Abstract
Orphanin FQ (OFQ), a member of the opioid family, is found in many areas of the hypothalamus and, when given centrally OFQ inhibits episodic LH secretion in rodents and sheep. Because GnRH neurons are devoid of the appropriate receptors to mediate steroid negative feedback directly, neurons that release OFQ may be involved. Using immunocytochemistry, we first determined that most OFQ neurons in the arcuate nucleus (ARC) and other hypothalamic regions of luteal phase ewes contained both estrogen receptor α and progesterone (P) receptor. Given a similar high degree of steroid receptor colocalization in other ARC subpopulations, we examined whether OFQ neurons of the ARC contained those other neuropeptides and neurotransmitters. OFQ did not colocalize with kisspeptin, tyrosine hydroxylase, or agouti-related peptide, but all ARC OFQ neurons coexpressed proopiomelanocortin. To test for a role for endogenous OFQ, we examined the effects of an OFQ receptor antagonist, [Nphe1,Arg14,Lys15]Nociceptin-NH2 (UFP-101) (30 nmol intracerebroventricular/h), on LH secretion in steroid-treated ewes in the breeding season and ovary-intact ewes in anestrus. Ovariectomized ewes with luteal phase concentrations of P and estradiol showed a significant increase in LH pulse frequency during infusion of UFP-101 (4.5 ± 0.5 pulses/6 h) compared with saline infusion (2.6 ± 0.4 pulses/6 h), whereas ewes implanted with only estradiol did not. Ovary-intact anestrous ewes displayed no significant differences in LH pulse amplitude or frequency during infusion of UFP-101. Therefore, we conclude that OFQ mediates, at least in part, the negative feedback action of P on GnRH/LH pulse frequency in sheep.
GnRH neurons are the final neural pathway controlling LH secretion from the gonadotropes of the anterior pituitary. For most of the estrous cycle, GnRH secretion is predominantly episodic with the amplitude and frequency of GnRH pulses controlled by ovarian steroids. During the estrous cycle in sheep, estradiol (E2) inhibits GnRH pulse amplitude (1, 2), whereas progesterone (P) suppresses GnRH pulse frequency (3, 4). The simplest explanation for control by ovarian steroids would be a direct inhibition of GnRH neurons, but GnRH neurons are devoid of estrogen receptor (ER)α (5, 6) and P receptor (PR) (7). Therefore, steroid negative feedback on GnRH/LH secretion by E2 and P is mediated via afferent neurons that have yet to be fully identified.
Many results have implicated endogenous opioid peptides (EOPs) in general (8), and dynorphin specifically, in the negative feedback control of GnRH secretion in sheep. Dynorphin appears to mediate this P negative feedback for three reasons: 1) greater than 90% of arcuate nucleus (ARC) and parvicellular dynorphin neurons contain PR (9), 2) P has been shown to increase dynorphin mRNA abundance and stimulate secretion (10), and 3) microimplants of a κ receptor antagonist in the medial basal hypothalamus increases LH pulse frequency in luteal phase ewes (11). However, recent results raise the possibility that other neuronal systems may also play a role in P negative feedback in the ewe (12). Another EOP that could play a role in steroid negative feedback is orphanin FQ (OFQ). OFQ, also known as nociceptin, was discovered by two independent groups and binds to a G-coupled protein receptor, opioid receptor like-1 (ORL-1), that is not affected by classical EOP receptor antagonists (13, 14). OFQ and ORL-1 are found throughout the preoptic area (POA) and hypothalamus in rats (15–17), humans (18), and sheep (19). In the ewe, greater than 90% of OFQ neurons in the POA colocalize with GnRH, but OFQ neurons not containing GnRH are also present in the hypothalamus (19). We (19) and others (20, 21) have shown that exogenous OFQ can inhibit LH secretion in vivo, and there is evidence that endogenous release of OFQ inhibits GnRH/LH secretion in ovariectomized rats (20, 21)
In light of these inhibitory actions of OFQ, we hypothesize that OFQ neurons contain ERα and PR and mediate steroid negative feedback in the ewe. To test this hypothesis, we used immunocytochemistry to examine whether OFQ neurons in the POA and hypothalamus of the ewe contain ERα and/or PR. We found a high percentage of OFQ cells in the ARC that colocalized ERα and PR, and because of studies showing steroid receptor colocalization in other ARC subpopulations, including ones that express kisspeptin, agouti-related peptide (AgRP), proopiomelanocortin (POMC), and tyrosine hydroxylase (TH) (6, 22–25), we examined whether or not OFQ colocalized with those other neuropeptides/transmitter enzyme using dual immunofluorescence. Finally, to test a role for endogenous OFQ, we examined LH secretion after intracerebroventricular administration of an OFQ receptor antagonist (the location of ORL-1 in the ovine hypothalamus is not known) in ovariectomized (OVX) ewes receiving P and E2 (P+E2) or E2 alone during the breeding season and in ovary-intact ewes during the nonbreeding (anestrous) season.
Materials and Methods
Animals
Adult ewes (>3 y) of mixed breeding were housed indoors with lighting that simulated natural day length and received a diet of alfalfa pellets with water and a mineral block ad libitum. Breeding season experiments were done with ewes showing regular estrous cycles (based on estrous detection with a vasectomized ram) between October and February; anestrous experiments were done in May. Reproductive status of anestrous ewes was confirmed by inspection of the ovaries for the absence of corpora lutea on the day of killing. For blood collection, jugular catheters were inserted using local anesthetic (Xylocaine; Webster Veterinary Supply) the day before samples (3 mL) were taken, blood was collected into heparinized tubes, and plasma was stored at −20°C. All procedures were approved by the West Virginia University Animal Care and Use Committee and followed National Institutes of Health guidelines for use of animals in research.
Reagents
Antibodies
Rabbit antiserum against OFQ was obtained from Neuromics, and mouse antisera against ERα and PR were purchased from DAKO and Beckman Coulter/Immunotech, respectively. Rabbit antiserum against POMC was purchased from Phoenix Pharmaceuticals, Inc, guinea pig antisera against mouse AgRP was obtained from Antibodies Australia, mouse antiserum against TH (a marker of dopamine neurons) was purchased from Millipore, and rabbit antiserum against kisspeptin was a gift from A. Caraty. All of these antibodies have been validated for use in sheep (19, 26–29). In addition, omission of one or both primary antibodies and preabsorption with purified antigens served as controls (data not shown) in experiments 1 and 2.
ORL-1 antagonists
[Nphe1,Arg14,Lys15]Nociceptin-NH2 (UFP-101) and N-(4-Amino-2-methyl-6-quinolinyl)-2-[(4-ethylphenoxy)methyl]benzamide hydrochloride (JTC-801) (Tocris Bioscience) were stored as recommended by the manufacturer and diluted to 250 nmol/mL in 0.9% sterile saline the day before infusions. Both antagonists have been shown to display potent and selective antagonist action on ORL-1 with pKi values for UFP-101 and JTC-801 of 10.2 and 7.35, respectively (30, 31). Furthermore, UFP-101 and JTC-801 both show a high specificity for ORL-1 with minimal binding to κ-, μ-, and δ-opioid receptors.
