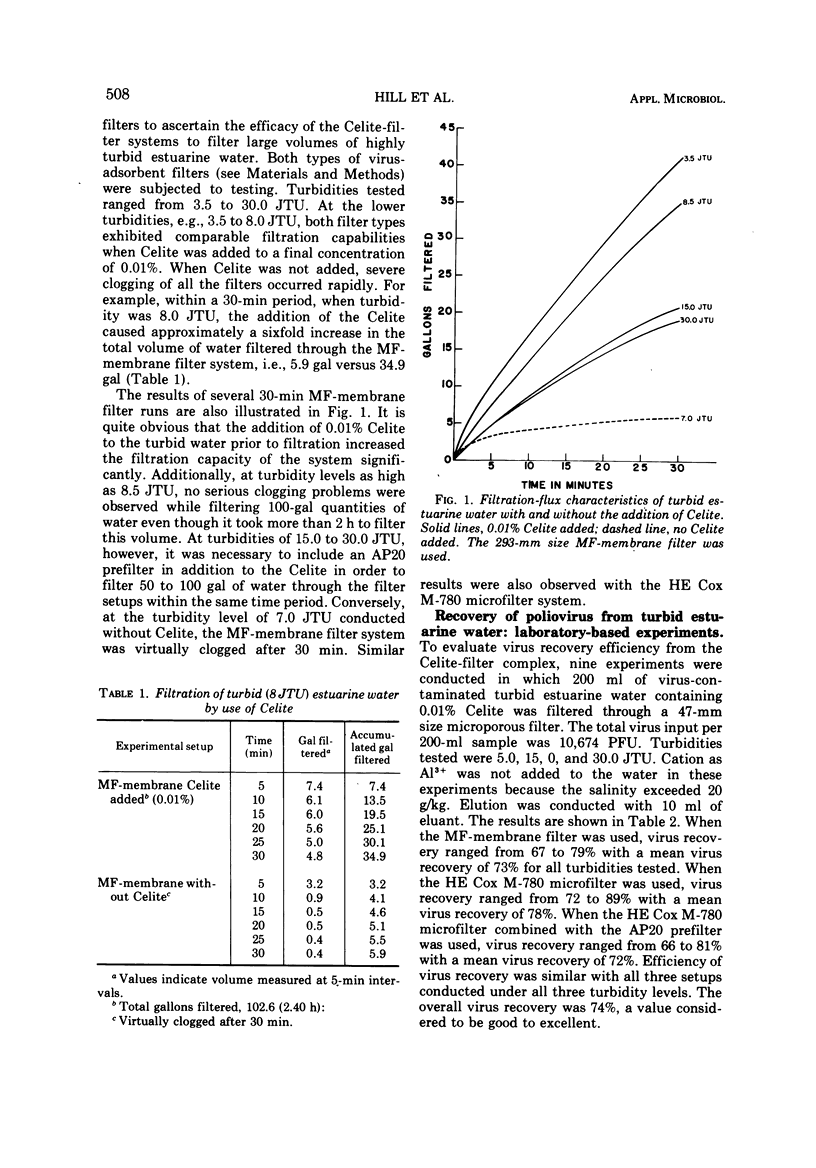
The application of a new step for recovering poliovirus from moderately to highly turbid estuarine water by the filter virus-adsorption technique was investigated. The experiments were conducted under both (i) laboratory-based conditions (200-ml volumes) where the turbidity was controlled and (ii) simulated field conditions (15- to 100-gal volumes) where the turbidity varied depending upon the hydrology of the raw estuarine water. The new step consisted of adding Celite to the turbid water prior to sampling for virus. In the experiments, the pH of the water was first adjusted to 3.5 and then AlCl3 was added to 0.0005 M. Celite was added to a concentration of 0.01% and mixed thoroughly. Either an HE Cox M-780 microfilter (Cox Instrument, Div. of Lynch Corp., Detroit, Mich.) or an MF-membrane filter (Millipore Corp., Bedford, Mass.) was used as the virus adsorbent. Virus was eluted from the Celite-filter complex in situ at pH 9 with 5× nutrient broth. In the laboratory-based experiments, when turbidity ranged from 5.0 to 30.0 Jackson turbidity units (JTU), virus recovery ranged from 66 to 89%. In the simulated field experiments, when the turbidity ranged from 8.5 to 80.0 JTU, virus recovery ranged from <1 to 74%, depending upon the multiplicity of virus input and the level of turbidity. The new step greatly improved the filtration-flux of turbid water and significantly reduced the premature clogging problem usually observed with microporous filters.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg G., Dahling D. R., Berman D. Recovery of small quantities of viruses from clean waters on cellulose nitrate membrane filters. Appl Microbiol. 1971 Oct;22(4):608–614. doi: 10.1128/am.22.4.608-614.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLIVER D. O. FACTORS IN THE MEMBRANE FILTRATION OF ENTEROVIRUSES. Appl Microbiol. 1965 May;13:417–431. doi: 10.1128/am.13.3.417-425.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. M., Boyle W. C., Goepfert J. M. Rapid quantitative method for Salmonella detection in polluted waters. Appl Microbiol. 1971 Apr;21(4):662–667. doi: 10.1128/am.21.4.662-667.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGHERTY W. J., ALTMAN R. Viral hepatitis in New Jersey 1960-1961. Am J Med. 1962 May;32:704–716. doi: 10.1016/0002-9343(62)90160-2. [DOI] [PubMed] [Google Scholar]
