Abstract
In multi-male groups where females mate promiscuously, male-infant associations have rarely been studied. However, recent studies have shown that males selectively support their offspring during agonistic conflicts with other juveniles and that father’s presence accelerates offspring maturation. Furthermore, it was shown that males invest in unrelated infants to enhance future mating success with the infant’s mother. Hence, infant care might provide fitness gain for males. Here we investigate male-infant associations in rhesus macaques (Macaca mulatta), a primate with low paternity certainty as females mate with multiple partners and males ensure paternity less efficiently through mate-guarding. We combined behavioral data with genetic paternity analyses of one cohort of the semifree-ranging population of Cayo Santiago (Puerto Rico) and recorded affiliative and aggressive interactions between focal subjects and adult males from birth to sexual maturation (0–4 years) of focal subjects. Our results revealed, that 9.6% of all interactions of focal subjects involved an adult male and 94% of all male-infant interactions were affiliative, indicating the rareness of male-infant aggression. Second and most interestingly, sires were more likely to affiliate with their offspring than non-sires with unrelated infants. This preference was independent of mother’s proximity and emphasized during early infancy. Male-infant affiliation rose with infant age and was pronounced between adult males and male rather than female focal subjects. Overall our results suggest that male-infant affiliation are also an important component in structuring primate societies and affiliation directed towards own offspring presumably represent low cost paternal care.
Keywords: male-infant association, rhesus macaques, paternal care, paternity uncertainty, kin recognition
Introduction
Among mammals, females provide the main part of parental care starting from gestation, and they ensure nutritional supply via lactation as well as protection and social integration (Trivers 1972). Males could additionally provide access to food or protection e.g., against infanticide (Hrdy 1979; van Schaik & Janson 2000). If male investment is selectively directed towards own offspring increasing the chance of their survival (thus enhancing infant and male fitness), this has been considered as paternal care (Trivers 1972; Clutton-Brock 1991). In species with high paternity certainty paternal care can therefore be expected (Geary 2000). Indeed, paternal care has been demonstrated in several mammalian species, living in one male groups (rodents: Makin & Porter 1984; Gubernick & Teferi 2000; Trainor & Marler 2001; Jones & Wynne-Edwards 2001; primates: Santos & Martins 2000) where males provide food, transport and associate with offspring.
However, in multi-male, multi-female groups females mate highly promiscuously leading to paternity confusion with several males potentially investing in a given infant based on their mating history with the infant’s mother (Alberts & Fitzpatrick 2012). It was therefore questioned whether male-infant bonds would reflect paternity because promiscuity leads to paternity uncertainty unless males invest in mate-guarding, a reproductive strategy to ensure paternity (van Schaik & Paul 1996). That males mate-guard to reduce paternity uncertainty has been described for several species (rodents: Sherman 1989; Ribble & Perrin 2005, primates: Engelhardt et al. 2005, 2006; Ostner et al. 2006; Garcia et al. 2009). However, the ability of timing the mating effort in relation to the female fertile phase is not universal. Since many primate species live in multi-male, multi-female groups the efficiency of mate-guarding has been studied particularly among primates.
While in some primates males are capable of adjusting their mate-guarding to the female’s fertile phase (Engelhardt et al. 2004; Garcia et al. 2009), others seem to be less precise (Heistermann et al. 2001; Li et al. 2005; Ostner et al. 2006; Fürtbauer et al. 2010, 2011; Dubuc et al. 2012). Differences in male ability to recognize females’ fertile phases could be due to varying accuracy of female signalling as there is inter-specific variation in the expression and information content of female fertility signals (Engelhardt et al. 2004; Dubuc et al. 2009). For example, female sexual behavior was found to be displayed specifically during the fertile phase of female wild long-tailed macaques, Macaca fascicularis (Engelhardt et al. 2004). For rhesus macaques female mating initiation could be linked to the females’ fertile phase (Zehr et al. 2000). However in both studies male responsiveness towards female behavioral change was imprecise (ibid).
Today with available genetic data we are able to learn more about the potential impact of paternity on male-infant association in promiscuous species. Although father-offspring association has been reported in spotted hyenas, Crocuta crocuta, (van Horn et al. 2004), most evidence has been reported in primates. Male savannah baboons (Papio cynocephalus)were found to preferentially support their own offspring in agonistic encounters with other juveniles (Buchan et al. 2003) and infant chacma baboons (Papio ursinus) associate more with their fathers during feeding (Huchard et al. 2013). It has furthermore been suggested that male savannah baboons selectively share proximity with own offspring (Onyango et al. 2012) and enhance offspring fitness, as individuals whose father was co-resident with them for a longer time during their juvenile period reach maturation earlier than individuals whose father was present for a shorter time (Charpentier et al. 2008). However, male-infant associations in baboons can potentially be linked to male-female friendships (Smuts 1985). A study in chacma baboons has shown that friendships often reflect paternity, as the majority of male friends turned out to be the father of his female friend’s offspring (Huchard et al. 2010). Incorporating mating data revealed that the proportion of consort activity impacts both paternity and formation of friendship, as a female’s primary consort partner most often becomes her friend when present at parturition (Moscovice et al. 2010). In his absence, females preferably form friendships with the secondary or tertiary consort partner (Moscovice et al. 2010).
In comparison to baboons, our knowledge on male-infant association is much more restricted for macaques. In Barbary macaques (Macaca sylvanus), a species for which male-infant interactions are most frequently reported, paternity is not a predictor of such relations (Paul et al. 1996). Instead infants are used to regulate relationships among males, a phenomena known as agonistic buffering (Paul et al. 1996), for which the benefits to the infants still remain unclear. Moreover, males of this species associate with unrelated infants and thereby increase their probability to mate with the infant’s mother (Ménard et al. 2001), a reproductive strategy also known as mating investment or care-then-mate pattern. A recent study in rhesus macaques (Macaca mulatta) revealed that, despite many opportunities, males do not provide support to their offspring when involved in agonistic conflicts regardless of offspring age (Kulik et al. 2012). A previous study on the same species using only behavioral data on male-immature association revealed variation among dyads ranging from temporarily to persistent associations over the first two years of infancy (Hill 1986). Inferring paternity from blood protein markers, a study including only four males in a captive group, showed a small paternity effect in rhesus macaques, whereby infants selectively initiated associations with their fathers (Berenstain et al. 1981).
To investigate male-infant associations, we used rhesus macaques living in multi-male, multi-female groups in which females remain in their birth group while males disperse to breed elsewhere (Greenwood 1980). This is an interesting species for different reasons. First, males in this species face paternity uncertainty as females mate with up to 4 partners during their period of likely conception (Manson 1992). In addition, male mate-guarding does not cover the entire female fertile phase to efficently counteract paternity uncertainty, as only 30–40% of all fertilizations are achieved through mate-guarding (Dubuc et al. 2012). Although females provide reliable information about their fertile phase (Dubuc et al. 2009; Higham et al. 2010), detection by rhesus males is impacted by male’s familiarity towards females (Higham et al. 2011). Interestingly, males do not distinguish between fertile and post-fertile phase as both mate-guarding (Dubuc et al. 2012) and female facial attraction are prolonged towards the female luteal phase when probability of fertilization is zero (Higham et al. 2011). As such, it is not clear whether successful mate-guarding is a reliable proxy of paternity confidence for males in this species. Second, male rhesus macaques disperse several times in life (Drickamer & Vessey 1973) and mainly queue for rank rather than fight for it (Bercovitch 1992; Berard 1999). Therefore, in contrast to other primate species, alpha males are not necessarily the strongest and most attractive males (Dubuc et al. 2011). Nevertheless, male reproduction is skewed, with few high-ranking males siring the majority of offspring while most males do not reproduce at all (Widdig et al. 2004). Notably, in these studies, the alpha male did neither receive the highest share of matings nor paternity success (Widdig et al. 2004; Dubuc et al. 2011). Although high-ranking males have some mating advantages (Chapais 1983; Berard et al. 1994; Dubuc et al. 2011), it appears that dominance is not a reliable proxy of paternity likelihood. Third, female rhesus macaques are known for their extremely restrictive mothering (Maestripieri 1994). This may limit males’ opportunities to interact with infants, especially when the infants are very young and most vulnerable. On the other hand, male care might be at the highest demand in early infancy when impact of infant protection is most efficient. For these reasons we tested the influence of paternity, mother’s proximity to the infant as well as male and infant attributes on male-infant associations in rhesus macaques.
