Abstract
IGF-binding protein (IGFBP)-2 overexpression confers resistance to high-fat feeding and inhibits the differentiation of preadipocytes in vitro. However, whether administration of IGFBP-2 can regulate adipogenesis in vivo and the domains that mediate this response have not been defined. IGFBP-2 contains 2 heparin-binding domains (HBD), which are localized in the linker region (HBD1) and C-terminal region (HBD2) of IGFBP-2. To determine the relative importance of these domains, we used synthetic peptides as well as mutagenesis. Both HBD1 and HBD2 peptides inhibited preadipocyte differentiation, but the HBD2 peptide was more effective. Selective substitution of charged residues in the HBD1 or HBD2 regions attenuated the ability of the full-length protein to inhibit cell differentiation, but the HBD2 mutant had the greatest reduction. To determine their activities in vivo, pegylated forms of each peptide were administered to IGFBP-2−/− mice for 12 weeks. Magnetic resonance imaging scanning showed that only the HBD2 peptide significantly reduced (48 ± 9%, P < .05) gain in total fat mass. Both inguinal (32 ± 7%, P < .01) and visceral fat (44 ± 7%, P < .01) were significantly decreased by HBD2 whereas HBD1 reduced only visceral fat accumulation (24 ± 5%, P < .05). The HBD2 peptide was more effective peptide in reducing triglyceride content and serum adiponectin, but only the HBD2 peptide increased serum leptin. These findings demonstrate that the HBD2 domain of IGFBP-2 is the primary region that accounts for its ability to inhibit adipogenesis and that a peptide encompassing this region has activity that is comparable with native IGFBP-2.
The bioavailability of IGF-I and IGF-II is modulated by high-affinity IGF-binding proteins (IGFBPs), which regulate ligand transport and bioavailability. IGFBP-2 is the second most abundant IGFBP in human circulation (1), and it is the principal form of IGFBP secreted by white preadipocytes during adipogenesis (2). Epidemiologic studies have shown an association between IGFBP-2 and metabolic homeostasis. IGFBP-2 levels in humans correlate inversely with body mass index, adiposity, plasma insulin, and markers of insulin resistance (3–6). In addition, IGFBP-2 inhibits adipogenesis in 3T3-L1 cells in vitro as evidenced by a reduction in the number of lipid-laden cells and reduced expression of the adipocyte marker proteins peroxisome proliferator-activated receptor gamma (PPARγ) and adipocyte protein 2 (aP2) (7). Overexpression of IGFBP-2 in mice led to reduced susceptibility to diet-induced obesity and improved insulin sensitivity (7). In contrast, IGFBP-2 knockout mice (IGFBP-2−/−) are heavier and have a greater increase in percent body fat compared with wild-type (WT) littermates at 16 weeks of age (8).
Although previous studies suggest that IGFBP-2 inhibits adipogenesis, whether it directly inhibits differentiation of nonimmortalized preadipocytes isolated from animals and the specific domains within IGFBP-2 that mediate this effect have not been determined. Members of the IGFBP family exhibit 67%–70% structural homology. However, many of the physiological effects of the individual binding proteins are distinct (9). The greatest homology among the 6 forms of IGFBPs is contained in the N- and C-terminal regions. The N-terminal region contains the primary IGF-I binding site, whereas the C-terminal region facilitates IGF-I binding and accounts for the ability of several members of the family to bind to extracellular matrix (10). A heparin-binding domain (HBD) has been identified in the C-terminal region IGFBP-2, IGFBP-3, and IGFBP-5, whereas a arginine glycine aspartic acid (RGD) sequence is present in IGFBP-1 and IGFBP-2 (11). In addition to C-terminal HBD (referred hereafter as HBD2), IGFBP-2 contains a unique HBD that is located in the linker region (referred hereafter as HBD1). Functional studies have shown that C-terminal HBD within IGFBP-3 and IGFBP-5 can bind to extracellular matrix proteins (10, 12), which has been proposed to mediate both IGF-dependent and IGF-independent actions (13), whereas RGD sequence has been shown to be responsible for IGFBP-1 (14) and IGFBP-2 (15) binding the α5β1 integrin, and this mediates cell migration (14). A synthetic peptide containing the HBD1 sequence stimulated osteoblast proliferation, increased trabecular bone mass, and reduced bone resorption in IGFBP-2−/− mice (16). However, the roles of the HBD1 and HBD2 domains in altering adipogenesis have not been determined. Therefore, the present study was undertaken to determine whether IGFBP-2 could inhibit preadipocyte differentiation and to define the relative importance of the HBD1 and HBD2 domains in regulating this effect. Similarly, an in vivo study was performed to determine whether peptides containing these sequences could inhibit fat mass acquisition.
Materials and Methods
More detailed methods can be found in Supplemental materials and methods, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Generation of synthetic peptides and peptides pegylation
The synthetic peptide containing the HBD1 domain of mouse IGFBP-2 (CKHLSLEEPKKLRP), a scrambled HBD1 peptide (CKPLRLSKEEHPLK) (HBD1 control peptide), the HBD2 domain of human IGFBP-2 (CKHGLYNLKQCKMSLNGQR), and the C-terminal HBD of IGFBP-5 (RKGFYKRKQCKPSRGRKR) (HBD2 control peptide) were synthesized by the Protein Chemistry Core Facility at the University of North Carolina at Chapel Hill. HBD1 and HBD2 peptides that did not contain the N-terminal cysteine were also prepared. Purity and sequence identity were confirmed by mass spectrometry. HBD1, HBD2, and HBD1 control peptides (that each contained the N-terminal cysteine) were pegylated following a procedure described in Supplemental materials and methods.
Generation of pLenti-IGFBP-2 WT, 2 HBDs mutants, and non-IGF-I binding mutant
The WT mouse IGFBP-2 amplified from pCMV-SPORT6 (American Type Culture Collection) was inserted into the pENTR/D-TOPO vector and was used as a template to make the substitution mutants. The 2 IGFBP-2 mutants incorporated substitutions of amino acids within the HBD1 domain containing the sequence 188KHLSLEEPKKLR199 and HBD2 domain of IGFBP-2 containing the sequence 243KHGLYNLKQCKMSLNGQR260. The substitutions, highlighted in bold, were as follows: AALSLEEPAALA (HBD1 mutant) and AAGLYNAAQCAMSLNGQA (HBD2 mutant), respectively. The QuikChange Site-Directed Mutagenesis kit (Agilent Technologies) was used to incorporate the base changes needed to encode these substitutions. The non-IGF-I binding mutant form of IGFBP-2 was prepared as described previously (17).
Purification of WT, non-IGF-I binding mutant, and 2 HBD mutant forms IGFBP-2
The constructs were expressed in CHO-K1 cells as described previously (18). Conditioned medium was collected from confluent CHO-K1 cells expressing WT IGFBP-2 or non-IGF-I binding mutant or the HBD1 mutant or HBD2 mutant that had been maintained in serum-free α-MEM for 48 hours. The expressed proteins were purified as described previously (19). To determine IGF-I binding capacity of non-IGF-I binding IGFBP-2, an IGF-I binding assay was performed following a procedure described previously (20).
Cell culture of primary preadipocytes
Preadipocytes were isolated from epididymal fat pads of IGFBP-2−/− mice as previously described (2). The cultures had the medium changed every 2 days until they reached confluency. Two-day postconfluent cells were then exposed to differentiation medium (serum-free DMEM containing 0.5 mM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone, and 5 μg/mL insulin) and incubated for 2 days. Experimental treatments that were added to this medium included either WT IGFBP-2 (3 μg/mL), HBD1 peptide (6 μg/mL), HBD1 control peptide (6 μg/mL), HBD2 peptide (6 μg/mL), HBD2 control peptide (6 μg/mL), HBD1 mutant IGFBP-2 (3 μg/mL), the HBD2 mutant IGFBP-2 (3 μg/mL), or non-IGF-I binding mutant IGFBP-2 (3 μg/mL). Cultures were maintained for 2 additional days in the standard medium supplemented with 5-μg/mL insulin. Thereafter, the cultures were kept in the fresh standard medium without insulin for additional 2 days.
Oil Red O staining
Cells were rinsed with PBS and then fixed with 10% formalin for 30 minutes; 100% propylene glycol (Poly Scientific) was added and incubated for 5 minutes, and cultures were incubated for 10 minutes at 60°C with Oil Red O (Poly Scientific) and then with 80% propylene glycol for 5 minutes. Images were captured using an Olympus IX81 inverted microscope, and results were quantified using ImageJ (NIH, version 1.45S).
