Abstract
Pharmacological and surgical interventions that increase glucagon-like peptide 1 (GLP-1) action are effective to improve glucose homeostasis in type 2 diabetes mellitus. In light of this, nutritional strategies to enhance postprandial GLP-1 secretion, particularly in the context of diet-induced obesity, may provide an alternative therapeutic approach. Importantly, recent evidence suggests the amino acid l-arginine, a well-known insulin secretagogue, can also stimulate release of GLP-1 from isolated rat intestine. Here we tested the hypothesis that oral l-arginine acts as a GLP-1 secretagogue in vivo, to augment postprandial insulin secretion and improve glucose tolerance. To test this, we administered l-arginine or vehicle by oral gavage, immediately prior to an oral glucose tolerance test in lean and diet-induced obese mice. In both lean and obese mice oral l-arginine increased plasma GLP-1 and insulin and substantially improved glucose clearance. To directly assess the contribution of GLP-1 receptor (GLP-1R)-signaling to these improvements, l-arginine was given to Glp1r knockout mice and their wild-type littermates. In this experiment oral l-arginine significantly augmented insulin secretion and improved glucose clearance in WT mice, but not in Glp1r knockout littermates. Taken together these findings identify l-arginine as a GLP-1 secretagogue in vivo and demonstrate that improvement of glucose tolerance by oral l-arginine depends on GLP-1R-signaling. These findings raise the intriguing possibility that l-arginine-based nutritional and/or pharmaceutical therapies may benefit glucose tolerance by improving the postprandial GLP-1 response in obese individuals.
Glucagon-like peptide 1 (GLP-1) is a hormone secreted from enteroendocrine L-cells into the hepatic portal circulation in response to the ingestion of nutrients (1). GLP-1 acts at its receptor (GLP-1R) to augment glucose-stimulated insulin secretion from pancreatic β-cells and is essential for normal oral glucose tolerance (reviewed in Refs. 2 and 3). Therefore, developing strategies to enhance GLP-1 secretion from intestinal L cells could provide alternative therapies for type 2 diabetes mellitus (T2DM). Like carbohydrates and fats, dietary protein stimulates GLP-1 secretion (4). Moreover, oral administration of the amino acid glutamine potently induces the release of GLP-1, thereby augmenting insulin secretion in obese- and type 2 diabetic subjects (5). Thus, at least one individual amino acid can improve oral glucose tolerance by increasing endogenous GLP-1 secretion.
It is well established that the amino acid l-arginine is a potent insulin secretagogue (6). Moreover, increasing evidence supports that dietary supplementation with l-arginine improves glycemic control in multiple species (7). However, no studies have, to our knowledge, investigated a potential contribution of the GLP-1 system to the benefits of l-arginine for glucose metabolism. In the present study we tested the hypothesis that l-arginine is a GLP-1 secretagogue in vivo, and moreover that GLP-1R signaling contributes to l-arginine-mediated improvements in insulin secretion and oral glucose tolerance.
Materials and Methods
Animals
Male C57BL/6 mice were housed in an AAALAC-approved facility with a 12-hour light, 12-hour dark cycle and allowed free access to water and standard chow or 60% high-fat diet (D12492 HFD; Research Diets), unless otherwise noted. Diet-induced obese (DIO) mice were maintained on HFD for 5–6 months prior to testing. Global Glp1r knockout (KO) mice (Glp1rCMVKO) were generated by crossing a floxed Glp1r mouse line with CMVcre mice, followed by outbreeding of the CMVcre as previously described (8). Functional KO was validated as reported elsewhere (8). All animal procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Oral glucose tolerance tests in lean and DIO mice
To investigate the role of l-arginine on glucose tolerance in both lean and obese, insulin-resistant individuals, we delivered l-arginine or vehicle orally to lean and DIO mice prior to an oral glucose tolerance test (OGTT). Mice were fasted for 6 hours, weighed, and separated into weight-matched groups. At −15 minutes, mice received an oral gavage containing 1 g/kg · body weight (bw) l-arginine or saline (0.9% NaCl) in a 300-μL volume. Although it is difficult to precisely address the physiologic relevance of this dose, we note that a similar dose of oral l-glutamine is known to act as a GLP-1 secretagogue and to improve glucose tolerance in diabetic rats (9). At 0 minutes, mice received an oral gavage containing 2 g/kg · bw dextrose. Glucose was measured in blood collected from the tip of the tail vein using AccuChek glucose meters and strips (Roche) at baseline and at 0, 15, 30, 60, and 120 minutes.