Overall, based on promiscuous mating and insufficient mate-guarding in this species we predicted that male-infant interaction should not be impacted by paternity. Alternatively, males could be expected to provide care to offspring either if mating probability is sufficiently high or via post-birth offspring recognition. As infant mortality is highest during the first year of life (Blomquist 2013) we assumed sire-offspring association to be more pronounced during early infancy and the amount of male-infant association to increase as maternal control decreases. Furthermore, we tested whether mother’s proximity influenced the likelihood of male-infant interaction, predicting sire-offspring association to be independent from mother’s presence. Finally, male-infant interaction may be influenced by male’s age, since male reproduction was shown to follow a bell shaped distribution (Bercovitch et al. 2003). As males of an intermediate age are more likely to have sired offspring than younger and older males, we predicted them to invest more into their current progeny. Additionally, older males facing a decrease in future reproductive success might also invest into unrelated infants to enhance their mating opportunity with the infants’ mother despite their age. As higher-ranking males are more likely to sire infants (Dubuc et al. 2011) we predicted that father-offspring associations, are more likely involving high-ranking, in comparison to low-ranking males. Here we present behavioural data collected from birth to maturation of individuals, since we expected these hypothesized effects to vary over the course of immaturity.
Material and Methods
(a) Study species, population and subjects
Rhesus macaques live in multi-male, multi-female groups characterized by female philopatry (Gouzoules & Gouzoules 1987) and male dispersal (Lindburg 1969; Colvin 1983). They breed on a seasonal basis (Drickamer 1974) and both males and females mate highly promiscuously (Lindburg 1971). The inter-birth intervals of females are approximately one year and females predominantly give birth to a single offspring (Rawlins & Kessler 1986). Infants can be assigned to non-overlapping birth cohorts even though infants from the same cohort may differ in age by up to 6 months.
The study was conducted on the rhesus macaque population of Cayo Santiago, a 15.2 ha island offshore Puerto Rico (USA). All monkeys inhabiting the island were direct descendants of the 409 founder animals captured in India in 1938 (Rawlins & Kessler 1986). Ever since, individuals have only been added to the population via natural births, however genetic analyses from pedigree data revealed no evidence of inbreeding over the study period (Muniz & Widdig, unpublished data). The date of birth or death, respectively, sex of all study subjects, period of group membership along with number of maternal kin were taken from the demographic data base of the Caribbean Primate Research Center (CPRC) continuously recorded since 1956. When males disperse to a new group, CPRC census takers note the group and check this assignment of group membership at least for the next two months. If group membership remains constant, the first day the animal was seen in the new group is defined as the date of immigration (A. Ruiz, pers. communication).
During the study period (Oct 2004 to Aug 2008), our study troop (group R) consisted of 269.38 ± 23.39 (mean ± SD) animals across study years with adult females outnumbering adult males (sex ratio 1.18 females:1 male). We followed all 57 newborns born between September 2004 and January 2005 (hereafter focal subjects), of which 27 were females and 30 were males, starting immediately after birth. A total of 28 focal subjects survived until the study was completed (15 females, 13 males) reaching an age of 3.76 ± 0.06 years, (mean ± SD) while thirteen died during the study period and 16 subjects were removed by the CPRC due to colony management. All group members were recognized on an individual basis using individual and artificial marks. For individuals younger than one year we additionally considered mother’s proximity.
(b) Behavioral data
To cover the entire period until puberty, we observed our focal subjects continuously from birth to sexual maturation. In our study population, females undergo first menarche at the age of around 2.5 years (Zehr et al. 2005) and males are capable of reproducing at around 3.5–4 years of age (Rawlins & Kessler 1986). Thus our study period ensured focal subjects to reach maturation. A total of 3543 observation hours were collected over the entire study period resulting in 64.42 ± 37.33 (mean ± SD) hours (range 8.3 to 95.3 hours, median 75.3 hours) per focal subject using a 20min standard protocol (focal animal sampling) (Altmann 1974). For each focal subject only one protocol per day was recorded at maximum (189.62 ± 92.0, mean ± SD protocols per focal subject, range 19 – 287). Focal observations were evenly distributed over the day, and observation time was weekly balanced among focal subjects. During each protocol, we continuously recorded affiliative and aggressive interactions between the focal subjects and all group members. Affiliative interactions included socio-positive approach, i.e., no immediate agonistic interaction followed the approach, while the dyad would stay in close proximity of ≤ 2.0m for at least ≥5s (compare Cooper et al. 2005), social grooming or friendly behavior such as touch, hug, carry, hold, play and lip smacking. Aggression included either physical (contact) or non-physical (non-contact) aggression. All interactions were noted within the 2.0m range of a focal subject. For each interaction of the focal subject, we recorded whether the mother of the focal subject was within or outside of the 2.0m range (hereafter mothers’ condition). We assumed that a mother outside of 2.0m range increases her permissiveness, which potentially promotes the infant’s independence.
We further collected data on displacement, aggression or submission between adult males ad libitum (Altmann 1974) to construct a male dominance hierarchy (see below).
Data were collected by AW, DL and two field assistants. Subsequent assistants were trained for a total of two months including interobserver-reliability tests (Kaufman & Rosenthal 2009) during the last two weeks. When conducting simultaneous focal sampling new assistants reached a reliability ranging between 90–97% with the trainer. Data were collected using Psion Workabout™ handhelds and processed with Observer software (version 5.0). All observers were blind to paternity across the entire study period.
(c) Determination of paternity
Genotypic data were available for most of the adult group members, from previous studies (see, e.g. Widdig et al. 2001, 2006a, b; Kulik et al. 2012) as part of the genetic data base of the Cayo Santiago population started in 1992. Nearly the entire population was systematically sampled for animals a) born between 1992 and 2000 or b) born before 1992 if they survived until systematic sampling began in 1992. Furthermore, we were able to sample all, but two newborns born during the 2005 birth cohort. In addition, we sampled remaining group members (e.g., mothers of focal subjects) and potential sires for which we lacked a DNA sample. Samples taken were either hair, blood, tissue or fecal samples. We extracted DNA from samples partly via a GenoM48-Automat (Nagy et al. 2005).
The genetic data analysed in this study were taken from a data base currently consisting of 2302 genotyped animals at 14.62 ± 2.44 loci on average (mean ± SD) out of a panel of 23 STR markers (Dubuc et al. 2011). The mean number of alleles per locus was 7.38 ± 2.87 (mean ± SD), the mean observed heterozygosity across loci was 0.75 ± 0.08 (mean ± SD), the mean expected heterozygosity was 0.74 ± 0.07 (mean ± SD), and the mean polymorphic information content was 0.69 ± 0.8 (mean ± SD) (all calculations performed with CERVUS 3.0; Kalinowski et al. 2007). There was no evidence of a null allele occurring at these loci and all, but one locus, were in Hardy-Weinberg equilibrium (HWE). Locus D20S206 deviation from HWE could be due to chance, mutation or typing errors. The overall typing error rate derived from mother-offspring mismatches was 11% for the entire data base, however, this value decreased to 3% when considering only individuals from group R included in the analysis because of the increased effort in completing their genotypes.
Maternity derived from long-term field observations was genetically tested and confirmed for all mother-infant pairs (N=55) in the study. This information was subsequently used in the paternity analyses. All sampled males older than 1250 days (based on earliest age at reproduction; Bercovitch et al. 2003), present on the island at least 200 days prior to the infant’s birth (mean days ± SD of gestation length of 166.5 ± 7.4; Silk et al. 1993) were considered as potential sires for a given infant. We made effort to sample 164 of a total of 175 (93.71%) different potential sires from the entire island who fulfilled these criteria with respect to our focal cohort in order to account for extra-group paternity reported for the study population (Widdig et al. 2004). Given that conception period was different across focal subjects, for each focal infant 156.76 ± 2.06 (mean ± SD) potential sires were typed (93.44%).