Surgical procedures
All procedures were performed using sterile technique under gas anesthesia (oxygen plus 2%–4% isoflurane). Ovariectomies were performed by midventral laparotomy, and a chronic lateral cerebroventricle cannula was inserted using a modification of our standard neurosurgical approach (32). After exposure of the top of the skull, an initial mark was positioned 5 mm rostral and 3.5 mm lateral to bregma, after which a 1-cm hole was drilled at this mark to expose the dura. Four stainless steel screws were inserted in the skull surrounding the hole in order to anchor the dental acrylic. A small hole was burned in the exposed dura by brief cauterization, and a 16-gauge needle, which had been cut to a length of 1.25 inches and equipped with detachable tubing filled with sterile water, was lowered into the brain until the ventricle was pierced and water flow was observed (depth of 18–22 mm). Then, 1 mL of a radio-opaque dye, Omnipaque 350 (Iohexol), was slowly injected and the dorsal/ventral position of the needled adjusted to the middle of the lateral ventricle, if necessary, based on a lateral x-ray. The needle hub was plugged to prevent loss of cerebrospinal fluid and was covered with a plastic protective cap and cemented in place using dental acrylic. All ewes were treated pre- and postoperatively with Penicillin Procaine G (Webster Veterinary Supply), Dexamethasone (Webster Veterinary Supply), and FluMeglumine (Webster Veterinary Supply) as previously described (32) and allowed at least 10 days for recovery.
Tissue collection and processing
Tissue for immunocytochemistry was collected as described previously (33). Briefly, ewes (n = 3) in the midluteal phase of the estrous cycle were heparinized (20 000 U) and killed using an iv overdose of sodium pentabarbitol (Euthasol, Webster Veterinary). Heads were removed and perfused via the carotid arteries with four liters of 2% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) containing 0.1% sodium nitrite. Blocks of tissue containing the POA and the hypothalamus were then removed and stored in 2% paraformaldehyde for 24 hours at 4°C and transferred to 20% sucrose until sectioned. Frozen coronal sections (40 μm) were cut with a freezing microtome and stored in cryopreservative until the time of immunocytochemical staining.
Experiment 1. Colocalization of OFQ with ERα or PR
Dual immunocytochemistry for OFQ and ERα or PR was done with free floating sections as previously described (19, 27, 34); all incubations were at room temperature. On day 1, a series of every sixth section from the POA through the ARC was washed 12 × 15 minutes in 0.1M PBS and then placed in 1% H202 for 10 minutes followed by 4 × 5-minute washes in PBS. Tissue was next incubated for 1 hour with 0.4% Triton X-100 (Sigma-Aldrich) in 20% normal goat serum (NGS) in PBS, followed by 1:1000 ERα antiserum or 1:50 PR antiserum for 17 and 40 hours, respectively. Thereafter, tissue was rinsed with PBS 4 × 5 minutes, and biotinylated goat antimouse antibody (Vector Laboratories) at 1:500 and Vectastain ABC-elite (Vector Laboratories) at 1:500, both in 0.4% Triton X-100 in 4% NGS, were applied sequentially for 1 hour each with 4 × 5-minute washes of PBS between incubations. Sections were then placed in a 3, 3′-diaminobenzidine tetrahydrochloride (DAB)-nickel solution (10-mg DAB [Sigma-Aldrich] and 2 mL of 2% nickel sulfate in 50-mL PB with 20-μL 30% H202 added just before incubation) for 10 minutes. Sections were washed in PBS 4 × 5 minutes and then incubated for 10 minutes in 1% H202 followed by 4 × 5-minute washes in PBS. After incubation for 40 hours with 1:2500 OFQ antiserum in 0.4% Triton X-100 in 4% NGS, tissue was rinsed with PBS 4 × 5 minutes, and biotinylated goat antirabbit antibody (Jackson ImmunoResearch) at 1:500 and Vectastain ABC-elite (Jackson ImmunoResearch) at 1:500, both in 0.4% Triton X-100 in 4% NGS, were applied sequentially for 1 hour each with 4 × 5-minute washes of PBS between incubations. Sections were then incubated in DAB without nickel enhancement, washed 4 × 5 minutes in PB, and mounted on Superfrost/Plus microscope slides (Fisher Scientific). Sections were dehydrated using a series of increasing alcohol baths and coverslipped using DPX Mounting Medium (Electron Microscopy Sciences).
Experiment 2. Colocalization of OFQ with AgRP, TH, POMC, and kisspeptin
Because the antisera against POMC and kisspeptin were produced in the same species as the OFQ antisera, dual immunofluorescence with these antisera was performed using biotinylated tyramide amplification and conjugated second antibody detection to visualize the first and second antigen, respectively (26); otherwise, amplification was not needed. All incubations were at room temperature. For AgRP and TH visualization with OFQ, a series of tissue sections from the middle ARC (n = 2 ewes) was washed in 0.1M PBS, incubated in 0.1% Triton X-100 containing 4% (TH) and 20% (AgRP) NGS followed by a 17-hour incubation in PBS containing 4% NGS with rabbit antiserum for OFQ (1:2500) and guinea pig antiserum for AgRP (1:1000) or mouse antiserum for TH (1:2000). The next day after washing tissue 4 × 5 minutes in PBS (from here on, tissue was covered with tin foil to prevent light exposure), secondary antibody for OFQ (goat antirabbit Alexa Fluor 555; 1:100) and AgRP (goat antiguinea pig Alexa Fluor 488; 1:100) or TH (goat antimouse Alexa Fluor 488; 1:100) in 0.4% Triton X-100 and 4% NGS in PBS was applied to tissue for 1 hour. For POMC and kisspeptin, a series of sections through the middle ARC (n = 2 ewes) was washed in 0.1M PBS, incubated in 0.4% Triton X-100 in 20% NGS for 1 hour, followed by a 17-hour incubation in rabbit antiserum for OFQ (1:20 000) or rabbit antiserum for kisspeptin (1:200 000). The next day tissue was washed in 0.1M PBS 4 × 5 minutes, followed by a 1-hour incubation in biotinylated goat antirabbit (1:500) in 0.4% Triton X-100 in 4% NGS in PBS. Tissue was washed 4 × 5 minutes followed by 1-hour incubation in ABC-elite solution 1:500), then washed 4 × 5 minutes in PBS. Tissue was then incubated in biotinylated tyramine (1:250) for 10 minutes followed by 4 × 5-minute washes in PBS. Then, tissue was incubated in PBS containing Alexa Fluor 555-streptavidin (red; 1:100 for OFQ) or Alexa Fluor 488-streptavidin (green; 1:100 for kisspeptin) for 1 hour. Tissue was then washed 4 × 5 minutes in PBS followed by a 17-hour incubation with rabbit anti-POMC (1:16 000) or rabbit anti-OFQ (1:1000), respectively, in 0.4% Triton X-100 with 4% NGS containing PBS. The next day, tissue was washed 4 × 5 minutes in PBS and then incubated with goat antirabbit Alexa Fluor 488 (green; 1:100 for POMC) or goat antirabbit Alexa Fluor 555 (red; 1:100 for OFQ) in 0.4% Triton X-100 with 4% NGS containing PBS for 30 minutes. After a final wash 4 × 5 minutes in PBS, all tissue was mounted on glass slides, coverslipped using gelvatol mounting medium, and stored in the dark at 4°C.