Immunoprecipitation and immunoblotting
Differentiated adipocytes were lysed in ice-cold lysis buffer (21), and solubilized proteins were quantified (Thermo Scientific). Equal protein amounts of lysates were loaded onto a sodium dodecyl sulfate-polyacrylamide gel, and the proteins were separated, then transferred to an Immobilon filter and visualized by immunoblotting using 1:1000 for antiadiponectin (Affinity BioReagents), 1:500 for anti-PPARγ (Cell Signaling Technology, Inc), 1:2000 for anti-aP2 (ProSci, Inc), and 1:5000 for the anti-β-actin antibody (Sigma Chemical Co). The immune complexes were visualized using enhanced chemiluminescence (Thermo Fischer Scientific).
Mice
The mice, B6.129-Igfbp-2tm1Jep (referred to as Igfbp-2−/− mice), prepared as described previously (8, 22), were backcrossed onto C57BL/6J background for at least 10 generations. Igfbp-2+/+ mice were C57BL/6J controls. All in vivo and ex vivo experimental studies were performed using male mice. The animal study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of University of North Carolina at Chapel Hill. Mice were assigned to one of 3 treatment groups: 1) Peg HBD1 peptide (N = 8), 2) Peg HBD2 peptide (N = 10), and 3) control peptide (N = 18). Igfbp-2−/− mice were administered 50 μg of each pegylated peptide in 0.1-mL PBS. Igfbp-2+/+ mice (N = 8) given 0.1 mL of PBS served as controls (WT). All injections were administered ip 3 times weekly from 10 to 22 weeks of age. All mice were provided with free access to 2018 Teklad global rodent diet (Harlan), containing 18.6% protein, 6.2% fat, and 3.5% crude fiber. Food consumption and the weights of the mice were determined weekly.
Body composition and serum adipokine measurement
Body fat and lean mass were measured at weeks 0 and 12 of treatment by magnetic resonance imaging (MRI) analysis (EchoMRI-100; Echo Medical Systems), using unanesthetized animals (23). After 12 weeks of treatment, the mice were euthanized, and blood was collected by cardiac puncture and centrifuged at 3500 rpm × 15 minutes. The serum was stored at −20°C. Abdominal inguinal and visceral fat pads were dissected from each animal according to defined anatomical landmarks. Subcutaneous fat between the rib cage and the upper thigh was termed sc inguinal fat, whereas all fat from the lesser curvature of the stomach to the sigmoid colon was termed visceral fat. Fat depots were blotted dry before weighing. The right inguinal fat pad was dissected separately, and the triglyceride content of this tissue was determined by a colorimetric analysis (Pointe Scientific) as previously described (22). Serum adiponectin and leptin were measured by ELISA following manufacture's instructions (Millipore).
Glucose tolerance test
For oral glucose tolerance tests after a 4-hour fast, mice were given 20% glucose (2.5 g/kg body weight) by oral gavage and blood obtained from the tail vein at baseline, 15, 30, 60, 90, and 120 minutes (Bayer Contour Glucometer).
Statistical analysis
All data are expressed as the mean ± SEM. Results were analyzed for statistically significant differences using Student's t test for the data obtained from in vitro assays or ANOVA followed by Bonferroni multiple comparison post hoc test for the data obtained from in vivo assays. In addition, repeated measures-ANOVA was used where appropriate. Statistical significance was set at P < .05.
Results
IGFBP-2 and HBD1 or HBD2 peptides inhibit differentiation of IGFBP-2−/− preadipocytes
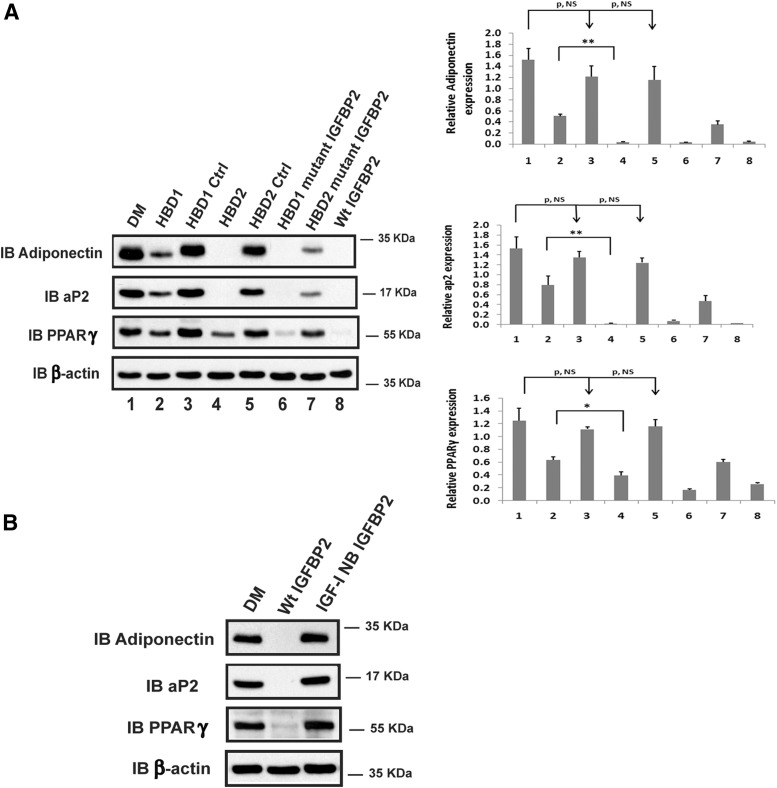
To investigate the effects of each of the HBDs on adipogenesis, peptides that contained the HBD1 or HBD2 sequences were incubated with cultures of preadipocytes that had been isolated from IGFBP-2 null mice. Native IGFBP-2 inhibited preadipocyte differentiation into mature adipocytes as indicated by suppression of 3 differentiation markers adiponection, aP2 and PPARγ (Figure 1A, lane 8). The HBD1 peptide significantly inhibited adiponectin (eg, 66 ± 10% reduction, P < .01), aP2 (eg, 51 ± 2% reduction, P < .01), and PPARγ expression (eg, 47 ± 9% reduction, P < .05) (Figure 1A, lane 2 vs 1). The control HBD1 peptide had no effect (Figure 1A, lane 3 vs 1). Interestingly, the HBD2 peptide completely inhibited the expression of adiponectin and aP2, and PPARγ expression was reduced 67 ± 7% (P < .01) (Figure 1A, lane 4 vs 1). A peptide containing the homologous region of IGFBP-5 did not alter their expression (Figure 1A, lane 5 vs 1). IGFBP-2 mutants in which the charged residues in either the HBD1 or HBP2 domains were changed to neutral residues were tested. The IGFBP-2 mutant containing the altered HBD1 sequence inhibited differentiation marker expression as well as WT IGFBP-2 (Figure 1A, lane 6 vs 8). In contrast, the IGFBP-2 mutant containing the altered HBD2 residues but an intact HBD1 sequence was significantly less effective in preventing preadipocyte differentiation (Figure 1A, lane 7 vs 6). When the ability to form differentiated adipocytes was determined, similar results were obtained (Figure 2). In addition, a non-IGF-I binding mutant form of IGFBP-2 (17), which had a more than 5000-fold reduction in IGF-I binding capacity, was used. The results showed that this mutant had no inhibitory effect on the differentiation of preadipocytes (Figure 1B). These results demonstrate that native IGFBP-2, the HBD1 mutant, and the HBD2 peptide were the most potent inhibitors of adipogenesis, whereas the HBD1 peptide and HBD2 mutant had less activity. Taken together, the results strongly suggest that the inhibitory effect of IGFBP-2 on the differentiation of preadipocyte is mediated primarily via its HBD2 domain and also requires the presence of its IGF-I binding capacity.
Figure 1.
IGFBP-2 and its HBDs inhibit the differentiation of preadipocytes isolated from IGFBP-2 null mice. (A) Primary preadipocytes from IGFBP-2−/− mice were isolated from inguinal fat pads following the procedure described in Materials and Methods. Two days after reaching confluence, cells were treated with differentiation medium (DM) (lanes 1–8). This medium was then supplemented with HBD1 peptide (HBD1, lane 2), scrambled HBD1 peptide (HBD1 Ctrl [Control], lane 3), HBD2 peptide (HBD2, lane 4), IGFBP-5 C-terminal HBD peptide (HBD2 Ctrl, lane 5), IGFBP-2 protein with mutated HBD1 sequence (HBD1 mutant IGFBP-2, lane 6), IGFBP-2 with mutated HBD2 sequence (HBD2 mutant IGFBP-2, lane 7), or native IGFBP-2 (WT IGFBP-2, lane 8). The cell lysates were immunoblotted (IB) with antiadiponectin, aP2, and PPARγ antibodies, respectively. As a loading control, the blots were IB with an anti-β-actin antibody. Quantitative analysis of the results from 3 separate experiments was performed, and the results are expressed in relation to β-actin expression. Each value represents mean ± SE. *, P < .05 and **, P < .01 denotes a significant difference between 2 treatments. P, NS indicates no significant difference between 2 treatments. (B) Preadipocytes isolated from IGFBP-2−/− mice were treated with either DM, DM plus WT IGFBP-2 (Wt IGFBP-2), or DM plus IGF-I nonbinding IGFBP-2 (IGF-I NB IGFBP-2). The cell lysates were IB with antiadiponectin, aP2, and PPARγ antibodies, respectively. As a loading control, the blots were IB with an anti-β-actin antibody.