A given mother-father-offspring trio was genotyped on 16.35 ± 3.07 (mean ± SD) common loci but at least at 12 common loci. Paternity was determined for all 55 sampled focal subjects using a combination of exclusion and likelihood analyses as follows. In 53 cases, all males were excluded on at least two loci, with the exception of the assigned sire, who matched the offspring-mother pair at all loci. In the remaining two cases the assigned sire had no mismatch with the respective mother-offspring pair, but one other candidate sire could only be excluded on one locus. In order to confirm assigned paternities derived via exclusion, all paternities were additionally supported at the 95% confidence level in favor of the male with the highest LOD score calculated by CERVUS 3.0 (all calculations performed with CERVUS 3.0; Kalinowski et al. 2007). Among all focal subjects typed only one was sired by an extra-group male and the sires of two other focal subjects had died shortly before the infants were born. For these three cases, only behavioral interactions between non-sires and infants could be included.
(d) Establishing dominance hierarchies
Male dominance hierarchy was calculated using the ELO method (Elo 1978; Albers 2001; Neumann et al. 2011) and an R function written by LK. It estimates the competitive abilities of individuals by looking at interactions sequentially over time while the outcome of these interactions will continuously update the scores used to estimate competitive abilities (Neumann et al. 2011). Additionally, we tested whether ELO revealed similar results than ranking methods commonly used such as I&SI (de Vries 1998) and confirmed that they were highly correlated (Spearman’s rank correlation (rho), N=65 individuals, rho=0.64, p<0.001) (see Neumann et al. 2011). Ranks of adult females were determined based on the outcome of dyadic agonistic interactions in 1997 (Widdig et al. 2001) and observed to be highly stable over time. As shown in many Cercopithecines, offspring of the same female rank directly below their mother inverse to their birth order (Cheney 1977; Datta 1988), therefore infants were assigned to an individual rank according to the rank of their mother as no deviation from the predicted birth rank was observed. All ranks were calculated on a daily basis to control for rank changes due to births and deaths. We standardized the ranks of males and females (including focal subjects) separately per day to a range from 0 to 1 (lowest to highest ranking). As ELO ranks have a varying minimum and maximum, the standardization is done using: Standardized rank = (rank - min(ranks)) / (max(ranks) - min(ranks)). With rank being the individual rank at a given day and min(ranks) and max(ranks) being the minimum and maximum of all the ranks of that day.
(e) Statistical analysis
To test what influenced the affiliation between focal subjects and sexually mature males we applied a Generalized Linear Mixed Model (GLMM) (Baayen 2008). The dataset used in our analysis was derived through several steps. First, we determined the number of male-infant affiliations, our response variable, by summing up the frequencies of affiliative behaviors for each male-infant dyad per day (i.e., per protocol) considering only males present in our study group based on the demographic records while distinguishing interactions with i) mothers being within or outside of the 2.0m range of the focal and ii) focal subjects initiating or receiving a given interation. We then transformed these counts into a binary variable by setting all values greater than zero to one. Subsequently, we summed up all results per day in frequencies (i.e., number of days with interactions) per three-months period (determined based on the focal age) leading to a total of 91.538 data points. The response finally used was the number of days with and without affiliations (679 vs. 31 days, respectively), kept as two separate variables (which allows to model the probability of affiliative interactions; see below), separately for each combination of focal-male dyad, mother’s condition (within or outside of 2.0m of focal) and initiation (by focal or by male). In the model we included mother’s condition and initiation of interaction by immature individuals or males as fixed effects as well as for each focal the sex, its age (average age in the three-months period) and squared age (since the relation between focal age and male affiliation was expected to be non-linear), rank and number of close maternal kin (10.70 ± 6.53, mean ± SD). We controlled for focal age from birth to maturation to investigate whether male-infant relations were varying over an immature individuals’ life. For adult males the model contained rank and age. We indicated in an additional variable whether the male was the sire of a given focal (hereafter paternity). Finally, we included the identity of both focal subject and male, and three-months period (nested within focal) as random effects into the model. We expected several interactions between these main effects since the study expended on the entire ontogeny from birth to maturation. Specifically, we included the four-way interaction between initiation of affiliation, focal age, paternity, and mother’s condition, since we expected male initiation to be pronounced at early infancy, but male and focal intiation probabilities to get more similar as focal subjects get older. However, we expected paternity to potentially mediate this pattern in the sense that if immature individuals are able to recognize their fathers the increase of focal initiated affiliation should be steeper for sires than non-sires. This three-way interaction was finally combined with mother’s condition, since it seemed plausible that mothers either restrict male-infant affiliations during early infancy (bluring the expected patterns) or promote sire-offspring affiliations. To account for the possibility that male-infant affiliations would peak at an intermediate age we included the same interaction with focal age squared rather than age. Patterns of affiliation with males might differ along ontogeny between the sexes, since females stay in their natal group whereas males disperse. Hence, we included also the three-way interaction between initiation of affiliation, sex of the focal animal and focal age (linear and squared). To achieve a reliable model we also included all interactions encompassed by these interactions. Since it could be taken for granted that the development of affiliation is not linear along focal age we used both terms focal age and focal age squared, when testing focal age with any other predictor variable in an interaction.
The data analyzed were likely to show temporal autocorrelation, i.e., residuals of data points recorded closer to one another in time could be more similar to one another than data points recorded further apart, which could lead to the violation of the assumption of independent residuals devaluing the reliability of the model. Thus, we aimed to include two autocorrelation terms, one for the focal subject and one for the male, to explicitly account for temporal autocorrelation in the data. To calculate them, we first ran the model as described above to derive the residuals. Then, we averaged the residuals of all other data points, separately for each data point and also separately for each focal and male, with the contribution of the residuals being weighted by their time lag to the particular data point. For these averages the weight followed a normal distribution with a mean of zero (i.e., maximum weight at a time lag of zero). Their standard deviations were simultaneously determined by maximizing the likelihood of the model including the two autocorrelation terms. However, in the final model we included only the autocorrelation term for the male as an additional fixed effect (we excluded the focals’ autocorrelation term, because it revealed a negative estimate, presumably an artefact of the rarity of interactions).
The model was run in R (version 2.15.0, R Development Core Team 2012) using the function “lmer” from the R package “lme4” (Bates et al. 2011) with the number of days with and without affiliations handed over as the response (binomial response in two vectors). Hence we modeled the proportions of days with affiliations from the total of all possible affiliative events and the model reveals probabilities of affiliations to happen. The GLMM was fitted with binomial error structure and logit link function. Using a likelihood ratio test (Dobson 2002) (R function “anova”, package “stats”), we determined the statistical significance of the full model by comparing its fit with that of the null model (comprising only the random effects and the autocorrelation term). Also all terms in the model were tested for their statistical significance by running additional likelihood ratio tests (LRT) comparing the fit of the full model with that of a reduced model lacking the particular term of interest but comprising all other terms. In case an interaction did not reveal significance we removed it from the model in order to be able to reliably infer about the respective lower terms it included. Such removal was only done conditional on the full-null model comparison revealing significance. The autocorrelation term was calculated using an R function written by RM. The minimization of the AIC to find the best fitting standard deviations of the weighted function for the autocorrelation terms was done using the R function “optim” from the package “stats”. Prior to running the model we z-transformed all covariates (including the autocorrelation term) to a mean of zero and a standard deviation of one. To check for the assumptions of the model we calculated Variance Inflation Factors (VIF) (Quinn and Keough 2002) for a model lacking the random effects indicating that collinearity was not an issue (largest VIF=1.54). VIF were determined using the function “vif” of the R package “car” (Fox & Weisberg 2011). We regarded P-values ≤0.05 significant and 0.05 < P ≤0.1 as a trend. Trends should be considered, because dichotomising results based on P-values being significant or not can result in misleading conclusions (Stoehr 1999).
In order to analyse the influence of the same predictors upon aggressive interactions between males and focal subjects, we intended to use the statistical methods as applied for affiliation. However, the rarity of aggressive events rendered a corresponding model impossible.