Experiment 3a. Effects of ORL-1 antagonists in OVX+P+E2 ewes
Eight breeding season ewes were OVX, and a guide tube was inserted into one lateral ventricle. At the time of OVX, ewes were given a 1-cm E2 implant sc (35) and 2 P packets sc (36). Starting approximately 2 weeks later, ewes received a lateral ventricle infusion of 0.9% saline (120 μL/h), UFP-101 (30 nmol/h), or JTC-801 (30 nmol/h). Doses of antagonists were based on data from preliminary experiments in ovary-intact ewes (see Supplemental Figures 1 and 2, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The day before infusion, 1 jugular vein was catheterized, and ewes were placed in narrow pens so that they could lie down but not turn around; an infusion line was inserted just before the start of blood collections that extended from the tip of the guide tube to a portable microinfusion pump strapped to the back of each animal. Blood was collected every 12 minutes for 6 hours with infusion starting immediately before and continuing throughout the collection period. Each ewe received all 3 treatments in random order, separated by 3 days between each treatment. Gentamicin (Webster Veterinary Supply) was given im prophylactically at the end of each collection period.
Experiment 3b. Effects of an ORL-1 antagonist in OVX+E2 ewes
At the end of experiment 3a, P packets were removed, but E2 implants were left in place. Ten days later, all sheep received a lateral ventricle infusion of saline (120 μL/h) or UFP-101 (30 nmol/h) with 3 days between treatments in a crossover design. Because results did not differ between antagonists in experiment 3a, we chose to only administer UFP-101 for experiment 3b and 4. Blood samples were collected every 12 minutes for 5 hours with infusion starting immediately before and continuing throughout the collection period.
Experiment 4. Effects of an ORL-1 antagonist in ovary-intact, anestrous ewes.
Starting approximately 2 weeks after insertion of a lateral ventricle guide tube, 6 ovary-intact, anestrous ewes were infused with saline (120 μL/h) or UFP-101 (30 nmol/h) into the lateral ventricle with 3 days between treatments in a crossover design. Blood samples were collected every 12 minutes for 4 hours with infusion starting immediately before and continuing throughout the collection period.
Data analysis
Immunocytochemistry
Hemisections from a series of every sixth section from the POA and hypothalamus were used for immunocytochemical detection of OFQ, ERα, and PR. OFQ was visualized using unenhanced DAB as the chromogen (brown reaction product), whereas ERα and PR were visualized using nickel-enhanced DAB (blue-black reaction product). Dual-labeled cells were defined as those in which a blue-black nucleus was surrounded by a brown cytoplasm as seen in the same plane of focus. Immunopositive cells were counted manually using a light microscope. The rostral ARC was defined as that level adjacent to the rostral median eminence and pars tuberalis of the pituitary, the middle ARC as that level adjacent to the tuberoinfundibular sulcus of the third ventricle, and the caudal ARC as that level adjacent to the mammillary recess of the third ventricle (9). Three to four hemisections through the middle ARC were used for analysis of immunofluorescent staining of OFQ and kisspeptin, AGRP, TH, or POMC using a Leica fluorescent microscope, resulting in analysis of 40–170 OFQ cells per animal per staining combination. Immunofluorescent cells were visualized by confocal z-stacks of 1-μm-thick optical sections and were captured using a Zeiss LSM 510 confocal microscope at ×63 magnification.
Assays
LH concentrations were measured as previously described (37) in duplicate with a RIA using 100–200 μL of plasma and reagents provided by the National Hormone and Peptide Program. LH assay sensitivity averaged 0.12 ng/tube (NIH S24) with intra- and interassay coefficients of variation being 15.6% and 28.9%, respectively.
Statistics
Pulses were identified using previously described criteria (38) and pulse frequency, pulse amplitude, and mean LH concentrations determined for each treatment period. Pulse frequency for experiment 3a was analyzed using Friedman repeated measures ANOVA on ranks. Pulse frequency for experiment 3b and 4 was analyzed using Wilcoxon signed-rank test. Mean LH and LH pulse amplitude were analyzed using a one-way ANOVA in experiment 3a. A paired t test was used to analyze mean LH and LH pulse amplitude in experiment 3b and 4. Differences were considered to be significant at P < .05.
Results
Experiment 1. Do OFQ neurons in the ovine hypothalamus possess ERα or PR?
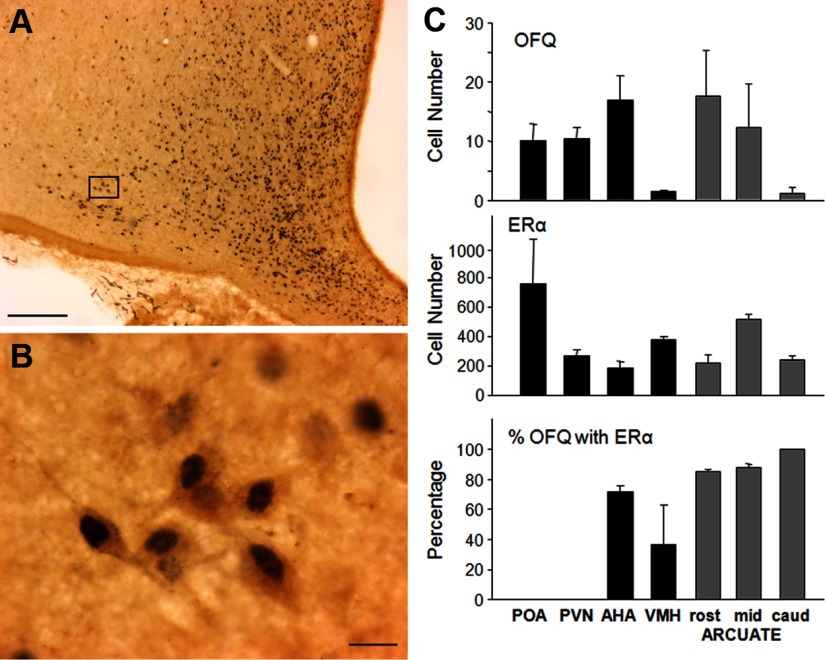
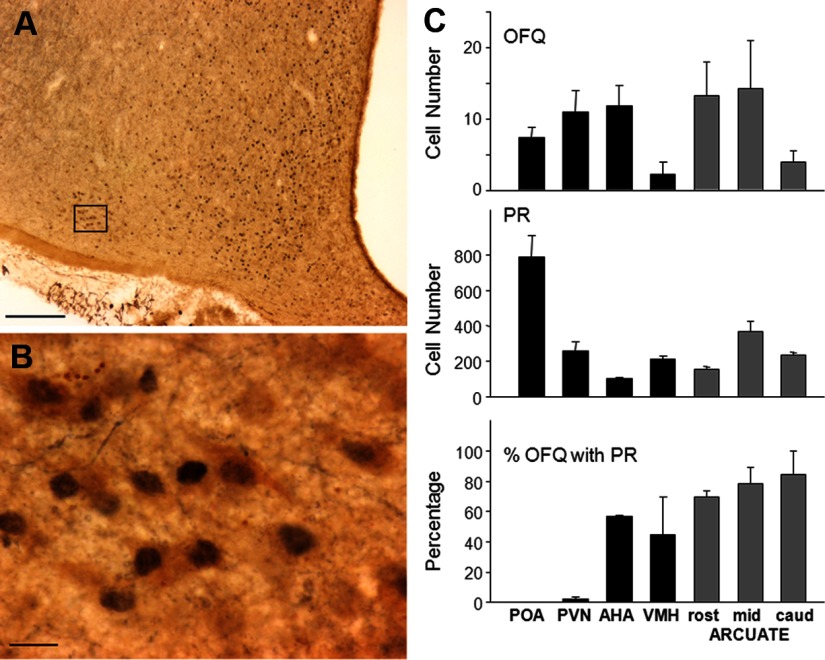
OFQ immunoreactive cells were seen in all areas examined with the most dense staining occurring in the POA, periventricular nucleus, anterior hypothalamic area (AHA), and rostral to middle ARC (Figure 1, A–C). ERα (Figure 1, A, B, and C) and PR (Figure 2, A, B, and C) immunoreactive nuclei were seen in all areas examined with the greatest density in the POA. In the rostral, middle, and caudal levels of the ARC, ERα was colocalized in 85%–100% of OFQ cells (Figure 1C), and PR was observed in 70%–84% of OFQ neurons (Figure 2C). In the AHA, 71.6 ± 4.2% of OFQ-positive cells coexpressed ERα (Figure 1C), whereas 56.0 ± 0.9% coexpressed PR (Figure 2C). In the ventromedial hypothalamus (VMH), 36.6 ± 26.3% and 44.9 ± 24.6% of OFQ-positive cells contained ERα (Figure 1C) and PR (Figure 2C), respectively. In contrast, less than 2% of OFQ cells in the POA or paraventricular area (PVN) colocalized ERα or PR. The total number of ERα or PR cells far outnumbered that of OFQ cells in each area (Figures 1C and 2C). Thus, the percentages of ERα or PR cells colocalizing OFQ were extremely low (ranging from 0.1% to 1%) in all regions examined.