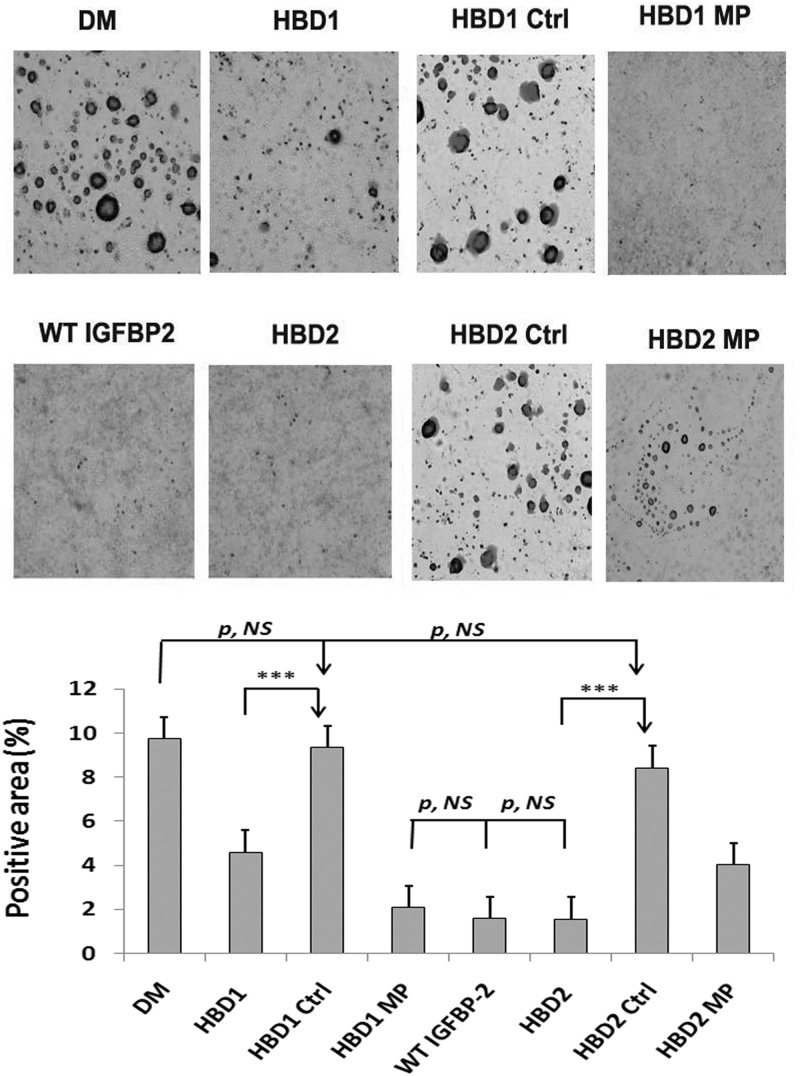
Figure 2.
Oil Red O staining of IGFBP-2−/− preadipocytes exposed to IGFBP-2, IGFBP-2 mutants, or peptides containing the different HBDs. Primary preadipocytes from IGFBP-2−/− mice were cultured in the standard medium. Two days after they reached confluence, cells were treated with either with differentiation medium (DM) alone or this medium plus with a peptide containing IGFBP-2 HBD1 sequence (HBD1), a peptide containing the scrambled HBD1 sequence (HBD1 Ctrl [Control]), a mutant form of IGFBP-2 containing a substituted HBD1 sequence (HBD1 MP [mutated protein]), WT bovine IGFBP-2 (WT IGFBP-2), a peptide containing IGFBP-2 HBD2 sequence (HBD2), a peptide containing the IGFBP-5 C-terminal HBD sequence (HBD2 Ctrl), or a mutant form of IGFBP-2 containing a substituted HBD2 sequence (HBD2 MP). After 48 hours, the media were changed to standard medium plus 0.5 mM insulin. The media were changed to standard medium after additional 48 hours. The cultures were stained with Oil Red O following the procedure described in Materials and Methods. The results were quantified using ImageJ and expressed as Oil Red O positive area (pixels) divided by whole area (pixels). ***, P < .001 indicates the significant differences between 2 treatments. P, NS, indicates no significant difference between 2 treatments. A representative image of 3 independent experiments is shown.
The HBD1 and HBD2 peptides decrease weight gain in IGFBP-2−/− mice
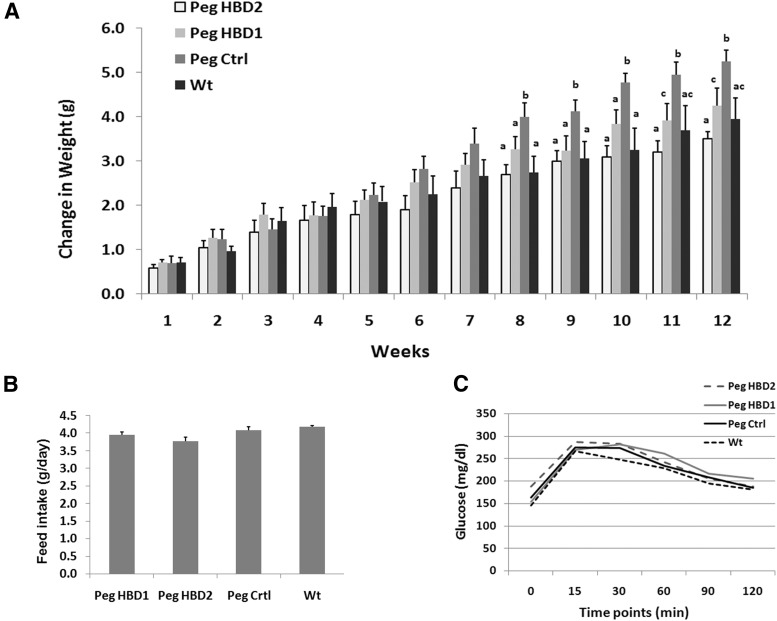
To investigate the effects of IGFBP-2 HBDs on weight gain and fat mass in vivo, the peptides containing each HBD sequence were administered to IGFBP-2−/− mice. Because both control peptides had a similar effect on preadipocyte differentiation in vitro, only the scrambled HBD1 peptide was used as a control. To extend the half life of the peptides and increase their resistance to proteolysis in vivo, all 3 peptides were pegylated. We have previously shown that 50 μg of HBD1 peptide administered via ip injection 5 times per week for 3 weeks promoted bone growth in IGFBP-2−/− male mice (16). We modified the previous protocol and injected 50 μg of each peptide (ip) 3 times per week for 12 weeks. Age-matched WT C57BL/6J (WT) male mice were treated with vehicle and compared with the 3 groups of mice that received peptide treatments. Because this study required a large number of IGFBP-2−/− mice, the experiment was separated into 2 phases. In the first phase, HBD1 and control peptide treatments as well as WT control mice were studied. In the second phase, the effects of the HBD2 and control peptides were determined. The weights and ages of the mice in each group in each phase were similar at the start of treatment. The results showed that the IGFBP-2−/− mice receiving the control peptide gained more weight during the 12 weeks compared with the control WT mice (Figure 3A). During the study interval, the IGFBP-2−/− mice treated with the HBD1 or HBD2 peptide gained significantly less weight after 8 weeks of treatment compared with mice treated with the control peptide. This difference persisted in the HBD-treated mice, and after 12 weeks of treatment, the differences remained significant (Figure 3A). In addition, after 10 weeks, weight gain was significantly less in the mice that received HBD2 peptide, compared with the mice that received HBD1 peptide (Figure 3A). Importantly, the gain of body weight in WT control mice was similar to the IGFBP-2−/− mice treated with either the HBD1 or HBD2 peptides, respectively (Figure 3A). To exclude the possibility that differences in weight gain were caused by differences in food intake, we measured the food consumption weekly. The results showed no significant difference among all treatments (Figure 3B). In addition, oral glucose tolerance tests showed no significant differences among different treatments (Figure 3C).
Figure 3.