Plasma GLP-1 response to oral l-arginine in lean mice
Blood for GLP-1 measurement was collected from 6-hour fasted mice. After obtaining a basal blood sample from the tip of the tail vein, mice were given an oral dose of l-arginine (1 g/kg · bw), and a second blood sample was obtained at 15 minutes. Whole blood (75 μL) was collected into chilled tubes containing 10 μL of antiproteolytic cocktail (4.65 g EDTA + 93 mg aprotinin + 40 000 U of heparin in 50 mL saline). Plasma was stored at −80°C until further analysis.
Plasma GLP-1 and insulin response in DIO mice
Blood for GLP-1 and insulin measurements was collected using a balanced crossover design conducted over 2 test days in 6-hour fasted mice. Hence each animal received both l-arginine (1 g/kg · bw) and the saline control. At −15 minutes, mice received an oral gavage containing l-arginine or saline. At 0 minutes, mice received an oral gavage containing 2 g/kg · bw dextrose. Whole blood (75 μl) was collected from the tip of the tail vein into chilled tubes containing 10 μL of antiproteolytic cocktail (4.65 g EDTA + 93 mg aprotinin + 40000 U of heparin in 50 mL saline), at −15, 0, and 15 minutes. Plasma was stored at −80°C until further analysis.
Oral glucose tolerance test and l-arginine-induced insulin release in Glp1r KO and WT mice
We performed an OGTT in chow-fed Glp1r KO and wild-type (WT) mice, as described above. l-Arginine-induced insulin release in chow-fed, lean Glp1r KO and WT mice was assessed in 6-hour fasted animals with free access to water. After obtaining a basal blood sample from the tail vein, mice were given an oral dose of l-arginine (1 g/kg bw), and a second blood sample was obtained at 15 minutes.
Measurements of GLP-1 and insulin
Commercially available kits were used to measure plasma total GLP-1 (MesoScale Discovery) and insulin (ELISA, Crystal Chem), per the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using GraphPad Prism Version 5.0c (GraphPad Software) or SigmaStat 3.1 (SYSTAT). Data were analyzed by repeated measures ANOVA (RM ANOVA) with Tukey's post hoc test or by paired, two-tailed Student's t test, as appropriate. In all cases α = 0.05. Results are presented as mean ± SEM.
Results
Oral l-arginine improves glucose tolerance and increases plasma GLP-1 and insulin
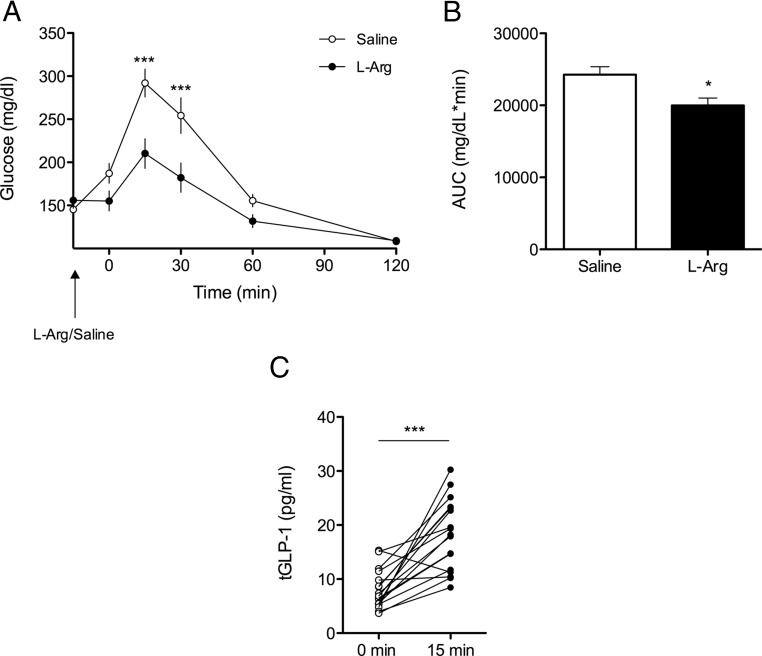
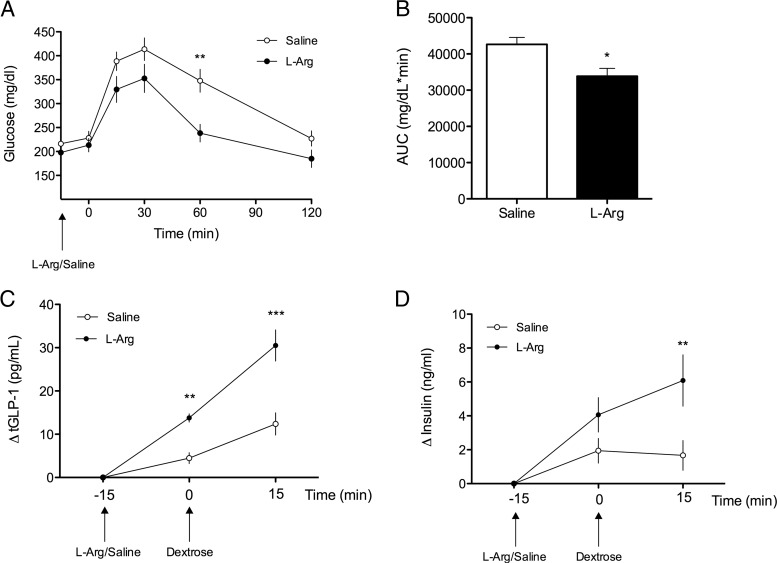