Figure 1.
(A and B) Photomicrographs of OFQ immunoreactive cells (brown cytoplasm) and ERα (blue-black nuclei) in the ovine ARC. Box in A depicts position of neurons shown at larger magnification in B. Magnification bars, 200 μm (A) and 25 μm (B). (C) Mean (±SEM, n = 3) OFQ-positive cell number (top graph), ERα-containing cell number (middle graph), and percentage of OFQ neurons also containing ERα (bottom graph) in the POA, PVN, AHA, VMH, and 3 regions (rost, rostral; mid, middle; caud, caudal) of the ARC (gray bars).
Figure 2.
(A and B) Photomicrographs of OFQ immunoreactive cells (brown cytoplasm) and PR (blue-black nuclei) in the ovine ARC. Box in A depicts position of neurons shown at larger magnification in B. Magnification bars, 200 μm (A) and 25 μm (B). (C) Mean (±SEM, n = 3) OFQ-positive cell number (top graph), PR-containing cell number (middle graph), and percentage of OFQ neurons also containing PR (bottom graph) in the POA, PVN, AHA, VMH, and 3 regions (rost, rostral; mid, middle; caud, caudal) of the ARC (gray bars).
Experiment 2. Does OFQ colocalize with POMC, kisspeptin, AgRP, or TH in the ARC of sheep?
Because the highest percent colocalization of OFQ and ERα/PR was within the ARC, we determined whether OFQ was also found in specific ARC neurons that also contain these steroid receptors. All OFQ neurons in the ARC also expressed POMC (Figure 3, A–C), but the population of POMC neurons extended further dorsal and lateral than the OFQ cells so that OFQ/POMC dual-labeled cells comprised 25.4% of the total POMC population. OFQ did not colocalize with kisspeptin (Figure 3D), AgRP (Figure 3E), or TH (Figure 3F) in the ovine ARC.
Figure 3.
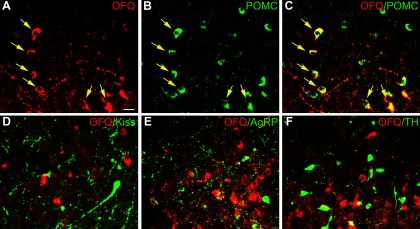
(A–F) Confocal images (1-μm optical section) through the ovine ARC processed for dual immunofluorescence detection of OFQ (red) and either POMC, kisspeptin, AgRP, or TH (green). (C) Merged image of single-labeled OFQ neurons (A) and POMC neurons (B) to depict yellow dual-labeled OFQ/POMC neurons (eg, arrows). Note presence of a few single-labeled POMC neurons (eg, top-middle portion of B and C). Merged images in bottom row show OFQ and kisspeptin (Kiss) (D), AgRP (E), or TH (F). Magnification bar, 25 μm.
Experiment 3a. Can OFQ receptor antagonists increase LH secretion in OVX+P+E2 ewes?
Given that OFQ can inhibit LH secretion in OVX ewes (19), we tested the hypothesis that infusion of an OFQ receptor antagonist would increase LH secretion in OVX ewes treated with luteal phase concentrations of P and E2. Figure 4 displays the pulse profiles from one ewe that received saline, UFP-101, and JTC-801 infusions. Mean LH (Figure 5A) and LH pulse amplitude (Figure 5B) were not significantly increased with infusion of UFP-101 or JTC-801. In contrast, LH pulse frequency (Figure 5C) for UFP-101 (4.5 ± 0.5 pulses/6 h) and JTC-801 (4.9 ± 0.3 pulses/6 h) was significantly increased compared with infusion of saline (2.6 ± 0.4 pulses/6 h). The latency from the start of infusion to the first LH pulse was similar for UFP-101 (51 ± 13.2 min) and JTC-801(41.1 ± 8.4 min) and slightly, but not significantly (P = .088), longer for the saline treatment (79.5 ± 8.4 min).
Figure 4.
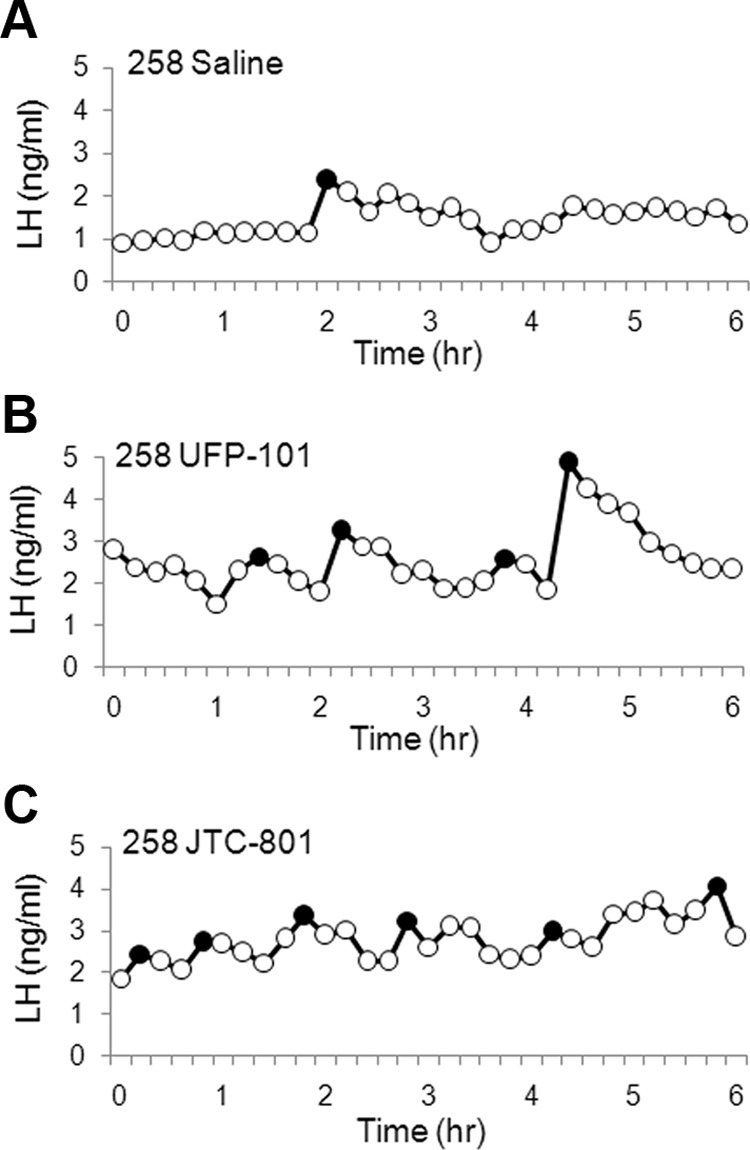
LH profiles from experiment 3a in ewe number 258 that was OVX and treated sc with implants containing P and E2. LH profiles during infusion of saline (A), UFP-101 (B), and JTC-801 (C) into the lateral ventricle are shown, with pulses identified using a closed circle.