- Hamblet F. E., Hill W. F., Akin E. W. Effect of plaque assay diluent upon enumeration of poliovirus type 1. Appl Microbiol. 1967 Jan;15(1):208–208. doi: 10.1128/am.15.1.208-.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. F., Jr, Akin E. W., Benton W. H., Metcalf T. G. Virus in water. II. Evaluation of membrane cartridge filters for recovering low multiplicities of poliovirus from water. Appl Microbiol. 1972 May;23(5):880–888. doi: 10.1128/am.23.5.880-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. F., Jr, Hamblet F. E., Benton W. H. Inactivation of poliovirus type 1 by the Kelly-Purdy ultraviolet seawater treatment unit. Appl Microbiol. 1969 Jan;17(1):1–6. doi: 10.1128/am.17.1.1-6.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu O. C., Brashear D. A., Seraichekas H. R., Barnick J. A., Metcalf T. G. Virus in water. I. A preliminary study on a flow-through gauze sampler for recovering virus from waters. Appl Microbiol. 1971 Mar;21(3):405–410. doi: 10.1128/am.21.3.405-410.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON J. O., McLEAN W. R. Infectious hepatitis traced to the consumption of raw oysters. An epidemiologic study. Am J Hyg. 1962 Jan;75:90–111. doi: 10.1093/oxfordjournals.aje.a120238. [DOI] [PubMed] [Google Scholar]
- METCALF T. G. Use of membrane filters to facilitate the recovery of virus from aqueous suspensions. Appl Microbiol. 1961 Sep;9:376–379. doi: 10.1128/am.9.5.376-379.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROOS B. Hepatitepidemi, spridd genom ostron. Sven Lakartidn. 1956 Apr 20;53(16):989–1003. [PubMed] [Google Scholar]
- Rao N. U., Labzoffsky N. A. A simple method for the detection of low concentration of viruses in large volumes of water by the membrane filter technique. Can J Microbiol. 1969 May;15(5):399–403. doi: 10.1139/m69-071. [DOI] [PubMed] [Google Scholar]
- Ver B. A., Melnick J. L., Wallis C. Efficient filtration and sizing of viruses with membrane filters. J Virol. 1968 Jan;2(1):21–25. doi: 10.1128/jvi.2.1.21-25.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Henderson M., Melnick J. L. Enterovirus concentration on cellulose membranes. Appl Microbiol. 1972 Mar;23(3):476–480. doi: 10.1128/am.23.3.476-480.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