In both lean and DIO mice, oral administration of l-arginine 15 minutes prior to an OGTT significantly improved glucose tolerance relative to controls (Figure 1, A and B; Figure 2, A and B). Coincident with this, oral l-arginine elicited GLP-1 secretion in both lean and DIO mice. Among lean mice, oral administration of l-arginine significantly increased total plasma GLP-1 (tGLP-1) within 15 minutes (Figure 1C) (P < .001, 8.0 ± 0.9 pg/mL at 0 minutes, 18.2 ± 1.6 pg/mL at 15 minutes). Likewise, among DIO mice, the plasma total GLP-1 response (ΔtGLP-1) was significantly increased 15 minutes following oral administration of l-arginine (P < .01, 7.6 ± 0.9 pg/mL at −15 minutes, 21.4 ± 0.9 pg/mL at 0 minutes), compared with oral saline [8.5 ± 0.8 pg/mL at −15 minutes, 13.0 ± 1.8 pg/mL at 0 minutes]. This was further enhanced by an oral gavage of dextrose (P < .001, 38.1 ± 3.8 pg/mL vs. 20.9 ± 3.0 pg/ml at 15 minutes) (Figure 2C). Consistent with the improved glucose tolerance the plasma insulin response (Δinsulin) to 2 g/kg · bw oral dextrose was significantly enhanced by prior administration of l-arginine relative to saline in DIO mice [4.6 ± 1.2 pg/mL at −15 minutes, 8.3 ± 1.6 pg/mL at 0 minutes, 10.7 ± 1.5 pg/mL at 15 minutes, P < .01 and 6.3 ± 1.4 pg/mL at −15 minutes, 8.2 ± 1.7 pg/mL at 0 minutes, 7.9 ± 1.6 pg/mL at 15 minutes, respectively] (Figure 2D).
Figure 1.
l-Arginine Improves Glucose Tolerance in Mice. A, Blood glucose following oral l-arginine (1 g/kg) or saline to lean C57bl/6 mice 15 minutes before an OGTT (2 g glucose/kg · bw) (2-way repeated measures ANOVA, n = 7). B, Glucose area under the curve (AUC) (t test). C, Plasma GLP-1 (total) levels before and 15 minutes following oral gavage of l-arginine (1 g/kg) (paired t test, n = 17). Data presented as mean ± SEM. *, P < .05; ***, P < .001.
Figure 2.
l-Arginine Induces GLP-1 Secretion and Improves Glucose Tolerance in DIO Mice. A, Blood glucose following oral gavage of l-arginine (1g/kg) or saline to DIO C57bl/6 mice 15 minutes before an OGTT (2 g glucose/kg · bw) (2-way repeated measures ANOVA, n = 4–5). B, Glucose area under the curve (AUC) (t test). C, Plasma total-GLP-1 response (ΔtGLP-1) to oral gavage of l-arginine or saline 15 minutes prior to an OGTT (2 g dextrose/kg · bw) in DIO mice (2-way repeated measures ANOVA, n = 9). D, Plasma insulin response (Δinsulin) following oral gavage of l-arginine or saline 15 minutes prior to an OGTT (2 g glucose/kg · bw) in DIO mice (2-way repeated measures ANOVA, n = 9). Data presented as mean ± SEM. *, P < .05; **, P < .01; ***, P < .001.