Figure 5.
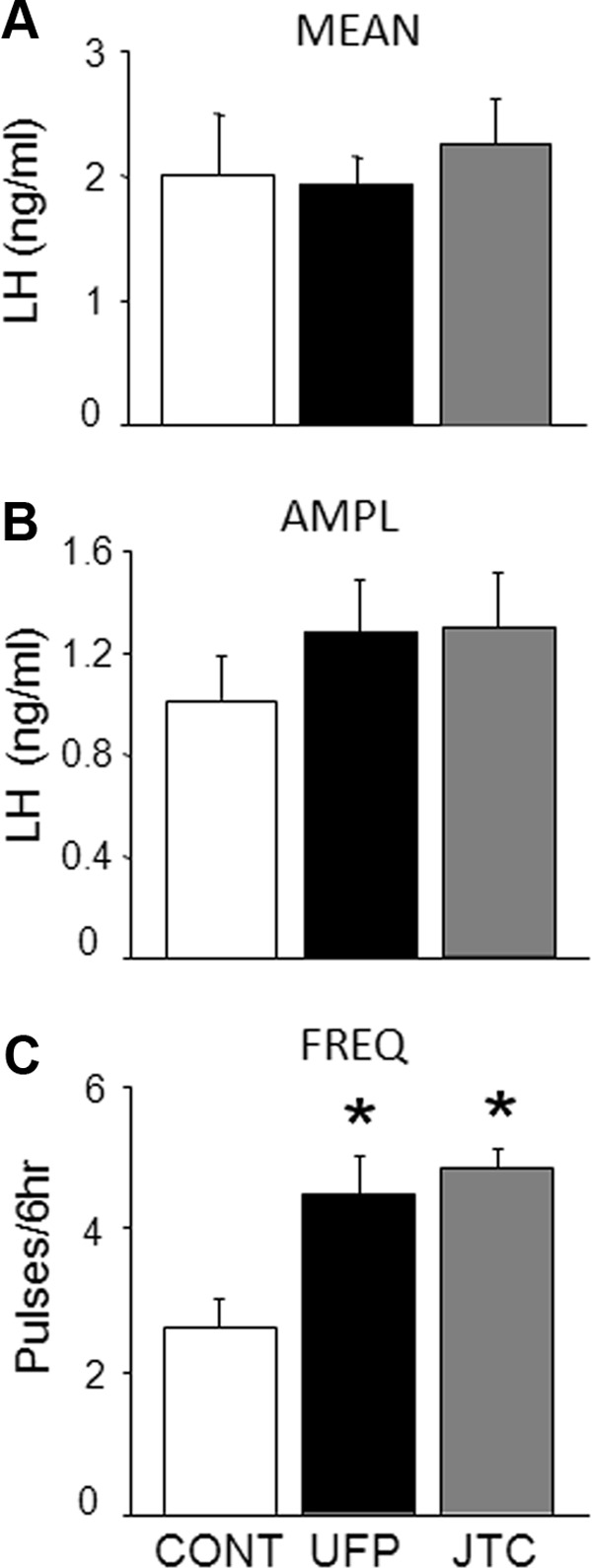
Mean (±SEM) LH concentrations (A), LH pulse amplitude (AMPL; B), and LH pulse frequency (FREQ; C) for saline (CONT; open bars), UFP-101 (black bars), and JTC-801 (gray bars) infused into the lateral ventricle of OVX+P+E2 ewes (n = 8). *Significant difference (P < .05) from saline treatment.
Experiment 3b. Does UFP-101 increase LH secretion in OVX+E2 ewes?
Because we observed similar results with UFP-101 and JTC-801 in experiment 3a, we conducted experiment 3b and 4 using only UFP-101. Because E2 inhibits LH pulse amplitude during the breeding season, OVX+E2 ewes infused with saline showed the expected high-frequency, low-amplitude pattern of episodic LH secretion (Figure 6). UFP-101 infusion had no significant effect on mean LH (Figure 6A) or LH pulse frequency (Figure 6C). UFP-101 also did not alter LH pulse amplitude (UFP-101, 3.8 ± 1.1 ng/mL; and saline, 3.0 ± 1.0 ng/mL) (Figure 6B).
Figure 6.
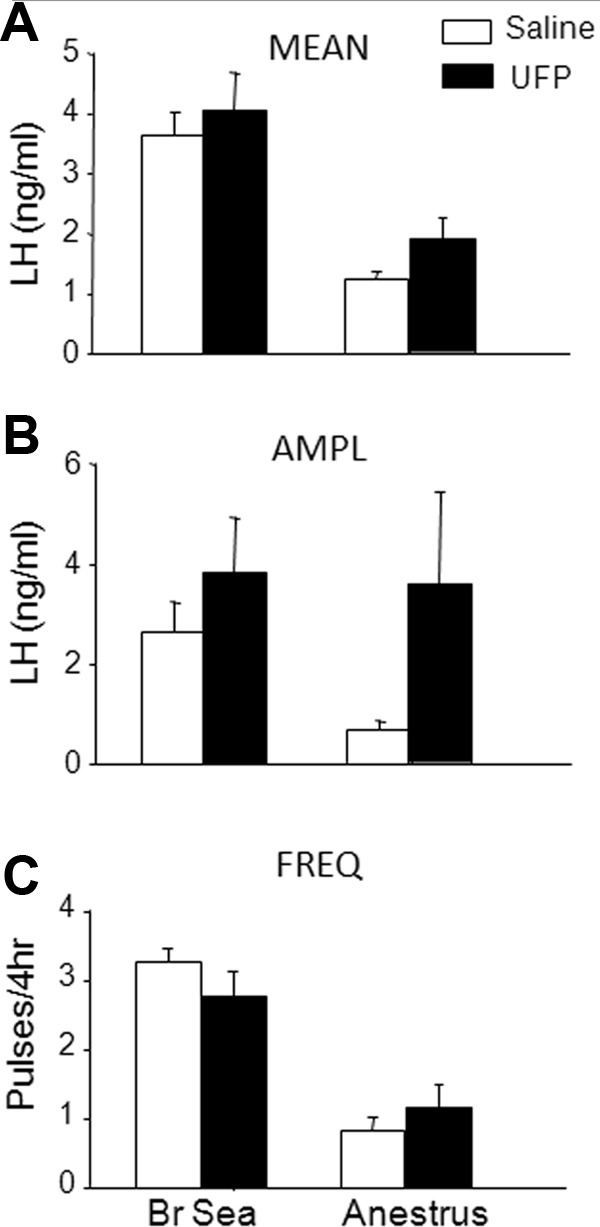
Mean (±SEM) for LH concentrations (A), LH pulse amplitude (AMPL; B), and LH pulse frequency (FREQ; C) for saline (open bars) and UFP-101 (black bars) infused into the lateral ventricle of OVX+E2 breeding season (Br Sea) (n = 8) and ovary-intact anestrous (anestrus; n = 6 for mean LH and pulse frequency). LH pulse amplitudes were not statistically analyzed in anestrous ewes due to low animal numbers (n = 3), because many ovary-intact anestrous ewes had no LH pulses during the 4-hour sampling period. Note that for illustrative purposes only, the frequency data for breeding season ewes was converted from pulses/5 hours to pulses/4 hours by multiplying the total number of pulses/5 hours by 80% for each ewe.