The HBDs of IGFBP-2 suppress weight gain in IGFBP-2−/− mice but do not affect glucose metabolism and food intake. IGBPP-2−/− mice were treated with either a pegylated synthetic peptide containing IGFBP-2 HBD1sequence (PegHBD1, n = 8), the IGFBP-2 HBD2 sequence (PegHBD2, n = 10), or a Pegylated synthetic control peptide (Peg Ctrl, n = 18) following the procedures described in Materials and Methods for 12 weeks. WT mice (n = 8) were injected with PBS. (A) Average body weight gain of mice at weekly time points was calculated for each group. (B) Average daily of feed intake was calculated for each group. (C) Oral glucose tolerate tests were performed in all groups of mice after 12 weeks of treatment following a procedure described in Materials and Methods. Each bar value represents mean ± SE. Different letters represent significant differences between 2 treatments.
The HBDs of IGFBP-2 inhibit body fat mass accumulation and change serum adipokine concentrations
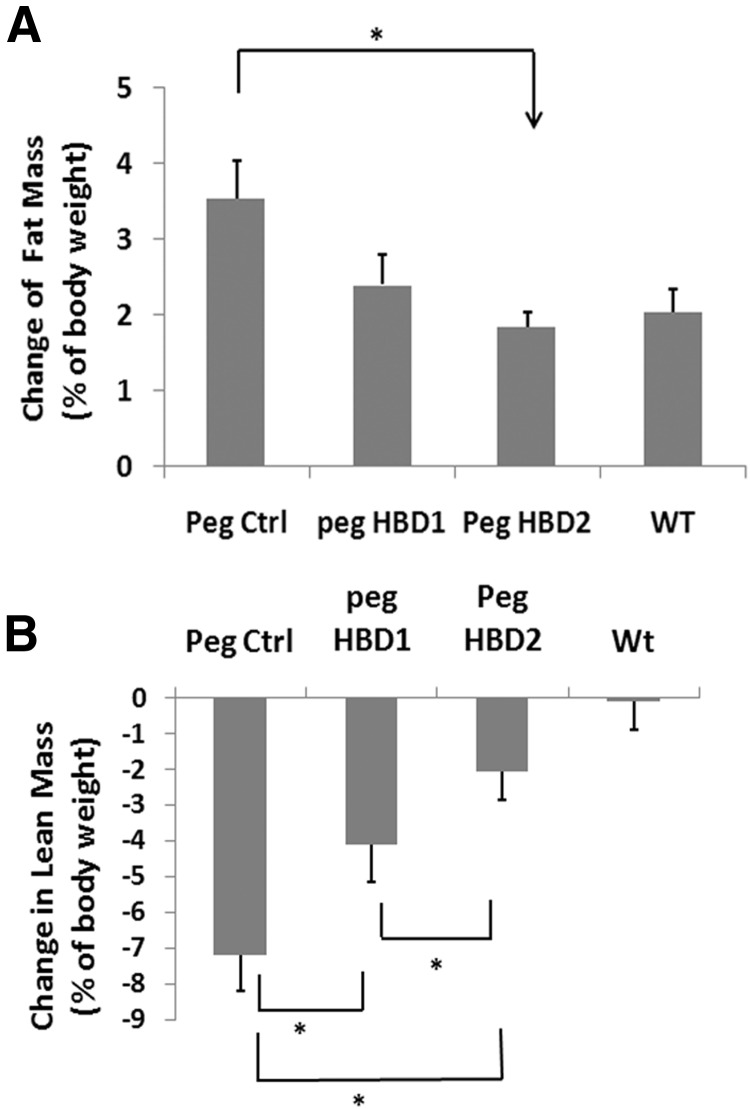
To examine the changes in body composition, MRI was performed on all mice at the beginning and the end of study. After 12 weeks of treatment, both HBD peptides reduced the fat mass gain, but only HBD2 reached statistical difference compared with control peptide treatment (eg, 0.58 ± 0.12 vs 1.19 ± 0.35 g increase; P < .05) (Table 1). When the change of fat mass was expressed as a change in fat expressed as a percentage of body weight, the same result was obtained. Only the HBD2 peptide treatment resulted in a significant decrease body fat mass gain over the study interval (eg, 48 ± 9% reduction; P < .05, compared with control) (Figure 4A). HBD2 peptide treatment was also associated with significant preservation of lean mass compared with control peptide (eg, lean mass increase: 3.10 ± 0.57 vs 1.85 ± 0.49 g; P < .05) (Table 1). When the lean mass change was expressed as a percentage of body weight, the results showed that IGFBP-2−/− mice treated with the control peptide experienced a relatively greater lean mass change (eg, 7.2 ± 1.0% relative reduction) compared with mice treated with either HBD peptide, although HBD2 peptide was more effective (eg, 4.1 ± 1.1% relative reduction for HBD 1, 2.1 ± 0.8% relative reduction for HBD2; P < .05) (Figure 4B).
Table 1.
Fat Mass and Lean Mass Increase After Treatments
| Treatments | Fat Mass Increase (g) | Lean Mass Increase (g) |
|---|---|---|
| Peg Ctrl | 1.19 ± 0.35 | 1.85 ± 0.49 |
| Peg HBD1 | 0.82 ± 0.17 | 2.51 ± 0.35 |
| Peg HBD2 | 0.58 ± 0.12a | 3.10 ± 0.57a |
| WT | 0.71 ± 0.15 | 2.21 ± 0.40 |
Data were expressed as mean ± SE.
P < .05 when peg HBD2 treatment is compared with peg control peptide (Peg Ctrl) treatment.
Figure 4.
The HBDs of IGFBP-2 suppress body fat mass gain and prevent the loss of body lean mass in IGFBP-2−/− mice. IGFBP-2−/− mice were treated as described in the legend to Figure 3. Body fat and lean mass from each group were analyzed using Echo MRI scanning at weeks 0 and 12, respectively. The changes in total fat mass or lean mass between weeks 0 and 12 in each group are presented as the changes in fat or lean mass expressed as a percentage of body weight (A and B). Each bar value represents mean ± SE. *, P < .05 indicates a significant difference between 2 treatments.
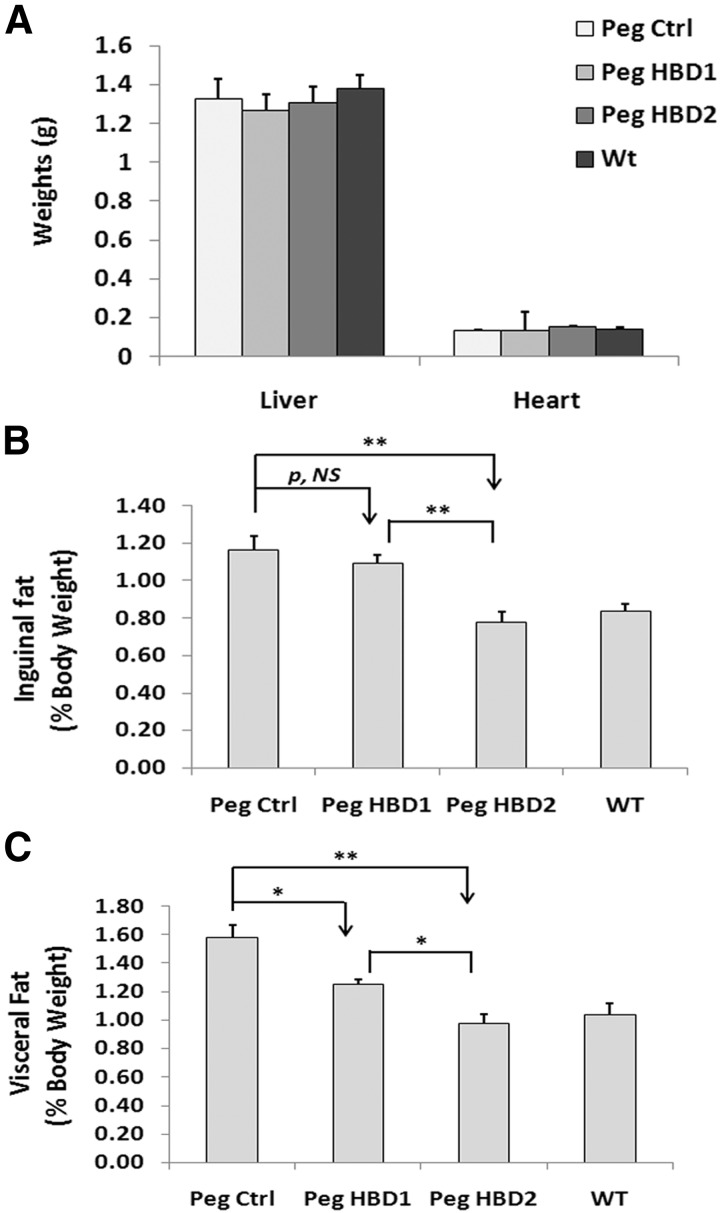
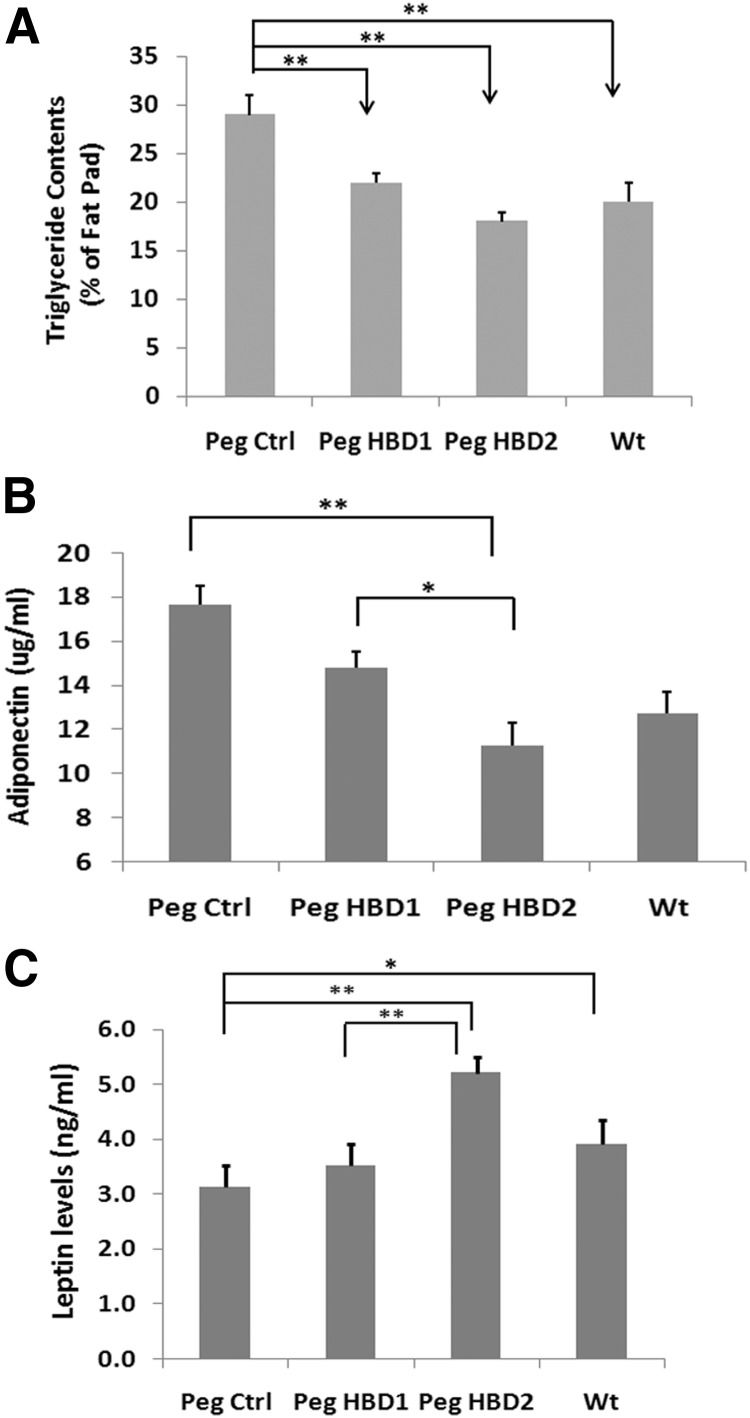
To confirm the effects on fat mass, after completion of 12 weeks of treatment, the mice were euthanized, and 2 body fat compartments were weighed. The results showed no significant difference in the liver and heart weight among different treatments (Figure 5A), which excluded the possibility that organ weight change contributed to the treatment related body weight changes. Fat compartment analysis showed that the IGFBP-2−/− mice given the control peptide had the greatest inguinal and visceral fat content among all groups. The HBD2 peptide significantly reduced the inguinal fat pad weight compared with IGFBP-2−/− mice treated with the control peptide (eg, 0.23 ± 0.02 vs 0.34 ± 0.03 g; P < .05), whereas the HBD1 peptide had no effect (Table 2). A similar