Effect of oral l-arginine on insulin levels and glucose tolerance in Glp1r-deficient mice
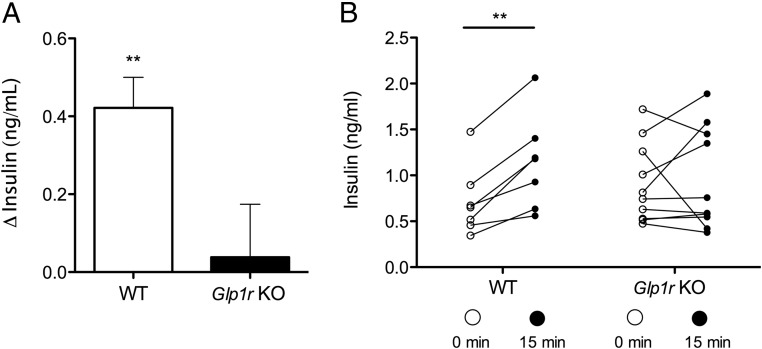
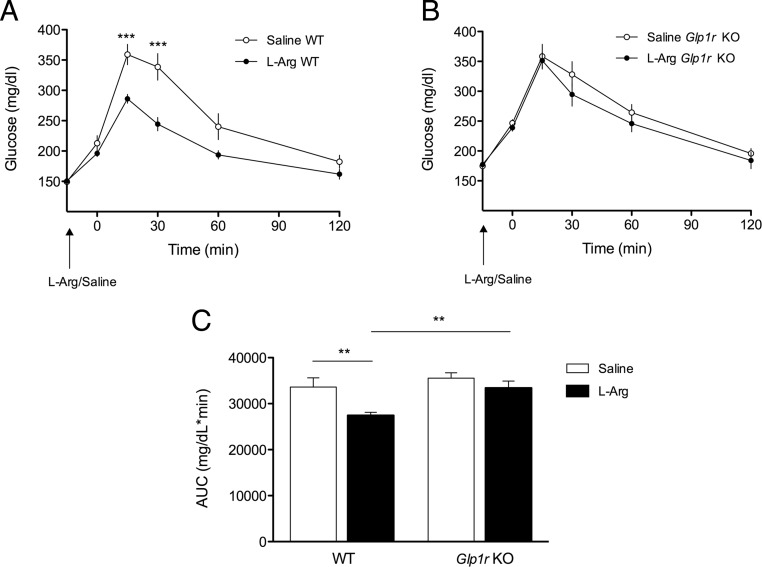
Assessment of plasma insulin levels in chow-fed lean Glp1r KO and WT mice following oral gavage of l-arginine revealed a significant increase in plasma insulin in WT mice 15 minutes following l-arginine administration (P < .01, 0.7 ± 0.1 pg/mL at 0 minutes, 1.1 ± 0.2 pg/mL at 15 minutes) (Figure 3, A and B) but no effect of l-arginine on plasma insulin levels in Glp1r KO mice (0.9 ± 0.1 pg/mL at 0 minutes, 1.0 ± 0.2 pg/mL at 15 minutes) (Figure 3, A and B). Oral gavage of l-arginine 15 minutes prior to an OGTT significantly improved glucose tolerance in WT mice (P < .01) (Figure 4, A and C), but had no significant effect on glucose tolerance in Glp1r KO mice (Figure 4, B and C). Although we observed no significant impairment in oral glucose tolerance associated with Glp1r deficiency per se in this experiment, consistent with previous reports (10), a mild impairment was observed when this cohort of mice was challenged with a higher dose of glucose (2.5 g/kg · bw) (data not shown).
Figure 3.
The GLP-1R Is Necessary for l-Arginine-Induced Insulin Secretion. A, Mean change of plasma insulin from baselines to 15 minutes after l-arginine administration (paired t test). B, Individual plasma insulin levels before and 15 minutes following oral gavage of l-arginine in lean WT and Glp1r KO mice (paired t test, n = 7–9). Data presented as mean ± SEM. **, P < .01.
Figure 4.