Results
Based on 3987 available male-infant dyads, we observed 14.360 male-infant interactions representing 9.6% of all interactions focal subjects were involved in. A total of 94.0% of all observed male-infant interactions were affiliative, indicating the rareness of male-infant aggressive interactions. Similarly, interactions between focal subjects and all group members were mainly affiliative (97%).
1. Affiliation
Overall the results revealed that the set of predictor variables used had a clear influence on the probability of male-infant affiliation (LRT comparing the fit of the full with the fit of the null model containing only the random effects and the autocorrelation term: χ2=2926.6, df=33, p<0.001, see Table 2 in appendix). To achieve the final model all included interactions were tested for significance using LRT. This resulted in the removal of the following interactions (1) the four-way interaction between initiation of affiliation, paternity, focal age (linear and non-linear) and mother’s condition (LRT χ2>0.51, all df=2, p>0.77) and (2) four three-way interactions between (a) initiation of affiliation, focal sex and focal age (linear and non-linear) (LRT χ2>0.32, df=2, p>0.85), (b) initiation of affiliation, paternity and focal age (linear and non-linear) (LRT χ2>2.01, all df=2, p>0.37), (c) initiation of affiliation, paternity and mother’s condition (LRT χ2>0.87, all df=1, p>0.35) and (d) paternity, mother’s condition and focal age (linear and non-linear) (LRT χ2>0.77, all df=2, p>0.68).
Male and focal attributes
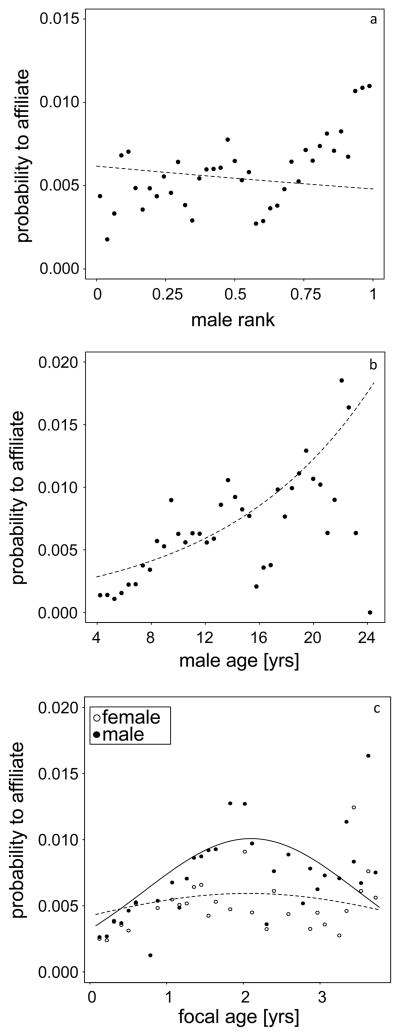
Males were more likely to affiliate when they were lower-ranking and older (Table 1, Figure 1a, b), whereas male-infant affiliation was more likely when the focal subject was a male (Table 1, Figure 1c), around two years of age (Table 1, Figure 1c) and lower-ranking (trend only) (Table 1). The number of close maternal kin of focal subjects did not affect the probability of male-infant affiliation (Table 1).
Table 1.
Results of GLMM analyses of male-infant affiliation (final model, z and p values not shown for intercept and variables/interactions comprised by a higher interaction)
| full model
| ||||
|---|---|---|---|---|
| Predictor Variable | Estimate | SE | z | p |
| Intercept | −5.50 | 0.13 | ||
| Autocorrelation term | 0.26 | 0.01 | 41.57 | 0.000 |
|
| ||||
| Main effects | ||||
| Male age | 0.45 | 0.11 | 4.11 | 0.000 |
| Male rank | −0.07 | 0.03 | −2.80 | 0.005 |
| Number of maternal kin of focal | 0.00 | 0.04 | −0.13 | 0.893 |
| Focal rank | −0.07 | 0.04 | −1.73 | 0.084 |
| Initiation | −0.42 | 0.06 | ||
| Focal sex | 0.42 | 0.09 | ||
| Paternity | −0.11 | 0.15 | ||
| Mother’s condition | −1.26 | 0.06 | ||
| Focal age | 0.54 | 0.05 | ||
| Focal age squared | −0.36 | 0.04 | ||
|
| ||||
| 2 way interaction | ||||
| Paternity: Mother’s condition | 0.47 | 0.26 | ||
| Focal sex : Focal age | 0.23 | 0.06 | ||
| Focal sex : Focal age squared | −0.24 | 0.06 | ||
| Paternity : Focal age | −0.42 | 0.17 | ||
| Paternity : Focal age squared | −0.13 | 0.18 | ||
| Mother’s condition : Focal age | −1.20 | 0.05 | ||
| Mother’s condition : Focal age squared | 0.43 | 0.05 | ||
| Initiation : Focal sex | 0.11 | 0.07 | ||
| Paternity : Initiation | 0.11 | 0.22 | ||
| Initiation : Mother’s condition | 0.80 | 0.07 | ||
| Initiation : Focal age | 0.47 | 0.06 | ||
| Initiation Focal age squared | 0.04 | 0.05 | ||
|
| ||||
| 3 way interaction | ||||
| Initiation : Paternity : Mother’s condition | 0.08 | 0.34 | ||
| Initiation : Focal sex : Focal age | 0.01 | 0.06 | 0.21 | 0.834 |
| Initiation : Focal sex : Focal age squared | 0.02 | 0.06 | 0.31 | 0.759 |
| Initiation : Paternity : Focal age | 0.19 | 0.26 | ||
| Initiation : Paternity : Focal age squared | 0.06 | 0.25 | ||
| Initiation : Focal age : Mother’s condition | −0.32 | 0.07 | ||
| Initiation : Focal age squared : Mother’s condition | 0.08 | 0.07 | ||
| Paternity : Mother’s condition : Focal age | 0.15 | 0.31 | ||
| Paternity Mother’s condition : Focal age squared | −0.29 | 0.34 | ||
|
| ||||
| 4 way interaction | ||||
| Initiation : Paternity : Mother’s condition : Focal age | −0.07 | 0.39 | −0.17 | 0.864 |
| Initiation : Paternity : Mother’s condition : Focal age squared | 0.28 | 0.40 | 0.70 | 0.486 |
Fig. 1.
Impact of male and focal attributes on the probability of male-infant affiliation: (a) males of higher rank were less likely to affiliate with immature individuals, (b) affiliation probability was higher for older males, (c) for male immatures the probability to affiliate rose with focal age and was higher than for female focals. The lines represent the fitted model(s).
Paternity, mother’s condition and initiation of affiliation
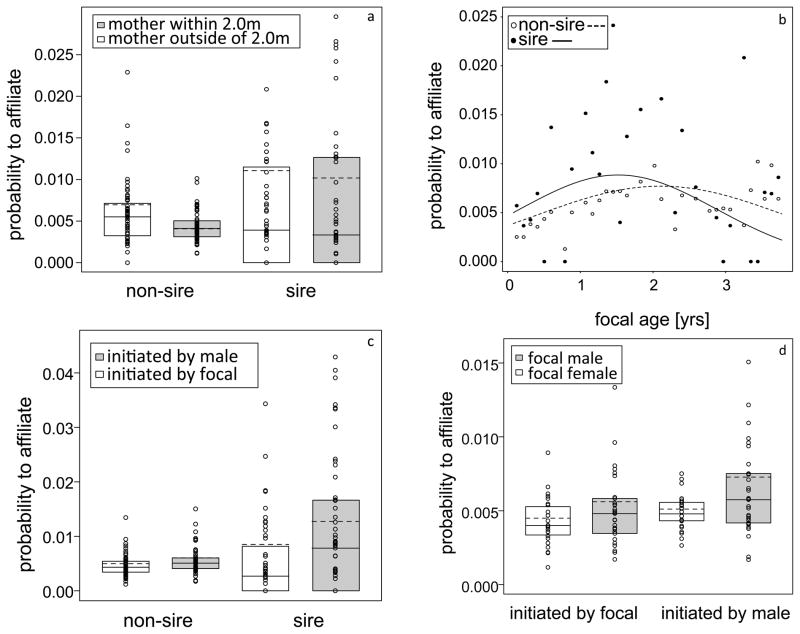
Our results also revealed the interaction between paternity and mother’s condition to be significant (Table 1). As shown in Figure 2a the difference between the probabilities of male-infant affiliation resulting from mothers being within or outside of the 2.0m range of the focal was higher for non-sires than for sires. Furthermore, sires had a higher probability to affilliate in both mother’s conditions than non-sires.