result was obtained when the inguinal fat pad expressed as a percentage of body weight is compared among the treatments (eg, 32 ± 7% difference with HBD2 treatment when compared with control peptide; P < .01) (Figure 5B). The absolute visceral fat weight was significantly reduced in mice treated with either HBD1 or HBD2 peptide compared control peptide treatment, but the HBD2 peptide was more effective (eg, 0.25 ± 0.02 g for HBD2 and 0.34 ± 0.01 g for HBD1 vs 0.46 ± 0.04 g for control; P < .05) (Table 2). Similar results were detected when the changes were expressed as a percentage of body weight (eg, 44 ± 7% difference between HBD2 and 24 ± 5% difference between HBD1 and control; P < .05) (Figure 5C). Interestingly, when the weights of both fat compartments were expressed as a percentage of body weight, the results in IGFBP-2−/− mice treated with HBD2 peptide were similar to the WT mice (Figure 5, B and C). When adipocyte sizes were compared among treatments, no significant differences were detected (eg, 12399 ± 1408, 12406 ± 748, 11967 ± 716, and 12422 ± 831 pixels/cell for control peptide, HBD1, HBD2, and WT, respectively; P value, not significant). Analysis of the triglyceride content in the inguinal fat pad showed that it was significantly reduced in IGFBP-2−/− mice treated with either HBD1 or HBD2 peptide, but HBD2 was more effective (eg, 37 ± 9% vs 22 ± 2% reduction, P < .05) (Figure 6A). In addition, the triglyceride content in WT mice was significantly less than that of IGFBP-2−/− mice treated a control peptide (eg, a 31 ± 5% less; P < .01) (Figure 6A). The mean serum adiponectin level was significantly lower with the HBD2 peptide treatment compared with either the mice treated with the control peptide (eg, 36 ± 4%; P < .01) or with HBD1 peptide (eg, 24 ± 5% reduction; P < .05) (Figure 6B). Treatment with the HBD2 peptide significantly increased serum leptin level compared with a control peptide-treated mice (eg, 57 ± 9% increase; P < .01), whereas the HBD1 peptide had no effect (Figure 6C). The serum leptin level in WT mice was also higher than that of control peptide-treated IGFBP-2−/− mice (Figure 6C). Taken together, these results demonstrate that both HBD1 and HBD2 peptides are able to inhibit adipogenesis, but the HBD2 peptide is a more potent inhibitor.
Figure 5.
The HBDs of IGFBP-2 suppress inguinal fat and visceral fat development in IGFBP-2−/− mice. IGFBP-2−/− mice were treated as described in the legend of Figure 3. At the time of euthanasia, the liver and heart of each mouse were collected and weighed (A). The inguinal fat and visceral fat were dissected following a procedure described in Materials and Methods and weighed. The results are shown as the inguinal fat or visceral fat mass expressed as a percentage of body weight (B and C). Each bar value represents mean ± SE. *, P < .05 and **, P < .01 indicate the significant differences between 2 treatments. P, NS, indicates no significant difference between 2 treatments. Peg Ctrl, pegylated control.
Table 2.
Changes in Inguinal and Visceral Fat Content After Treatments
| Treatments | Inguinal Fat (g) | Visceral Fat (g) |
|---|---|---|
| Peg Ctrl | 0.34 ± 0.03 | 0.46 ± 0.04 |
| Peg HBD1 | 0.30 ± 0.02 | 0.34 ± 0.01a |
| Peg HBD2 | 0.23 ± 0.02a | 0.25 ± 0.02a |
| WT | 0.25 ± 0.01 | 0.31 ± 0.08 |
Data were expressed as mean ± SE.
P < .05 when treatment is compared with peg control peptide (Peg Ctrl) treatment.
Figure 6.
The HBDs of IGFBP-2 decrease fat pad triglyceride content and serum adiponectin levels, whereas HBD2 stimulates serum leptin in IGFBP-2−/− mice. IGFBP-2−/− mice were treated as described in the legend of Figure 3. At the end of week 12, blood was collected from each mouse before they were euthanized. (A) Triglyceride levels in the right inguinal fat pad were measured following the procedure described in Materials and Methods. Serum adiponectin (B) and leptin levels (C) were measured following manufacturer's instructions. Each bar values were expressed as mean ± SE. *, P < .05 and **, P < .01 indicate significant differences between 2 treatments. Peg Ctrl, pegylated control.