The GLP-1R Is Necessary for l-Arginine-Induced Improvements in Glucose Tolerance. Blood glucose following oral gavage of l-arginine (1 g/kg) or saline to lean WT (A) and Glp1r KO (B) mice 15 minutes before an OGTT (2 g glucose/kg · bw) (2-way repeated measures ANOVA, n = 8–9). C, Glucose area under the curve (AUC) (2-way repeated measures ANOVA, n = 8–9). Data presented as mean ± SEM. **, P < .01; ***, P < .001.
Discussion
The present data demonstrate that oral administration of l-arginine acutely improves glucose tolerance in both lean and DIO mice. Moreover, l-arginine potently increases plasma concentrations of GLP-1, suggesting that GLP-1R signaling may contribute to its insulinotropic action. Consistent with this possibility, in mice with genetic disruption of Glp1r, the effect of l-arginine to induce insulin secretion and to improve glucose tolerance is significantly blunted. These findings reveal a novel role for l-arginine to improve glucose tolerance indirectly, via GLP-1 action, and may have implications for the treatment of T2DM.
Previous studies investigating the possibility that l-arginine acts as a GLP-1 secretagogue, either in vitro (11) or ex vivo (12), have generated conflicting results. First, Reimann et al. (11) investigated the individual effects of multiple l-α-amino acids to stimulate GLP-1 secretion from GLUTag cells, an immortalized murine enteroendocrine cell line that expresses GLP-1. They reported that l-glutamine, but not l-arginine, potently stimulates GLP-1 secretion. Conversely, Mace et al (12) reported that l-arginine elicits GLP-1 release from isolated loops of rat small intestine (12). Together with the present observation that l-arginine elicits GLP-1 release in vivo, these data suggest the effect of l-arginine on the L cell may require an intact intestinal environment or that GLUTag cells do not mimic L cells in all aspects. Additional studies will be necessary to parse the mechanisms by which l-arginine elicits GLP-1 secretion and the contribution of the extracellular environment. Relevant to this, we did not observe a significant effect of d-arginine to elicit GLP-1 secretion (data not shown), suggesting the metabolism of l-arginine or stereo-specific interaction with a receptor or a transporter may be necessary for its effects. Similar to other nutrients, in vivo l-α-amino acid-mediated enteroendocrine release of incretin hormones is likely to involve both G protein-coupled receptor-dependent and -independent, pathways (12–14).
The diminished effect of l-arginine to elicit insulin secretion and improve oral glucose tolerance in Glp1r KO mice implicates GLP-1 signaling in the β-cell response to this amino acid. Whereas acute iv administration of l-arginine has a direct effect to stimulate islet hormone secretion (15, 16), the effect of oral l-arginine on glucose tolerance and insulin release requires intact GLP-1 signaling and thus appears to be indirect. The GLP-1 incretin effect has traditionally been ascribed to effects on intestinal L cells elicited by ingested carbohydrate, but the findings reported here support the involvement of gut hormones to link protein ingestion with insulin secretion. This has also been recently suggested in studies of humans consuming meals of specific macronutrient composition (17). Also, we have recently reported that long-term dietary supplementation with l-arginine improves glucose metabolism in mice exposed to a low-protein diet (18). Additional studies will be necessary to determine the contribution of the GLP-1 system to the benefits of this chronic intervention and to further explore a potential physiological role for this amino acid in glucose metabolism.
Several effective pharmaceutical treatments for T2DM target the GLP-1 system, by either inhibiting its rapid clearance by dipeptidyl-peptidase IV or using dipeptidyl-peptidase IV-resistant GLP-1 mimetics (2). Developing strategies to enhance GLP-1 secretion from intestinal L cells, particularly in the context of DIO, could provide an alternative approach. The present findings raise the possibility that either dietary supplementation with l-arginine and/or l-arginine-based therapies may be effective to increase GLP-1 secretion in humans. Such an intervention would have the benefit of increasing GLP-1 concentration locally near GLP-1R-positive afferent neurons, thought to contribute to the metabolic benefits of GLP-1 (19, 20).
In summary, our data demonstrate a novel role for oral l-arginine to regulate glucose metabolism indirectly by inducing GLP-1 release. Additionally, we demonstrate a pronounced effect of oral l-arginine to enhance glucose clearance in both lean and obese animals, underlining that nutritional-based strategies aiming to improve endogenous GLP-1 release may provide an alternative therapeutic approach to aid metabolic disorders.