Fig. 2.
Two-way interactions between paternity and mother’s condition, focal age, focal sex and initiation: (a) the difference of the probability of male-infant affiliation was higher for non-sires than sires when considering mother’s condition, (b) the probability to affiliate was initially higher for sires and peaking at two years of focal age, whereas affiliation probability for non-sires exceeded sire-offspring affiliation probability after two years of focal age. The interaction between paternity and initiation of affiliation (c) indicated, that the difference between the probabilities to affiliate was larger for sires considering initiation, with an affiliation probability for sires and offspring being thrice as high than among non-sires and immature individuals. The interaction between initiation of affiliation and focal sex (d) revealed that males were more likely to initiate interactions than focals, especially with male focal subjects. For Fig 2a, 2b and 2c the boxes represent the first to third quartile of observed values, solid vertical lines in the boxes show the median, and dashed vertical lines in the boxes show the values fitted by the model.
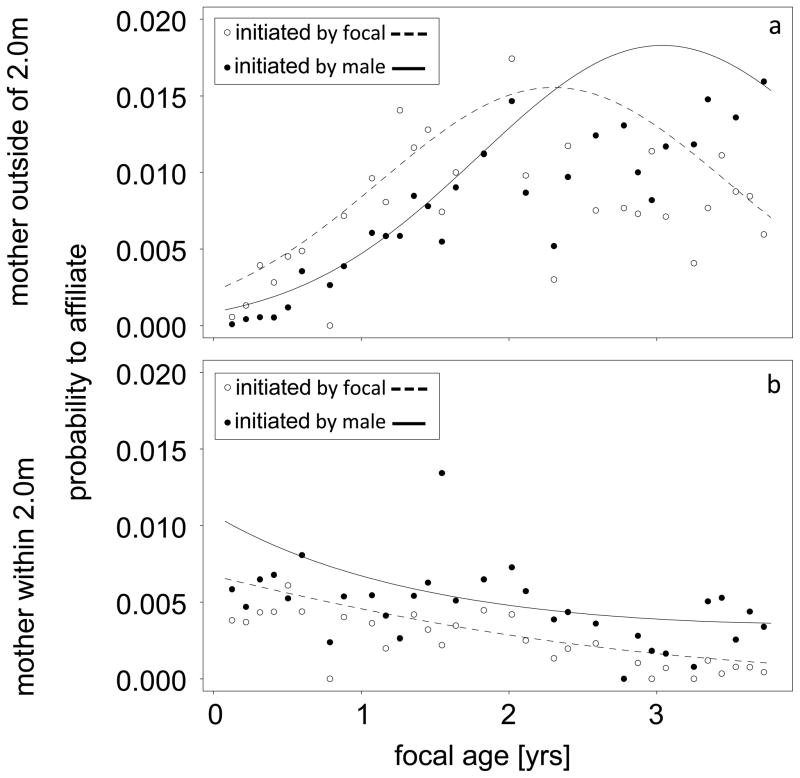
Two further interactions comprising paternity as a predictor yielded significance (Table 1). The result of the interaction between paternity and non-linear focal age showed that in the first ca. two years of infancy sire-offspring affiliation had a relatively higher probability, whereas later the probability of non-sire-infant affiliation was higher (Figure 2b). Immature individuals and non-sires were similarly likely to initiate interactions with each other, whereas in focal-sire dyads, males were more often the initiators (Figure 2c). Furthermore, affiliations between immature individuals and their sires were generally more likely than affiliations between focals and other males (regardless of who was initiating the affiliation). The significant interaction between initiation and focal sex (Table 1) showed that the effect of focal sex was more pronounced, when initiation was considered. As seen in Figure 2d the probability for affiliation between male focal subjects and adult males was higher than for female focal subjects. This sex bias was even more pronounced, when adult males initiated the interaction with immature individuals. Finally, the three-way interaction including mother’s condition, non-linear focal age and initiation of affiliation revealed significance (Table 1). Specifically, when mothers were outside of the 2.0m range of the focal (Figure 3a) the probability to affiliate rose with focal age and was higher when initiated by focals (up to two years of age) than by males, whereby when mothers were within the 2.0m range of the focal (Figure 3b) it decreased and was lower for focal than male initiated interactions.
Fig. 3.
Three-way interaction between mother’s condition, focal age and initiation of affiliation. When mothers were outside of 2.0m range of the focal (3a) probability to affiliate rose with focal age and was higher when initiated by focals (up to two years of age) then by males. In contrast it steadily decreased and was lower for focal than male initiated interactions, when mothers were within 2.0m range of the focal (Figure 3b).
2. Aggression
We observed only 12 incidents of sire-offspring aggression (performed by only six sires towards eight offspring) over the entire study period. This extremely low amount of aggression indicated that agonistic behavior did not seem to be of large importance in sire-offspring relations and thus made it unreasonable to perform any analysis.
Discussion
The results of our study support previous findings that male-infant interactions are present in rhesus macaques (Berenstain et al. 1981; Hill 1986), but revealed that the nature of these interactions is predominantly affiliative rather than aggressive. In addition, we showed that rank and age of both males and focal subjects as well as mother’s condition, paternity and initiation influenced the probability of male-infant affiliation.
Most interestingly, paternity in interdependence with three other factors was a significant predictor of male-infant affiliation in rhesus macaques. This was less likely given that promiscuous mating and insufficient mate-guarding (Dubuc et al. 2012) should increase paternity confusion and thus paternity certainty should be low. Indeed, several lines of evidence support that sires have a higher probability to interact with their offspring in comparison to non-sires. First, sire-offspring affiliation occurred independent of mother’s proximity. Second, in contrast to non-sires, sires had a higher affiliation probability with their offspring at a particular period reflecting the highest infant mortality in the study population (0–2 years) (Blomquist 2013). Finally, sires also initiated affiliation towards their offspring at a higher probability than vice versa, suggesting that father-offspring association during infancy might function to ensure the immatures’ survival, which in turn might affect male fitness. What remains open is why male rhesus macaques do not provide agonistic support towards their offspring involved in conflicts with group members of any age (Kulik et al. 2012). One explanation would be that aggression towards immature individuals is generally rare in this species. To date, we only have evidence that male baboons support their offspring in conflicts with other juveniles (Buchan et al. 2003), which is unlikley to be very costly, unless males protect them against adult aggressors, too. The lack of such high cost paternal care (e.g., offspring support against adult aggressors) might be due to selection as a principle of evolution favoring this behavior only when fitness benefits of offspring are higher than costs of paternal care (Geary 2000). Otherwise less costly paternal care (e.g., affiliation) might evolve or highly costly care is selected against, so that males may even abandon offspring and direct their effort predominately to compete for mates to ensure high reproductive output (Geary 2000; Boyd & Silk 2012). We propose our results to indicate low cost paternal care, although the fitness benefit for offspring and males remain to be investigated.
Although we did not aim to test the mating effort hypothesis, our result that affiliation between non-sires and focals was not more pronounced when mothers were in close proximity, is not in line with the hypothesis. Under the mating effort hypothesis male care for unrelated immatures should be more pronounced when mothers are present in order to enhance the care takers mating opportunity with the mother (Ménard et al. 2001) as shown in vervet monkeys, Cercopithecus aethipos, (Keddy Hector et al. 1989). It remains to be investigated whether males in the study species potentially increase their mating access as a result of care taking.