Discussion
Studies in genetically modified mice show a relationship between IGFBP-2 expression and acquisition of fat mass. Transgenic overexpression resulted in no change in lean mass, but the mice were resistant to high-fat feeding and gained less weight than control animals (7). Conversely, deletion of the IGFBP-2 gene resulted in animals that had a greater fat mass (eg, 41% increase) compared with control littermates at 16 weeks of age (8). Continued monitoring of these animals up to 1 year showed that they continued their accelerated weight gain and subsequently developed glucose intolerance and hyperinsulinemia (24). More recent studies in which IGFBP-2 was overexpressed in leptin-deficient mice or diet-induced obese mice showed that IGFBP-2 not only resulted in a reduction in fat mass but also improved glucose tolerance and hyperinsulinemia (25). In that study, IGFBP-2 resulted in a decrease in food intake and stabilization of body weight. However, the IGFBP-2 levels were more than 6000 ng/mL. Therefore, it is difficult to directly compare these results with the response of WT mice, who normally have levels in the range of 60–106 ng/mL. An additional study (26) showed that IGFBP-2 overexpression resulted in decreased muscle mass, growth retardation, and increased fat mass. These animals were produced on a mixed background, and the serum concentration of IGFBP-2 was not reported. Therefore, it is difficult to compare these results with other published studies that used mice with a homogeneous background and had results that were consistent across studies (7, 8, 24, 25).
Previous studies have shown that IGFBP-2 inhibits the differentiation of 3T3-L1 cells in vitro (7), although another study that used a lower concentration of IGFBP-2 (eg, 750 ng/mL) showed no effect (27). This difference could be due to using a concentration or other differences in experiment conditions. Our findings extend that observation to show that WT IGFBP-2 inhibits differentiation of nonimmortalized, diploid preadipocytes derived from IGFBP-2−/− mice. Because preadipocytes synthesize IGFBP-2 (28, 29), our findings in cells derived from IGFBP-2−/− mice represent a rigorous test of the hypothesis that exogenous addition of IGFBP-2 inhibits preadipocyte differentiation. To determine whether IGFBP-2 could alter adipogenesis in vivo, we used synthetic peptides. IGFBP-2 has a complex disulfide bonding pattern. Therefore, expression in mammalian cells is required, and obtaining sufficient material to be able to treat these animals for 12 weeks would be difficult. Therefore, we determined whether synthetic peptides that contained sequences derived from 2 HBDs retained the ability to inhibit preadipocyte differentiation.
Four domains within IGFBP-2 have been characterized. The IGFBD is contained within a hydrophobic pocket in the N terminus (although residues in the C-terminal domain contribute to high affinity binding) (30). Substitutions of 4 hydrophobic amino acids in this domain reduced IGF-I binding capacity more than 5000-fold. Both IGFBP-1 and IGFBP-2 contain an RGD sequence. This sequence mediates IGFBP-1 binding to the α5β1 integrin (14), but the function of this sequence in IGFBP-2 has been controversial. Some investigators have concluded that it mediates attachment to the α5β1 integrin, whereas others have been unable to confirm this finding (9). The third region that has been studied is the HBD in the C terminus. A region of homologous sequence has been extensively studied in IGFBP-3 and IGFBP-5 and has been shown to mediate attachment to extracellular matrix and cell surfaces (31). Mutagenesis of charged residues in this region altered the biologic activity of both IGFBP-3 and IGFBP-5 (9). This region also mediates IGFBP-3 and IGFBP-5 binding to acid labile subunit, which forms a stable ternary complex with IGF-I or IGF-II whose primary function is to transport IGF-I and IGF-II in plasma (32, 33). Mutagenesis studies have shown that this region within IGFBP-2 contributes to IGF binding (13). A fourth region that has been studied is a HBD contained in the linker region of the protein. Importantly, this region is unique for IGFBP-2 and is not contained in any other form of IGFBP. Russo et al (34) showed that mutation of this region resulted in the inability of IGF-I to stimulate glioma cell migration in vitro. Our laboratory showed that mutagenesis of this region resulted in failure of IGFBP-2 to be able to stimulate osteoblast proliferation in vitro and cartilage explant growth ex vivo (16). Furthermore, when a peptide containing this sequence was administered to IGFBP-2−/− mice, it resulted in substantial improvement in bone mineral density, including increased trabecular number and thickness (16). Therefore, this region appears to be critical for the ability of IGFBP-2 to enhance bone formation.
The studies reported here demonstrate that mutagenesis of charged residues in the HBD1 region resulted in loss of the ability of IGFBP-2 to inhibit preadipocyte differentiation in vitro, and a peptide containing this region resulted in 31% inhibition of fat mass acquisition. In contrast, a peptide containing the sequence within the C-terminal HBD was a more potent inhibitor of preadipocyte differentiation, and an IGFBP-2 mutant that had the charged residues in that region substituted with alanine had reduced ability to inhibit differentiation. The HBD2 peptide was 1.8-fold more potent than the HBD1 peptide in inhibiting preadipocyte differentiation. This is the first report of a biologic function of this domain. The mechanistic explanation for this difference will require additional investigation.
These findings were confirmed in in vivo studies in IGFBP-2−/− mice. Administration of the HBD2 peptide significantly inhibited the gain of fat mass over a 12-week period compared with mice that had received a control peptide. Although the HBD1 peptide also had a significant effect, the response to the HBD2 peptide was significantly greater. We conclude that the HBD2 peptide retains its superior potency in vivo and that both peptides can be used as a surrogate for native IGFBP-2. The treatments had no significant effect on food intake, but the study was not powered adequately to detect the small difference that we observed (eg, 8%). The peptide might increase energy expenditure, but this was not measured. The changes in fat mass over this interval did not result in a change in glucose tolerance. However, that was not unexpected, because assessment of glucose tolerance at the same age in IGFBP-2−/− mice showed no difference between the −/− animals and controls (24). The divergence in these findings and those reported by Hedbacker et al (25) may be due to the difference in serum IGFBP-2 concentrations. The levels in their study were 6000 ng/mL or 175 nM, whereas our estimate of the concentrations of the peptides in serum is that they ranged between 7.7 nM and 19 nM, which is similar to WT IGFBP-2 (eg, 7.6 nM–15.3 nM). This 9-fold difference in serum levels could account for the inability of the peptides to induce significant changes in insulin sensitivity. However, because we did not administer the entire protein, we cannot exclude the possibility that some other region within IGFBP-2 is required for enhancement of insulin sensitivity.
Several studies have suggested that there is a relationship between changes in serum IGFBP-2 and obesity and/or insulin resistance. The liver is the primary source of serum IGFBP-2 and excess dietary intake results in inhibition of hepatic production, which is reversed by leptin administration (25, 35). Administration of insulin to rats results in suppression of hepatic IGFBP-2 mRNA expression (36). Multiple investigators have demonstrated that obese preadolescent children have suppressed serum IGFBP-2 concentrations that increase after calorie restriction (37–39). Obese adults have suppressed IGFBP-2 levels, and those levels increase with administration of a low-calorie diet (4, 40–42). In overweight elderly adults, serum IGFBP-2 concentrations correlate with the degree of adiposity (6). Additionally, bilopancreatic diversion surgery is associated with significant increases in serum IGFBP-2 (43). Low IGFBP-2 levels have also been implicated in the metabolic syndrome (44, 45). When many of variables that are associated with obesity and insulin resistance were eliminated, linear regression analysis showed that IGFBP-2 levels correlated inversely with body mass index, serum insulin, and homeostatic model assessment in overweight adults (44, 45). Heald et al (44) found a correlation between the presence of metabolic syndrome and/or type 2 diabetes and low IGFBP-2. Attia et al (46) found a correlation between IGFBP-2 multiple cardiovascular risk factors in adolescents, suggesting that it was a determinant of the metabolic syndrome. Children born small for gestational age show a correlation between IGFBP-2 levels and insulin sensitivity as well as fat mass (47), and these relationships persist when they are studied as young adults (48). IGFBP-2 has also been associated with weight gain over the lifespan. Men who gained the most weight in a cohort followed for 60 years had the lowest mean IGFBP-2 (49). These findings suggest that IGFBP-2 may regulate fat mass accumulation and insulin sensitivity in humans. These studies have not determined whether IGFBP-2 has an inhibitory effect on food consumption. Treatment with the HBD2 peptide increased serum leptin by 57%, which could alter appetite. Therefore, additional studies to determine whether IGFBP-2 functions by decreasing appetite or by increasing energy use will be required.