Acknowledgments
We thank Dana Buesing, Todd Greer, Radhakrishna Krishna, William Li, and Sonia Lipp for expert technical assistance.
C.C., S.S., and H.B.O. were supported by the UNIK: Food Fitness and Pharma for Health and Disease research program; S.S. was supported by the Carlsberg foundation; H.B.O. was supported by the Novo Nordisk Foundation; S.C.W. was supported by National Institutes of Health Grant DK017844; R.J.S. was supported by National Institutes of Health Grant DK093848; K.K.R. was supported by National Institutes of Health Grant HL111319.
Disclosure Summary: D.A.D. consults for Amylin, Lilly, Novo Nordisk, and Zealand and has research support from Ethicon Endo-Surgery and MannKind; R.J.S. has consultancies, research support, or is a paid speaker with the following companies: Ethicon Endo-Surgery/Johnson & Johnson, Novo Nordisk, Merck, Novartis, Angiochem, Zafgen, Takeda, Ablaris, Pfizer, Eli Lilly, Eisai, Boehringer-Ingelheim, and Zealand Pharma.
Footnotes
- bw
- body weight
- DIO
- diet-induced obese/obesity
- GLP-1
- glucagon-like peptide 1
- GLP-1R
- GLP-1 receptor
- KO
- knockout
- OGTT
- oral glucose tolerance test
- T2DM
- type 2 diabetes mellitus
- WT
- wild type.
References
- 1. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2(8571):1300–1304 [DOI] [PubMed] [Google Scholar]
- 2. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439 [DOI] [PubMed] [Google Scholar]
- 3. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157 [DOI] [PubMed] [Google Scholar]
- 4. Ma J, Stevens JE, Cukier K, et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care. 2009;32(9):1600–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greenfield JR, Farooqi IS, Keogh JM, et al. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89(1):106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Floyd JC, Jr., Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45(9):1487–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKnight JR, Satterfield MC, Jobgen WS, et al. Beneficial effects of L-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids. 2010;39(2):349–357 [DOI] [PubMed] [Google Scholar]
- 8. Wilson-Perez HE, Chambers AP, Ryan KK, et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide-1 receptor deficiency. Diabetes. 2013;62(7):2380–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Badole SL, Bagul PP, Mahamuni SP, et al. Oral L-glutamine increases active GLP-1 (7-36) amide secretion and improves glycemic control in stretpozotocin-nicotinamide induced diabetic rats. Chem Biol Interact. 2013;203(2):530–541 [DOI] [PubMed] [Google Scholar]
- 10. Hansotia T, Baggio LL, Delmeire D, et al. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes. 2004;53(5):1326–1335 [DOI] [PubMed] [Google Scholar]
- 11. Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47(9):1592–1601 [DOI] [PubMed] [Google Scholar]
- 12. Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012;590(Pt 12):2917–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oya M, Kitaguchi T, Pais R, Reimann F, Gribble F, Tsuboi T. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J Biol Chem. 2013;288(7):4513–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15(4):421–431 [DOI] [PubMed] [Google Scholar]
- 15. Palmer JP, Walter RM, Ensinck JW. Arginine-stimulated acute phase of insulin and glucagon secretion. I. in normal man. Diabetes. 1975;24(8):735–740 [DOI] [PubMed] [Google Scholar]
- 16. Smajilovic S, Clemmensen C, Johansen LD, et al. The L-α-amino acid receptor GPRC6A is expressed in the islets of Langerhans but is not involved in L-arginine-induced insulin release. Amino Acids. 2013;44(2):383–390 [DOI] [PubMed] [Google Scholar]
- 17. Carrel G, Egli L, Tran C, et al. Contributions of fat and protein to the incretin effect of a mixed meal. Am J Clin Nutr. 2011;94(4):997–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clemmensen C, Madsen AN, Smajilovic S, Holst B, Bräuner-Osborne H. L-Arginine improves multiple physiological parameters in mice exposed to diet-induced metabolic disturbances. Amino Acids. 2012;43(3):1265–1275 [DOI] [PubMed] [Google Scholar]
- 19. Vahl TP, Tauchi M, Durler TS, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148(10):4965–4973 [DOI] [PubMed] [Google Scholar]
- 20. Williams DL. Minireview: finding the sweet spot: peripheral versus central glucagon-like peptide 1 action in feeding and glucose homeostasis. Endocrinology. 2009;150(7):2997–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]