Experiment 4. Does UFP-101 increase LH secretion in ovary-intact anestrous ewes?
Because E2 inhibits frequency at this time of year (39), LH pulse frequency in saline-infused ovary-intact anestrous ewes (Figure 6C) was expectedly low and was not significantly affected by UFP-101 infusion (Figure 6). Likewise, mean LH during infusion of UFP-101 (1.9 ± 0.4 ng/mL) did not differ significantly from that during infusion of saline (1.4 ± 0.1 ng/mL). The absence of LH pulses in many ovary-intact anestrous ewes during a 4-hour sampling period precluded valid statistical comparisons of mean LH pulse amplitude between UFP-101- and saline-infused ewes.
Discussion
These data provide evidence for an important role of endogenous OFQ in the negative feedback control of GnRH/LH secretion in the ewe. Our observation that most OFQ neurons in the ARC contain ERα and PR fits with the idea that OFQ could mediate hypothalamic actions of ovarian steroids on LH secretion. Furthermore, administration of OFQ receptor antagonists into the lateral ventricle of OVX+P+E2 ewes increased LH pulse frequency. In contrast, no effect was observed in OVX+E2 or ovary-intact anestrous ewes, which solidifies a role for endogenous OFQ in specifically mediating P negative feedback.
The distribution of OFQ, ERα-, and PR-containing neurons throughout the POA and hypothalamus (Figures 1 and 2) is similar to that seen previously in sheep (7, 19, 40) and humans (18). A similar distribution is also seen in the rat (16, 17), except for one report, based on in situ hybridization, that there are no OFQ-containing cell bodies in the VMH of females (17). However, two other reports in male (16) and female (21) rats agree with our report that this nucleus contains OFQ-positive cell bodies, albeit at low numbers. Our finding that OFQ-containing neurons in the POA do not express ERα or PR is consistent with the observation that, within this area, OFQ is coexpressed predominantly in GnRH neurons (19) and that GnRH neurons do not express either steroid receptor (5, 6). Therefore, steroid negative feedback most likely is not exerted directly through OFQ neurons located in the POA. In contrast, a relatively high percentage of OFQ neurons in other hypothalamic regions contain ERα and PR, with the highest colocalization in the ARC. This high percentage of ARC OFQ neurons containing ERα and PR (ERα, 80%–100%; PR, 60%–80%) is in contrast to many other steroid receptor containing neural populations in the ARC, such as those containing AgRP (ERα, 3%–10%; PR, 15%), dopamine (ERα, 15%; PR, 20%), and POMC (ERα, 15%–20%; PR, 28%) (6, 22–24), but quite similar to the kisspeptin population of the ARC (ERα and PR, 95%) (25).
Given the variety of steroid receptor-containing neurons in the ARC, we investigated whether ARC OFQ neurons coexpressed other neuropeptides that are also colocalized with ERα and PR. Within the ARC, we did not observe colocalization of OFQ with AgRP, TH, or kisspeptin. However, we did observe that approximately 25% of POMC neurons in, or near, the ARC coexpress OFQ, whereas all OFQ neurons in this region coexpress POMC. A comparison of the percentage of POMC neurons containing OFQ (this study) with the percentage of POMC neurons containing ERα or PR in sheep (6, 22) strongly suggests that all POMC neurons containing steroid receptors also contain OFQ. This fits well with a high percentage of ARC OFQ neurons containing steroid receptors and raises the possibility that this subset of POMC neurons mediate reproductive actions of ovarian steroids (β-endorphin has been implicated in the control of both tonic and surge secretion of LH in ewes) (41), whereas other POMC neurons are involved in mediating the effects of metabolic hormones on food intake (42). Alternatively, this subset of POMC neurons may mediate the effects of estrogen on food intake. Furthermore, POMC-containing synapses have been observed on GnRH neurons in the ovine hypothalamus (11), so it is possible that OFQ could have direct input to GnRH neurons.
The use of opioid antagonists has helped define a role for EOPs in mediating steroid negative feedback on GnRH/LH secretion in several species (37, 43–55), and our data extend this approach to indicate a role for OFQ in mediating P negative feedback. Because the stimulatory effects of ORL-1 blockade were not seen in OVX+E2 breeding season ewes or ovary-intact anestrous ewes, in which E2 holds LH pulse frequency in check, we conclude that OFQ is only involved in P negative feedback. These data might be viewed as conflicting with several studies indicating that dynophin plays an important role in P negative feedback in the ewe (11). However, we recently reported that local administration of P to the caudal ARC, targeting dynorphin neurons, was not sufficient to suppress LH secretion in OVX ewes, whereas local administration of a PR antagonist, RU486, to this same area increased LH secretion in ewes that received peripheral P treatment (12). Therefore, we suggested that dynorphin alone is not sufficient to mediate all the effects of P on GnRH/LH secretion, and other neurons, presumably within a short distance of the local action of the RU486 microimplants within the mediobasal hypothalamus, are also likely to contribute to P negative feedback. Given the concentration of OFQ-positive neurons containing PR in the more rostral and medial ARC (Figure 2), this could well be the population affected by RU486.
Evidence of a hypothalamic role for OFQ in control of GnRH secretion continues to grow. The first evidence of this was reported in the guinea pig, where OFQ hyperpolarized GnRH neurons within the medial basal hypothalamus (56). Since then, it has been shown in vivo that central administration of OFQ inhibits LH secretion and GnRH concentrations in push-pull perfusates of rats (20, 57) and GnRH pulse frequency in hypophysial portal blood of ewes (58). Evidence supporting a role for endogenous OFQ comes from reports that intracerebroventricular administration of an ORL-1 antagonist increased peripheral LH (20) and GnRH concentrations in push-pull perfusates from the median eminence of OVX rats (57). These data point to tonic release of OFQ in the absence of ovarian steroids in rats, but our data in E2-treated OVX ewes suggest that OFQ release is dependent on P in this species. We have not tested the ORL-1 antagonist in OVX ewes, but there is no evidence that E2 inhibits ORL-1 expression (see below), and the antagonist was ineffective in chronically OVX ewes treated with steroids that failed to inhibit LH release (our unpublished data). Thus, there may be important species differences in the physiological actions of OFQ on GnRH release.