In contrast to its effects in liver, insulin has been shown to stimulate IGFBP-2 production in differentiated white adipose tissue (2, 50). Quantification of IGFBP-2 mRNA expression in adipose tissue biopsies shows that IGFBP-2 expression was positively correlated with fat mass in prepubertal obese children, but there was no correlation between IGFBP-2 expression in fat and circulating IGFBP-2 (50). Also, there was a negative correlation with insulin sensitivity (50). Analysis of sc fat in adult women showed no differences in IGFBP-2 expression among obese or lean subjects, but it was suppressed in type 2 diabetes and increased with caloric restriction (51). These studies have been interpreted to suggest that insulin stimulates IGFBP-2 production in fat and that this leads to suppression of further preadipocyte differentiation. However, all of these studies have relied on correlations, and none have proved a causal relationship.
There is no definitive information regarding the mechanism by which IGFBP-2 and/or the HBD peptides suppress adipogenesis. We recently showed that the HBD1 peptide binds to receptor-type protein tyrosine phosphatase-β, a receptor tyrosine phosphatase, and inhibits its ability to dephosphorylate PTEN thereby enhancing osteoblast proliferation (19). However, the HBD2 peptide does not bind receptor-type protein tyrosine phosphatase-β and did not have this activity. Two studies have demonstrated that peptides that contain RGD sequences and bind to the α5β1 integrin, thereby stimulating focal adhesion kinase phosphorylation, are capable of inhibiting preadipocyte differentiation (52, 53). Although IGFBP-2 could potentially bind α5β1 through its RGD sequence, no study has demonstrated that binding through the HBD2 domain modulates this response. Because the HBD2 domain is near the RGD sequence, it is possible that it determines access of the RGD sequence to α5β1, but no study has investigated this possibility directly. Another possibility is that HBD2 is binding to a distinct protein that is present in the plasma membrane to mediate its functions. IGF-I binding ability was also required for IGFBP-2 to inhibit preadipocyte differentiation. Because it has been reported that the HBD2 domain of IGFBP-2 is not surface exposed at neutral pH (13), we postulate that IGF-I binding to IGFBP-2 may be required to induce a conformational change that allows surface exposure of this region, thereby facilitating HBD2 domain binding to a membrane receptor. To exclude the possibility that HBD peptides inhibit preadipocyte differentiation by antagonizing IGF-I actions, we conducted experiments where we added IGF-I in the presence of each peptide in the differentiation medium. Because the differentiation medium contains 10−6 M insulin, which is sufficient to activate IGF-I receptor, adding IGF-I had no additional effect on cell differentiation, and therefore, no additional effect of HBD peptides could be directly detected. Because IGFBP-2 or HBD peptides cannot bind insulin, we conclude that IGFBP-2 or the HBD peptides do not inhibit preadipocyte differentiation by preventing IGF-I or insulin binding to their receptors. However, inhibition of IGF-I or insulin receptor signaling is a potential mechanism that has not been excluded.
In summary, our studies demonstrate that both HBDs of IGFBP-2 have some ability to inhibit preadipocyte differentiation. However, the C-terminal HBD is significantly more potent in vitro and in vivo. The effects of this domain were such that administration of the peptide for 12 weeks limited fat mass accumulation to a rate that was similar to the rate that was measured in WT mice. This suggests that this domain can mimic many of the effects of the whole protein in limiting adipogenesis. Because IGFBP-2 levels correlate with the development of adiposity and insulin resistance in humans, our findings suggest that this peptide might not only be useful as a pharmacologic tool to study the role of adiposity in mediating insulin resistance but as a potential treatment modality.
Acknowledgments
We thank Ms Laura Lindsey (University of North Carolina at Chapel Hill) for her help in preparing the manuscript. We also thank the University of North Carolina at Chapel Hill Nutrition Obesity Research Center, which is supported by a National Institutes of Health grant (DK056350) and Dr Lei Li from Dr Rosalind A. Coleman's lab for her help in performing the glucose tolerance tests.
This work was supported by National Institute of Health Grants AR061164 and AG02331.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aP2
- adipocyte protein 2
- HBD
- heparin-binding domain
- IGFBP
- IGF-binding protein
- MRI
- magnetic resonance imaging
- PPARγ
- peroxisome proliferator-activated receptor gamma
- RGD
- arginine glycine aspartic acid
- WT
- wild type.
References
- 1. Chakrabarty S, Kondratick L. Insulin-like growth factor binding protein-2 stimulates proliferation and activates multiple cascades of the mitogen-activated protein kinase pathways in NIH-OVCAR3 human epithelial ovarian cancer cells. Cancer Biol Ther. 2006;5(2):189–197 [DOI] [PubMed] [Google Scholar]
- 2. Boney CM, Moats-Staats BM, Stiles AD, D'Ercole AJ. Expression of insulin-like growth factor-I (IGF-I) and IGF-binding proteins during adipogenesis. Endocrinology. 1994;135(5):1863–1868 [DOI] [PubMed] [Google Scholar]
- 3. Wheatcroft SB, Kearney MT. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends Endocrinol Metab. 2009;20(4):153–162 [DOI] [PubMed] [Google Scholar]
- 4. Nam SY, Lee EJ, Kim KR, et al. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21(5):355–359 [DOI] [PubMed] [Google Scholar]
- 5. Martin RM, Holly JM, Davey Smith G, Gunnell D. Associations of adiposity from childhood into adulthood with insulin resistance and the insulin-like growth factor system: 65-year follow-up of the Boyd Orr Cohort. J Clin Endocrinol Metab. 2006;91(9):3287–3295 [DOI] [PubMed] [Google Scholar]
- 6. Hu D, Pawlikowska L, Kanaya A, et al. Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: the Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2009;57(7):1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wheatcroft SB, Kearney MT, Shah AM, Ezzat VA, Miell JR, Modo M, Williams SC, Cawthorn WP, Medina-Gomez G, Vidal-Puig A, Sethi JK, Crossey PA. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56(2):285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeMambro VE, Clemmons DR, Horton LG, et al. Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology. 2008;149(5):2051–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23(6):824–854 [DOI] [PubMed] [Google Scholar]
- 10. Clemmons DR. Use of mutagenesis to probe IGF-binding protein structure/function relationships. Endocr Rev. 2001;22(6):800–817 [DOI] [PubMed] [Google Scholar]
- 11. Shimasaki S, Ling N. Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6). Prog Growth Factor Res. 1991;3(4):243–266 [DOI] [PubMed] [Google Scholar]
- 12. Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175(1):19–31 [DOI] [PubMed] [Google Scholar]
- 13. Kuang Z, Yao S, Keizer DW, et al. Structure, dynamics and heparin binding of the C-terminal domain of insulin-like growth factor-binding protein-2 (IGFBP-2). J Mol Biol. 2006;364(4):690–704 [DOI] [PubMed] [Google Scholar]
- 14. Jones JI, Gockerman A, Busby WH, Jr, Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the α 5 β 1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci USA. 1993;90(22):10553–10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schütt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW. Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol. 2004;32(3):859–868 [DOI] [PubMed] [Google Scholar]
- 16. Kawai M, Breggia AC, DeMambro VE, et al. The heparin-binding domain of IGFBP-2 has insulin-like growth factor binding-independent biologic activity in the growing skeleton. J Biol Chem. 2011;286(16):14670–14680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeMambro VE, Maile L, Wai C, et al. Insulin-like growth factor-binding protein-2 is required for osteoclast differentiation. J Bone Miner Res. 2012;27(2):390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gockerman A, Prevette T, Jones JI, Clemmons DR. Insulin-like growth factor (IGF)-binding proteins inhibit the smooth muscle cell migration responses to IGF-I and IGF-II. Endocrinology. 1995;136(10):4168–4173 [DOI] [PubMed] [Google Scholar]