Although we found evidence for male-infant associations being impacted by paternity in rhesus macaques, we cannot draw conclusions about the underlying mechanism. On the one hand males could assess paternity certainty by estimating their mating success with a given female. Even though recent evidence has shown that male rhesus macaques seem to time their mate-guarding effort insufficiently, males may use other reliable cues of female fertility more precisely, like female sexual behavior (Engelhardt et al. 2005) not yet detected in rhesus macaques. Father-offspring affiliation might therefore be the result of precise timing of mating, while males interacting with unrelated immature individuals might be due to imprecise timing or assessment of mating access in relation to paternity, potentially influenced by females rejecting sperm from or providing less reliable information to less preferred males. Bonding between males and immature individuals might also reflect the close relationship between mothers and males who previously mated with the mother (Berenstain et al. 1981; Moscovice et al. 2009). This is supported by the similar probability of affiliation between sires and offspring, independent from the mother’s proximity to the focal, as well as the fact that male sociality predicts paternity (Kulik et al. 2012).
On the other hand, an alternative mechanism underlying father-offspring association (potentially available for both males and immature individuals) might be post-birth kin recognition if certain phenotypic cues are linked to paternal relatedness and used to discriminate paternal kin from non-kin. Indeed, a recent experimental study showed that humans are able to detect paternity as well as maternity in rhesus macaque faces indicating that paternal kinship is present in visual appearance (Kazem & Widdig 2013). Furthermore, Swiss mice (Mus musculus) were found to be able to detect age and sex differences in baboon body odour as they perceive odour similarities among related compared to unrelated baboons (Célérier et al. 2010). Although both studies do not test kin recognition in conspecifics, their results support the existence of phenotypic cues being available in primates to assess relatedness necessary for kin discrimination at the behavioral level.
Male-infant affiliation was also affected by the sex of focal subjects as adult males affiliated with male rather than female focal subjects. This bias might be caused by the dispersing sex showing less affiliation to close maternal kin prior to dispersal (Lehmann & Boesch 2008) in comparison to the philopatric sex. This may open the opportunity or reflect the need for our male focal subjects to associate with individuals outside of their maternal family. Overall, male focal subjects were found to affiliate less with maternal group members throughout the first four years of life than female focal subjects (Kulik, unpublished data).
Our data also suggest, that some male attributes were predictors of the probability of male-infant affiliation. Older males were more likely to affiliate with focals than younger males, possibly because older males might invest in offspring as well as unrelated immatures since future reproduction is less likely for them; hence older males are predisposed to use the mate-then-care strategy. Alternatively, older males may be in need of any social contact leading them to affiliate with unrelated immatures, a hypothesis we were unable to test here.
In summary our data support previous studies that male-infant associations are also an important component in structuring primate societies. In addition, this is the first study to show that paternity in relation to other predictors impacts male-infant affiliation even in a species with low paternity confidence where paternal care was unexpected to have evolved. However, the evidence of paternal care to date represent low cost investment. Although there is no doubt about the influence of maternal care on infant developement, our results strengthen the need to look at the male contribution to infant care, too, which might be more important then previously thought. Moreover the results of this study strengthen the need for long-term behavioral data collection in order to be able to analyse complex contexts and interdependencies of several factors potentially influencing animal behavior.
Future studies should focus on the underlying mechanisms of male-infant affiliation to improve our understanding about the evolution of father-offspring relations. It should also be investigated whether sires and offspring can truly discriminate among kin and non-kin (kin recognition via phenotype matching), or whether bonding between males and immature individuals mirrors a relationship between the immature’s mother and an the adult male. Furthermore the impact of male-infant association, and specifically father-offspring association, on the immature individuals fitness should be examined, as potential benefit to either or both of them may present additional reasons for such relations to have evolved.
Supplementary Material
Acknowledgments
We are most grateful to the Caribbean Primate Research Center for their permission and support of this study. In addition, we appreciate the support of the staff of the Cayo Santiago Field Station, especially the census taker Edgar Davila, Julio Resto and Giselle Caraballo Cruz for their cooperation throughout the observational study and during the collection of the DNA samples. We are also grateful to Joyce Moewius and Akie Yanagie for their enthusiam during the collection of behavioral data as well as Klaus Leipholz, Margaret Chiavetta and Constance Dubuc for their cooperation during sampling collection. Fred Bercovitch, Matt Kessler, John Berard, Michael Krawczak, Peter Nürnberg and Jörg Schmidtke are acknowledged for their effort to start the genetic data base of the Cayo Santiago population mainly funded by NSF, NIH and the German Research Foundation (DFG). We owe special thanks to Andrea Trefilov, Elisabeth Kirst, Peter Nürnberg, Petra Otremba, Marion Nagy, Laura Muniz and Stefanie Bley for their input and collaboration in improving and extending the genetic data base and particularly Linda Vigilant for laboratory access. We also thank Michael Krawczak, Bernd Hundrieser and Olaf Junge for access and improvement of the FINDSIRE, a management program of genetic data. Hagen Stenzel kindly wrote a number of macros for data analysis. We thank Constance Dubuc and Liza Moscovice for discussion and comments on an earlier draft of this manuscript, as wells as two anonymous reviewers for their helpful suggestions. All research procedures were approved by the Caribbean Primate Research Center and the Institutional Animal Care and Use Committee (IACUC) of the University of Puerto Rico (protocol number 4060105) and the sample transfer was approved as required (Cites Export permission #05US101361/9, #06US112079/9, Cites Import permission #E-1426/05, #E-1082/06). The population of Cayo Santiago was supported by grant number 8 P40 OD012217-25 from the National Center for Research Resources (NCRR), the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health and the Medical Sciences Campus of the University of Puerto Rico. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of NCRR or ORIP. This project is part of a long-term study on the mechanisms of paternal kin discrimination conducted within the Jr. Research Group of Primate Kin Selection, an Emmy-Noether Group funded by the German Research Foundation (DFG) (grant numbers WI 1801/1-1, 1-2, 2-1, 3-1 awarded to AW). We thank the Max-Planck Institute for Evolutionary Anthropology, Leipzig, for their logistic support and for hosting of the Jr. Research Group of Primate Kin Selection and the KKGS Stiftung, Elsa-Neumann Stiftung and the University of Leipzig for graduate funding awarded to DL.
Footnotes
- R data file containing necessary data to perform GLMM analyses (doi:10.5061/dryad.4s6g0)
- table containing genotypes from all focal aimals and their assigned sires (doi:10.5061/dryad.4s6g0)
Author contributions box
Research questions were designed and data were collected by AW and DL. Genetic data were provided by AW. Data analyses were performed, manuscript was written and revised by all authors.