Although ORL-1 mRNA is detectable in the medial POA, supraoptic nucleus, PVN, VMH, and ARC (18), limited data exist for steroid effects on OFQ or its receptor. A study in rats reports an increase in ORL-1 mRNA after treatment of rats with either E2 or E2+P, whereas changes in peptide were only observed in E2+P-treated rats (17). Therefore, it might be possible that E2 increases ORL-1 expression, whereas P increases OFQ expression in ewes. If E2 increases expression of ORL-1 in GnRH neurons directly, it would presumably do so via ERβ or a putative membrane receptor, because GnRH neurons do not contain ERα. This idea is consistent with our data showing that an infusion of an OFQ receptor antagonist increased LH pulse frequency in OVX ewes with luteal phase concentrations of E2 and P. Therefore, the combination of these steroids results in increased endogenous OFQ release that is blocked by our infusion of UFP-101. However, experiments examining steroid effects on OFQ and ORL-1 expression in species other than rat remain to be done. It is worth noting that ovarian steroids increased expression of POMC mRNA in ovine ARC (59, 60), and based on our data, we would suggest that this is likely via a direct effect on the OFQ-POMC neurons in this region. Furthermore, the colocalization of OFQ in both GnRH (19) and POMC neurons (here), and evidence that OFQ inhibits both GnRH and POMC neurons in guinea pigs (56), raises the possibility that OFQ may well have an autocrine action on these neurons, in addition to any inhibitory actions that occur via interneurons. This mechanism might help explain the synchronized pulsatile GnRH release in immortalized GnRH cells (61), GnRH neurons in explants of the olfactory placode (62), and GnRH neurons in vivo. An autocrine action on POMC cells could also explain the stimulatory effects of exogenous OFQ on food intake observed in rats (63–65). However, it remains to be determined which neurons (ie, GnRH and/or POMC neurons) contain ORL-1.
In summary, we show that OFQ neurons colocalize with both ERα and PR in multiple areas of the hypothalamus as well as with POMC neurons in the ARC. Furthermore, we show that antagonizing the receptor for OFQ increases LH pulse frequency in P-treated, but not E2-treated, ewes. Thus, our data support a role for OFQ in P negative feedback on LH secretion in the ewe.
Supplementary Material
Acknowledgments
We thank Heather Bungard and Jennifer Lydon (West Virginia University Food Animal Research Facility) and Dr Margaret Minch for care of animals. We also thank Dr Al Parlow and the National Hormone and Peptide Program for reagents used to measure LH, Dr Alain Caraty for the gift of kisspeptin antibody, and Dr Ida Holaskova for help with collection of preliminary data for the antagonist experiments.
This work was supported by National Institutes of Health Grants R01 HD039916 and R01 HD017864.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AgRP
- agouti-related peptide
- AHA
- anterior hypothalamic area
- ARC
- arcuate nucleus
- DAB
- 3, 3′-diaminobenzidine tetrahydrochloride
- E2
- estradiol
- EOP
- endogenous opioid peptide
- ER
- estrogen receptor
- JTC-801
- N-(4-Amino-2-methyl-6-quinolinyl)-2-[(4-ethylphenoxy)methyl]benzamide hydrochloride
- NGS
- normal goat serum
- OFQ
- orphanin FQ
- ORL-1
- opioid receptor like-1
- OVX
- ovariectomy
- P
- progesterone
- POA
- preoptic area
- POMC
- proopiomelanocortin
- PR
- P receptor
- PVN
- paraventricular area
- TH
- tyrosine hydroxylase
- UFP-101
- [Nphe1,Arg14,Lys15]Nociceptin-NH2
- VMH
- ventromedial hypothalamus.
References
- 1. Evans NP, Dahl GE, Glover BH, Karsch FJ. Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology. 1994;134:1806–1811 [DOI] [PubMed] [Google Scholar]
- 2. Evans NP, Dahl GE, Mauger D, Karsch FJ. Estradiol induces both qualitative and quantitative changes in the pattern of gonadotropin-releasing hormone secretion during the presurge period in the ewe. Endocrinology. 1995;136:1603–1609 [DOI] [PubMed] [Google Scholar]
- 3. Clarke IJ, Thomas GB, Yao B, Cummins JT. GnRH secretion throughout the ovine estrous cycle. Neuroendocrinology. 1987;46:82–88 [DOI] [PubMed] [Google Scholar]
- 4. Karsch FJ, Cummins JT, Thomas GB, Clarke IJ. Steroid feedback inhibition of pulsatile secretion of gonadotropin-releasing hormone in the ewe. Biol Reprod. 1987;36:1207–1218 [DOI] [PubMed] [Google Scholar]
- 5. Herbison AE, Robinson JE, Skinner DC. Distribution of estrogen receptor-immunoreactive cells in the preoptic area of the ewe: co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone. Neuroendocrinology. 1993;57:751–759 [DOI] [PubMed] [Google Scholar]
- 6. Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and β-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133:887–895 [DOI] [PubMed] [Google Scholar]
- 7. Skinner DC, Caraty A, Allingham R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology. 2001;142:573–579 [DOI] [PubMed] [Google Scholar]
- 8. Goodman RL, Gibson M, Skinner DC, Lehman MN. Neuroendocrine control of pulsatile GnRH secretion during the ovarian cycle: evidence from the ewe. Reprod Suppl. 2002;59:41–56 [PubMed] [Google Scholar]
- 9. Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143:4366–4374 [DOI] [PubMed] [Google Scholar]
- 10. Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic Acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146:1835–1842 [DOI] [PubMed] [Google Scholar]
- 11. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145:2959–2967 [DOI] [PubMed] [Google Scholar]
- 12. Goodman RL, Holaskova I, Nestor CC, et al. Evidence that the arcuate nucleus is an important site of progesterone negative feedback in the ewe. Endocrinology. 2011;152:3451–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meunier JC, Mollereau C, Toll L, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535 [DOI] [PubMed] [Google Scholar]
- 14. Reinscheid RK, Nothacker HP, Bourson A, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794 [DOI] [PubMed] [Google Scholar]
- 15. Neal CR, Jr, Mansour A, Reinscheid R, et al. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999;412:563–605 [PubMed] [Google Scholar]
- 16. Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406:503–547 [PubMed] [Google Scholar]
- 17. Sinchak K, Romeo HE, Micevych PE. Site-specific estrogen and progestin regulation of orphanin FQ/nociceptin and nociceptin opioid receptor mRNA expression in the female rat limbic hypothalamic system. J Comp Neurol. 2006;496:252–268 [DOI] [PubMed] [Google Scholar]
- 18. Neal CR, Jr, Akil H, Watson SJ., Jr Expression of orphanin FQ and the opioid receptor-like (ORL1) receptor in the developing human and rat brain. J Chem Neuroanat. 2001;22:219–249 [DOI] [PubMed] [Google Scholar]
- 19. Foradori CD, Amstalden M, Coolen LM, et al. Orphanin FQ: evidence for a role in the control of the reproductive neuroendocrine system. Endocrinology. 2007;148:4993–5001 [DOI] [PubMed] [Google Scholar]