- 19. Shen X, Xi G, Maile LA, Wai C, Rosen CJ, Clemmons DR. Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase β and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol Cell Biol. 2012;32(20):4116–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imai Y, Moralez A, Andag U, Clarke JB, Busby WH, Jr, Clemmons DR. Substitutions for hydrophobic amino acids in the N-terminal domains of IGFBP-3 and -5 markedly reduce IGF-I binding and alter their biologic actions. J Biol Chem. 2000;275(24):18188–18194 [DOI] [PubMed] [Google Scholar]
- 21. Xi G, Shen X, Clemmons DR. p66shc negatively regulates insulin-like growth factor I signal transduction via inhibition of p52shc binding to Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 leading to impaired growth factor receptor-bound protein-2 membrane recruitment. Mol Endocrinol. 2008;22(9):2162–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danno H, Jincho Y, Budiyanto S, Furukawa Y, Kimura S. A simple enzymatic quantitative analysis of triglycerides in tissues. J Nutr Sci Vitaminol (Tokyo). 1992;38(5):517–521 [DOI] [PubMed] [Google Scholar]
- 23. Kelly SA, Nehrenberg DL, Hua K, Garland T, Jr, Pomp D. Exercise, weight loss, and changes in body composition in mice: phenotypic relationships and genetic architecture. Physiol Genomics. 2011;43(4):199–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeMambro V, R C, Clemmons D. IGFBP-2 null mice develop obesity and insulin resistance with aging. Growth Horm IGF Res. 2008;18(suppl 1):S26 [Google Scholar]
- 25. Hedbacker K, Birsoy K, Wysocki RW, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11(1):11–22 [DOI] [PubMed] [Google Scholar]
- 26. Rehfeldt C, Renne U, Sawitzky M, Binder G, Hoeflich A. Increased fat mass, decreased myofiber size, and a shift to glycolytic muscle metabolism in adolescent male transgenic mice overexpressing IGFBP-2. Am J Physiol Endocrinol Metab. 2010;299(2):E287–E298 [DOI] [PubMed] [Google Scholar]
- 27. Chan SS, Schedlich LJ, Twigg SM, Baxter RC. Inhibition of adipocyte differentiation by insulin-like growth factor-binding protein-3. Am J Physiol Endocrinol Metab. 2009;296(4):E654–E663 [DOI] [PubMed] [Google Scholar]
- 28. Peter MA, Winterhalter KH, Böni-Schnetzler M, Froesch ER, Zapf J. Regulation of insulin-like growth factor-I (IGF-I) and IGF-binding proteins by growth hormone in rat white adipose tissue. Endocrinology. 1993;133(6):2624–2631 [DOI] [PubMed] [Google Scholar]
- 29. Li Z, Picard F. Modulation of IGFBP2 mRNA expression in white adipose tissue upon aging and obesity. Horm Metab Res. 2010;42(11):787–791 [DOI] [PubMed] [Google Scholar]
- 30. Kuang Z, Yao S, McNeil KA, et al. Cooperativity of the N- and C-terminal domains of insulin-like growth factor (IGF) binding protein 2 in IGF binding. Biochemistry. 2007;46(48):13720–13732 [DOI] [PubMed] [Google Scholar]
- 31. Parker A, Clarke JB, Busby WH, Jr, Clemmons DR. Identification of the extracellular matrix binding sites for insulin-like growth factor-binding protein 5. J Biol Chem. 1996;271(23):13523–13529 [DOI] [PubMed] [Google Scholar]
- 32. Firth SM, Clemmons DR, Baxter RC. Mutagenesis of basic amino acids in the carboxyl-terminal region of insulin-like growth factor binding protein-5 affects acid-labile subunit binding. Endocrinology. 2001;142(5):2147. [DOI] [PubMed] [Google Scholar]
- 33. Domené HM, Hwa V, Jasper HG, Rosenfeld RG. Acid-labile subunit (ALS) deficiency. Best Pract Res Clin Endocrinol Metab. 2011;25(1):101–113 [DOI] [PubMed] [Google Scholar]
- 34. Russo VC, Schütt BS, Andaloro E, et al. Insulin-like growth factor binding protein-2 binding to extracellular matrix plays a critical role in neuroblastoma cell proliferation, migration, and invasion. Endocrinology. 2005;146(10):4445–4455 [DOI] [PubMed] [Google Scholar]
- 35. Levi J, Huynh FK, Denroche HC, et al. Hepatic leptin signalling and subdiaphragmatic vagal efferents are not required for leptin-induced increases of plasma IGF binding protein-2 (IGFBP-2) in ob/ob mice. Diabetologia. 2012;55(3):752–762 [DOI] [PubMed] [Google Scholar]
- 36. Ooi GT, Tseng LY, Rechler MM. Transcriptional regulation of the rat IGFBP-1 and IGFBP-2 genes. Growth Regul. 1993;3(1):14–17 [PubMed] [Google Scholar]
- 37. Radetti G, Bozzola M, Pasquino B, et al. Growth hormone bioactivity, insulin-like growth factors (IGFs), and IGF binding proteins in obese children. Metabolism. 1998;47(12):1490–1493 [DOI] [PubMed] [Google Scholar]
- 38. Ballerini MG, Ropelato MG, Domené HM, Pennisi P, Heinrich JJ, Jasper HG. Differential impact of simple childhood obesity on the components of the growth hormone-insulin-like growth factor (IGF)-IGF binding proteins axis. J Pediatr Endocrinol Metab. 2004;17(5):749–757 [DOI] [PubMed] [Google Scholar]
- 39. Argente J, Caballo N, Barrios V, et al. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in patients with anorexia nervosa: effect of short- and long-term weight recuperation. J Clin Endocrinol Metab. 1997;82(7):2084–2092 [DOI] [PubMed] [Google Scholar]
- 40. Rasmussen MH, Juul A, Hilsted J. Effect of weight loss on free insulin-like growth factor-I in obese women with hyposomatotropism. Obesity (Silver Spring). 2007;15(4):879–886 [DOI] [PubMed] [Google Scholar]
- 41. Voskuil DW, Bueno de Mesquita HB, et al. Determinants of circulating insulin-like growth factor (IGF)-I and IGF binding proteins 1–3 in premenopausal women: physical activity and anthropometry (Netherlands). Cancer Causes Control. 2001;12(10):951–958 [DOI] [PubMed] [Google Scholar]
- 42. Ahmed RL, Thomas W, Schmitz KH. Interactions between insulin, body fat, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2007;16(3):593–597 [DOI] [PubMed] [Google Scholar]
- 43. Li Z, Martin J, Poirier P, et al. Upregulation of plasma insulin-like growth factor binding protein 2 levels after biliopancreatic diversion in humans. Obesity (Silver Spring). 2012;20(7):1469–1473 [DOI] [PubMed] [Google Scholar]
- 44. Heald AH, Kaushal K, Siddals KW, Rudenski AS, Anderson SG, Gibson JM. Insulin-like growth factor binding protein-2 (IGFBP-2) is a marker for the metabolic syndrome. Exp Clin Endocrinol Diabetes. 2006;114(7):371–376 [DOI] [PubMed] [Google Scholar]
- 45. Arafat AM, Weickert MO, Frystyk J, et al. The role of insulin-like growth factor (IGF) binding protein-2 in the insulin-mediated decrease in IGF-I bioactivity. J Clin Endocrinol Metab. 2009;94(12):5093–5101 [DOI] [PubMed] [Google Scholar]
- 46. Attia N, Tamborlane WV, Heptulla R, et al. The metabolic syndrome and insulin-like growth factor I regulation in adolescent obesity. J Clin Endocrinol Metab. 1998;83(5):1467–1471 [DOI] [PubMed] [Google Scholar]
- 47. Ko JM, Park HK, Yang S, Hwang IT. Influence of catch-up growth on IGFBP-2 levels and association between IGFBP-2 and cardiovascular risk factors in Korean children born SGA. Endocr J. 2012;59(8):725–733 [DOI] [PubMed] [Google Scholar]
- 48. de Kort SW, van Doorn J, van de Sande AG, Leunissen RW, Hokken-Koelega AC. Serum insulin-like growth factor-binding protein-2 levels and metabolic and cardiovascular risk factors in young adults and children born small for gestational age. J Clin Endocrinol Metab. 2010;95(2):864–871 [DOI] [PubMed] [Google Scholar]
- 49. Rowlands MA, Holly JM, Gunnell D, et al. The relation between adiposity throughout the life course and variation in IGFs and IGFBPs: evidence from the ProtecT (prostate testing for cancer and treatment) study. Cancer Causes Control. 2010;21(11):1829–1842 [DOI] [PubMed] [Google Scholar]
- 50. Claudio M, Benjamim F, Riccardo B, Massimiliano C, Francesco B, Luciano C. Adipocytes IGFBP-2 expression in prepubertal obese children. Obesity (Silver Spring). 2010;18(10):2055–2057 [DOI] [PubMed] [Google Scholar]
- 51. Touskova V, Trachta P, Kavalkova P, et al. Serum concentrations and tissue expression of components of insulin-like growth factor-axis in females with type 2 diabetes mellitus and obesity: the influence of very-low-calorie diet. Mol Cell Endocrinol. 2012;361(1–2):172–178 [DOI] [PubMed] [Google Scholar]
- 52. Liu J, DeYoung SM, Zhang M, Zhang M, Cheng A, Saltiel AR. Changes in integrin expression during adipocyte differentiation. Cell Metab. 2005;2(3):165–177 [DOI] [PubMed] [Google Scholar]
- 53. Lin YT, Tang CH, Chuang WJ, Wang SM, Huang TF, Fu WM. Inhibition of adipogenesis by RGD-dependent disintegrin. Biochem Pharmacol. 2005;70(10):1469–1478 [DOI] [PubMed] [Google Scholar]