Literature
- Albers P. Elo-rating as a tool in the sequential estimation of dominance strengths. Animal Behaviour. 2001;61:489–495. [Google Scholar]
- Alberts SC, Fitzpatrick CL. Paternal Care and the Evolution of Exaggerated Sexual Swellings in Primates. Behavioral Ecology. 2012;23:699–706. doi: 10.1093/beheco/ars052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann J. Observational study of behavior sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Baayen H. Analyzing linguistic data: A practical introduction to statistics using R. Cambridge University Press; Berlin, New York: 2008. [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. 2011. [Google Scholar]
- Berard JD. A four-year study of the association between male dominance rank, residency status, and reproductive activity in rhesus macaques (Macaca mulatta) Primates. 1999;40:159–175. doi: 10.1007/BF02557708. [DOI] [PubMed] [Google Scholar]
- Berard JD, Nürnberg P, Epplen JT, Schmidtke J. Alternative reproductive tactics and reproductive success in male rhesus macaques. Behaviour. 1994;129:177–201. [Google Scholar]
- Bercovitch FB. Sperm competition, reproductive tactics, and paternity in savanna baboons and rhesus macaques. In: Martin RD, Dixson AF, Wickings EJ, editors. Paternity in primates: genetic tests and theories. Implications of human DNA Fingerprinting. Karger; Basel: 1992. pp. 225–237. [Google Scholar]
- Bercovitch FB, Widdig A, Trefilov A, Kessler MJ, Berard JD, Schmidtke J, Nurnberg P, Krawczak M. A longitudinal study of age-specific reproductive output and body condition among male rhesus macaques, Macaca mulatta. Naturwissenschaften. 2003;90:309–312. doi: 10.1007/s00114-003-0436-1. [DOI] [PubMed] [Google Scholar]
- Berenstain L, Rodman PS, Smith DG. Social relations between fathers and offspring in a captive group of rhesus monkeys (Macaca mulatta) Animal Behaviour. 1981;29:1057–1063. [Google Scholar]
- Blomquist GE. Maternal Effects on Offspring Mortality in Rhesus Macaques (Macaca mulatta) American Journal of Primatology. 2013;75:238–251. doi: 10.1002/ajp.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R, Silk JB. How Humans Evolved. Norton & Company; 2012. [Google Scholar]
- Buchan JC, Alberts SC, Silk JB, Altmann J. True paternal care in a multi-male primate society. Nature. 2003;425:179–181. doi: 10.1038/nature01866. [DOI] [PubMed] [Google Scholar]
- Célérier A, Huchard E, Alvergne A, Fejan D, Plard F, Cowlishaw G, Raymond M, Knapp LA, Bonadonna F. Detective mice assess relatedness in baboons using olfactory cues. The Journal of Experimental Biology. 2010;213:1399–1405. doi: 10.1242/jeb.038349. [DOI] [PubMed] [Google Scholar]
- Chapais B. Reproductive activity in relation to male dominance and likelihood of ovulation in rhesus monkeys. Behavioral Ecology and Sociobiology. 1983;12:215–228. [Google Scholar]
- Charpentier MJE, Van Horn RC, Altmann J, Alberts SC. Paternal effects on offspring fitness in a multimale primate society. Proceedings of the National Academy of Sciences. 2008;105:1988–1992. doi: 10.1073/pnas.0711219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney DL. The acquisition of rank and the development of reciprocal alliances among free-ranging immature baboons. Behavioral Ecology and Sociobiology. 1977;2:303–318. [Google Scholar]
- Clutton-Brock TH. The evolution of parental care. Princeton University Press; 1991. [Google Scholar]
- Colvin J. Familiarity, rank, and the structure of rhesus male peers networks. In: Hinde RA, editor. Primate social relationship. An integrated approach. Blackwell; Oxford: 1983. pp. 190–200. [Google Scholar]
- Cooper MA, Berntein IS, Hemelrijk CK. Reconciliation and relationship quality in Assamese macaques (Macaca assamensis) American Journal of Primatology. 2005;65:269–282. doi: 10.1002/ajp.20114. [DOI] [PubMed] [Google Scholar]
- Datta SB. The acquisition of dominance among free-raniging rhesus monkey siblings. Animal Behaviour. 1988;36:754–772. [Google Scholar]
- de Vries H. Finding a dominance order most consistent with a linear hierarchy: a new procedure and review. Animal Behaviour. 1998;55:827–843. doi: 10.1006/anbe.1997.0708. [DOI] [PubMed] [Google Scholar]
- Dobson AJ. An introduction to generalized linear models. Chapman & Hall/CRC; Boca Raton: 2002. [Google Scholar]
- Drickamer LC. A ten-year summary of reproductive data for free-ranging Macaca mulatta. Folia Primatologica. 1974;21:61–80. doi: 10.1159/000155596. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Vessey SH. Group changing in free-ranging rhesus monkeys. Primates. 1973;14:359–368. [Google Scholar]
- Dubuc C, Muniz L, Heistermann M, Widdig A, Engelhardt A. Do males time their mate-guarding effort with the fertile phase in order to secure fertilisation in Cayo Santiago rhesus macaques? Hormones and Behavior. 2012;61:696–705. doi: 10.1016/j.yhbeh.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Brent L, Accamando A, Gerald M, MacLarnon A, Semple S, Heistermann M, Engelhardt A. Sexual Skin Color Contains Information About the Timing of the Fertile Phase in Free-ranging Macaca mulatta. International Journal of Primatology. 2009;30:777–789. [Google Scholar]
- Dubuc C, Muniz L, Heistermann M, Engelhardt A, Widdig A. Testing the Priority-of-Access model in a seasonally breeding primate species. Behavioral Ecology and Sociobiology. 2011;65:1615–1627. doi: 10.1007/s00265-011-1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo AE. The Rating Of Chess Players, Past & Present. Arco Pub; 1978. [Google Scholar]
- Engelhardt A, Pfeifer JB, Heistermann M, Niemitz C, van Hooff JARAM, Hodges JK. Assessment of female reproductive status by male longtailed macaques, Macaca fascicularis, under natural conditions. Animal Behaviour. 2004;67:915–924. [Google Scholar]
- Engelhardt A, Heistermann M, Hodges JK, Nuernberg P, Niemitz C. Determinants of male reproductive success in wild long-tailed macaques (Macaca fascicularis) -male monopolization, female mate choice or post-copulatory mechanism? Behavioral Ecology and Sociobiology. 2006;59:740–752. [Google Scholar]
- Engelhardt A, Hodges JK, Niemitz C, Heistermann M. Female sexual behavior, but not sex skin swelling, reliably indicates the timing of the fertile phase in wild long-tailed macaques (Macaca fascicularis) Hormones and Behavior. 2005;47:195–204. doi: 10.1016/j.yhbeh.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S. An {R} Companion to Applied Regression. Sage; Thousand Oaks CA: 2011. [Google Scholar]
- Fürtbauer I, Heistermann M, Schülke O, Ostner J. Concealed Fertility and Extended Female Sexuality in a Non-Human Primate (Macaca assamensis) PLoS ONE. 2011;6:e23105. doi: 10.1371/journal.pone.0023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürtbauer I, Schülke O, Heistermann M, Ostner J. Reproductive and Life History Parameters of Wild Female Macaca assamensis. International Journal of Primatology. 2010;31:501–517. doi: 10.1007/s10764-010-9409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C, Shimizu K, Huffman M. Relationship between sexual interactions and the timing of the fertile phase in captive female Japanese macaques (Macaca fuscata) American Journal of Primatology. 2009;71:868–879. doi: 10.1002/ajp.20717. [DOI] [PubMed] [Google Scholar]
- Geary DC. Evolution and proximate expression of human paternal investment. Psychological Bulletin. 2000;126:55–77. doi: 10.1037/0033-2909.126.1.55. [DOI] [PubMed] [Google Scholar]
- Gouzoules S, Gouzoules H. Kinship. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. University of Chicago Press; Chicago: 1987. pp. 299–305. [Google Scholar]
- Greenwood PJ. Mating Systems, Philopatry and Dispersal in Birds and Mammals. Animal Behaviour. 1980;28:1140–1162. [Google Scholar]
- Gubernick DJ, Teferi T. Adaptive significance of male parental care in a monogamous mammal. Proceedings of the Royal Society B: Biological Sciences. 2000;267:147–150. doi: 10.1098/rspb.2000.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistermann M, Ziegler T, van Schaik CP, Launhardt K, Winkler P, Hodges JK. Loss of oestrus, concealed ovulation and paternity confusion in free-ranging Hanuman langurs. Proceedings of the Royal Society B: Biological Sciences. 2001;268:2445–2451. doi: 10.1098/rspb.2001.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Brent LJN, Dubuc C, Accamando AK, Engelhardt A, Gerald MS, Heistermann M, Stevens M. Color signal information content and the eye of the beholder: a case study in the rhesus macaque. Behavioral Ecology. 2010;21:739–746. doi: 10.1093/beheco/arq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. Social relationships between adult male and immature rhesus macaques. Primates. 1986;27:425–440. [Google Scholar]
- Hrdy SB. Infanticide among animals: A review, classification, and examination of implications for the reproductive strategies of females. Ethology and Sociobiology. 1979;1:13–40. [Google Scholar]
- Huchard E, Alvergne A, Féjan D, Knapp L, Cowlishaw G, Raymond M. More than friends? Behavioural and genetic aspects of heterosexual associations in wild chacma baboons. Behavioral Ecology and Sociobiology. 2010;64:769–781. [Google Scholar]
- Huchard E, Charpentier MJ, Marshall H, King AJ, Knapp LA, Cowlishaw G. Paternal effects on access to resources in a promiscuous primate society. Behavioral Ecology. 2013;24:229–236. [Google Scholar]
- Jones JS, Wynne-Edwards KE. Paternal behaviour in biparental hamsters, Phodopus campbelli, does not require contact with the pregnant female. Animal Behaviour. 2001;62:453–464. [Google Scholar]
- Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program cervus accommodates genotyping error increases success in paternity assignment. Molecular Ecology. 2007;16:1099–1106. doi: 10.1111/j.1365-294X.2007.03089.x. [DOI] [PubMed] [Google Scholar]
- Kaufman AB, Rosenthal R. Can you believe my eyes? The importance of interobserver reliability statistics in observations of animal behaviour. Animal Behaviour. 2009;78:1487–1491. [Google Scholar]
- Kazem AJN, Widdig A. Visual Phenotype Matching: Cues to Paternity Are Present in Rhesus Macaque Faces. PLoS ONE. 2013;8:e55846. doi: 10.1371/journal.pone.0055846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddy Hector AC, Seyfarth RM, Raleigh MJ. Male paternal care, female choice and the effect of an audience in vervet monkeys. Animal Behaviour. 1989;38:262–271. [Google Scholar]
- Kulik L, Muniz L, Mundry R, Widdig A. Patterns of interventions and the effect of coalitions and sociality on male fitness. Molecular Ecology. 2012;21:699–714. doi: 10.1111/j.1365-294X.2011.05250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Boesch C. Sexual Differences in Chimpanzee Sociality. International Journal of Primatology. 2008;29:65–81. doi: 10.1007/s10764-007-9230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Yin HB, Wang QS. Seasonality of reproduction and sexual activity in female Tibetan macaques (Macaca thibetana) at Huangshan, China. Acta Zoologica Sinica. 2005;51:365–375. [Google Scholar]
- Lindburg DG. Rhesus monkeys: mating season mobility of adult males. Science. 1969;166:1176–1178. doi: 10.1126/science.166.3909.1176. [DOI] [PubMed] [Google Scholar]
- Lindburg DG. The rhesus monkeys in North India: An ecological and behavioral study. In: Rosenblum LA, editor. Primate behavior. Academic Press; New York: 1971. pp. 1–106. [Google Scholar]
- Maestripieri D. Costs and benefits of maternal aggression in lactating female rhesus macaques. Primates. 1994;35:443–453. [Google Scholar]
- Makin JW, Porter RH. Paternal behavior in the spiny mouse (Acomys cahirinus) Behavioral and Neural Biology. 1984;41:135–151. doi: 10.1016/s0163-1047(84)90513-2. [DOI] [PubMed] [Google Scholar]
- Manson JH. Measuring female mate choice in Cayo Santiago rhesus macaques. Animal Behaviour. 1992;44:405–416. [Google Scholar]
- Ménard N, Segesser F, Scheffrahn W, Pastorini J, Vallet D, Gaci B, Martin RD, Gautier-Hion A. Is male-infant caretaking related to paternity and/or mating activities in wild Barbary macaques (Macaca sylvanus)? Comptes rendus de l’Académie des Sciences III Vie. 2001;324:601–610. doi: 10.1016/s0764-4469(01)01339-7. [DOI] [PubMed] [Google Scholar]
- Moscovice LR, Di Fiore A, Crockford C, Kitchen DM, Wittig R, Seyfarth RM, Cheney DL. Hedging their bets? Male and female chacma baboons form friendships based on likelihood of paternity. Animal Behaviour. 2010;79:1007–1015. [Google Scholar]
- Moscovice LR, Heesen M, Di Fiore A, Seyfarth R, Cheney D. Paternity alone does not predict long-term investment in juveniles by male baboons. Behavioral Ecology and Sociobiology. 2009;63:1471–1482. doi: 10.1007/s00265-009-0781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Animal Behaviour. 2011;82:911–921. [Google Scholar]
- Onyango PO, Gesquiere LR, Altmann J, Alberts SC. Testosterone positively associated with both male mating effort and paternal behavior in savanna baboons (Papio cynocephalus) Hormones and Behavior. 2012 doi: 10.1016/j.yhbeh.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostner J, Chalise MK, Koenig A, Launhardt K, Nikolei J, Podzuweit D, Borries C. What hanuman langur males know about female reproductive status. American Journal of Primatology. 2006;68:701–712. doi: 10.1002/ajp.20260. [DOI] [PubMed] [Google Scholar]
- Paul A, Kuester J, Arnemann J. The sociobiology of male-infant interactions in Barbary macaques, Macaca sylvanus. Animal Behaviour. 1996;51:155–170. [Google Scholar]
- Quinn GP, Keough MJ. Experimental design and data analysis for biologists. Cambridge University Press; 2002. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- Rawlins RG, Kessler MJ, editors. The Cayo Santiago Macaques. History, behavior and biology. State University of New York Press; Albany: 1986. [Google Scholar]
- Ribble D, Perrin M. Social organization of the Eastern Rock Elephant-shrew (Elephantulus myurus)3: the evidence for mate guarding. Belgian Journal of Zoology. 2005;135:167–173. [Google Scholar]
- Santos CV, Martins MM. Parental care in the buffy-tufted-ear marmoset (Callithrix aurita) in wild and captive groups. Revista Brasileira de Biologia. 2000;60:667–672. doi: 10.1590/s0034-71082000000400018. [DOI] [PubMed] [Google Scholar]
- Sherman PW. Mate guarding as paternity insurance in Idaho ground squirrels. Nature. 1989;338:418–420. doi: 10.1038/338418a0. [DOI] [PubMed] [Google Scholar]
- Silk JB, Short J, Roberts J, Kusnitz J. Gestation length in rhesus macaques (Macaca mulatta) International Journal of Primatology. 1993;14:95–104. [Google Scholar]
- Stoehr AM. Are significance thresholds appropriate for the study of animal behaviour? Animal Behaviour. 1999;57:F22–F25. doi: 10.1006/anbe.1998.1016. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone, Paternal Behavior, and Aggression in the Monogamous California Mouse (Peromyscus californicus) Hormones and Behavior. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aldine; Chicago: 1972. pp. 139–179. [Google Scholar]
- van Horn RC, Wahaj SA, Holekamp KE. Role-reversed nepotism among cubs and sires in the spotted hyena. Ethology. 2004;110:413–426. [Google Scholar]
- van Schaik CP, Paul A. Male care in primates: does it ever reflect paternity? Evolutionary Anthropology. 1996;5:152–156. [Google Scholar]
- van Schaik CP, Janson CH, editors. Infanticide by males and its implications. Cambridge University Press; Cambridge: 2000. [Google Scholar]
- Widdig A, Bercovitch FB, Streich WJ, Sauermann U, Nürnberg P, Krawczak M. A longitudinal analysis of reproductive skew in male rhesus macaques. Proceedings Biological Sciences / The Royal Society. 2004;271:819–826. doi: 10.1098/rspb.2003.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdig A, Nürnberg P, Bercovitch FB, Trefilov A, Berard JB, Kessler MJ, Schmidtke J, Streich WJ, Krawczak M. Consequences of group fission for the patterns of relatedness among rhesus macaques. Molecular Ecology. 2006a;15:3825–2832. doi: 10.1111/j.1365-294X.2006.03039.x. [DOI] [PubMed] [Google Scholar]
- Widdig A, Streich WJ, Nürnberg P, Croucher PJP, Bercovitch FB, Krawczak M. Paternal kin bias in the agonistic interventions of adult female rhesus macaques (Macaca mulatta) Behavioral Ecology and Sociobiology. 2006b;61:205–214. [Google Scholar]
- Widdig A, Nürnberg P, Krawczak M, Streich WJ, Bercovitch FB. Paternal relatedness and age proximity regulate social relationships among adult female rhesus macaques. Proceedings of the National Academy of Sciences. 2001;98:13769–13773. doi: 10.1073/pnas.241210198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Meter PEV, Wallen K. Factors Regulating the Timing of Puberty Onset in Female Rhesus Monkeys (Macaca mulatta): Role of Prenatal Androgens, Social Rank, and Adolescent Body Weight. Biology of Reproduction. 2005;72:1087–1094. doi: 10.1095/biolreprod.104.027755. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Tannenbaum PL, Jones B, Wallen K. Peak occurrence of female sexual initiation predicts day of conception in rhesus monkeys (Macaca mulatta) Reproduction Fertility and Development. 2000;12:397–404. doi: 10.1071/rd00080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.