- 20. An XF, Chen HP, Ma SL, Feng Y, Hao JW, Chen BY. Involvement of nociceptin/orphanin FQ in release of hypothalamic GnRH mediated by ORL1 receptor in ovariectomized rats. Acta Pharmacol Sin. 2005;26:1039–1044 [DOI] [PubMed] [Google Scholar]
- 21. An XF, Yu JY, Feng Y, Chen BY, Zhang SL. Role of hypothalamus nociceptin/orphanin FQ in pre-ovulatory luteinizing hormone surge of estrogen and progesterone-primed, ovariectomized rats. Acta Pharmacol Sin. 2007;28:1189–1197 [DOI] [PubMed] [Google Scholar]
- 22. Dufourny L, Caraty A, Clarke IJ, Robinson JE, Skinner DC. Progesterone-receptive β-endorphin and dynorphin B neurons in the arcuate nucleus project to regions of high gonadotropin-releasing hormone neuron density in the ovine preoptic area. Neuroendocrinology. 2005;81:139–149 [DOI] [PubMed] [Google Scholar]
- 23. Dufourny L, Caraty A, Clarke IJ, Robinson JE, Skinner DC. Progesterone-receptive dopaminergic and neuropeptide Y neurons project from the arcuate nucleus to gonadotropin-releasing hormone-rich regions of the ovine preoptic area. Neuroendocrinology. 2005;82:21–31 [DOI] [PubMed] [Google Scholar]
- 24. Skinner DC, Herbison AE. Effects of photoperiod on estrogen receptor, tyrosine hydroxylase, neuropeptide Y, and β-endorphin immunoreactivity in the ewe hypothalamus. Endocrinology. 1997;138:2585–2595 [DOI] [PubMed] [Google Scholar]
- 25. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 27. Hileman SM, Lubbers LS, Jansen HT, Lehman MN. Changes in hypothalamic estrogen receptor-containing cell numbers in response to feed restriction in the female lamb. Neuroendocrinology. 1999;69:430–437 [DOI] [PubMed] [Google Scholar]
- 28. Sheppard KM, Padmanabhan V, Coolen LM, Lehman MN. Prenatal programming by testosterone of hypothalamic metabolic control neurones in the ewe. J Neuroendocrinol. 2011;23:401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh SR, Hileman SM, Connors JM, et al. Estradiol negative feedback regulation by glutamatergic afferents to A15 dopaminergic neurons: variation with season. Endocrinology. 2009;150:4663–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calo G, Rizzi A, Rizzi D, et al. [Nphe1,Arg14,Lys15]nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br J Pharmacol. 2002;136:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamada H, Nakamoto H, Suzuki Y, Ito T, Aisaka K. Pharmacological profiles of a novel opioid receptor-like1 (ORL(1)) receptor antagonist, JTC-801. Br J Pharmacol. 2002;135:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson GM, Connors JM, Hardy SL, Valent M, Goodman RL. Oestradiol microimplants in the ventromedial preoptic area inhibit secretion of luteinizing hormone via dopamine neurones in anoestrous ewes. J Neuroendocrinol. 2001;13:1051–1058 [DOI] [PubMed] [Google Scholar]
- 33. Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18:534–541 [DOI] [PubMed] [Google Scholar]
- 34. Scott CJ, Tilbrook AJ, Simmons DM, et al. The distribution of cells containing estrogen receptor-α (ERα) and ERβ messenger ribonucleic acid in the preoptic area and hypothalamus of the sheep: comparison of males and females. Endocrinology. 2000;141:2951–2962 [DOI] [PubMed] [Google Scholar]
- 35. Skinner DC, Evans NP, Delaleu B, Goodman RL, Bouchard P, Caraty A. The negative feedback actions of progesterone on gonadotropin-releasing hormone secretion are transduced by the classical progesterone receptor. Proc Natl Acad Sci USA. 1998;95:10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karsch FJ, Legan SJ, Ryan KD, Foster DL. Importance of estradiol and progesterone in regulating LH secretion and estrous behavior during the sheep estrous cycle. Biol Reprod. 1980;23:404–413 [DOI] [PubMed] [Google Scholar]
- 37. Whisnant CS, Goodman RL. Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol Reprod. 1988;39:1032–1038 [DOI] [PubMed] [Google Scholar]
- 38. Goodman RL, Karsch FJ. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology. 1980;107:1286–1290 [DOI] [PubMed] [Google Scholar]
- 39. Goodman RL, Bittman EL, Foster DL, Karsch FJ. Alterations in the control of luteinizing hormone pulse frequency underlie the seasonal variation in estradiol negative feedback in the ewe. Biol Reprod. 1982;27:580–589 [DOI] [PubMed] [Google Scholar]
- 40. Lehman MN, Ebling FJ, Moenter SM, Karsch FJ. Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology. 1993;133:876–886 [DOI] [PubMed] [Google Scholar]
- 41. Goodman RL, Inskeep EK. Control of the ovarian cycle of the sheep. In: Neill JD, ed. Knobil and Neill's Physiology of Reproduction. 3rd ed Amsterdam: Elsevier; 2006;2389–2447 [Google Scholar]
- 42. Sohn JW, Williams KW. Functional heterogeneity of arcuate nucleus pro-opiomelanocortin neurons: implications for diverging melanocortin pathways. Mol Neurobiol. 2012;45:225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bhanot R, Wilkinson M. Opiatergic control of LH secretion is eliminated by gonadectomy. Endocrinology. 1983;112:399–401 [DOI] [PubMed] [Google Scholar]
- 44. Brooks AN, Lamming GE, Lees PD, Haynes NB. Opioid modulation of LH secretion in the ewe. J Reprod Fertil. 1986;76:693–708 [DOI] [PubMed] [Google Scholar]
- 45. Brooks AN, Haynes NB, Yang K, Lamming GE. Ovarian steroid involvement in endogenous opioid modulation of LH secretion in seasonally anoestrous mature ewes. J Reprod Fertil. 1986;76:709–715 [DOI] [PubMed] [Google Scholar]
- 46. Casper RF, Alapin-Rubillovitz S. Progestins increase endogenous opioid peptide activity in postmenopausal women. J Clin Endocrinol Metab. 1985;60:34–36 [DOI] [PubMed] [Google Scholar]
- 47. Currie WD, Rawlings NC. Naloxone enhances LH but not FSH release during various phases of the estrous cycle in the ewe. Life Sci. 1987;41:1207–1214 [DOI] [PubMed] [Google Scholar]
- 48. Devorshak-Harvey E, Bona-Gallo A, Gallo RV. Endogenous opioid peptide regulation of pulsatile luteinizing hormone secretion during pregnancy in the rat. Neuroendocrinology. 1987;46:369–378 [DOI] [PubMed] [Google Scholar]
- 49. Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ. Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology. 1995;136:2412–2420 [DOI] [PubMed] [Google Scholar]
- 50. Horton RJ, Cummins JT, Clarke IJ. Naloxone evokes large-amplitude GnRH pulses in luteal-phase ewes. J Reprod Fertil. 1987;81:277–286 [DOI] [PubMed] [Google Scholar]
- 51. Malven PV, Bossut DF, Diekman MA. Effects of naloxone and electroacupuncture treatment on plasma concentrations of LH in sheep. J Endocrinol. 1984;101:75–80 [DOI] [PubMed] [Google Scholar]
- 52. Quigley ME, Yen SS. The role of endogenous opiates in LH secretion during the menstrual cycle. J Clin Endocrinol Metab. 1980;51:179–181 [DOI] [PubMed] [Google Scholar]
- 53. Shoupe D, Montz FJ, Lobo RA. The effects of estrogen and progestin on endogenous opioid activity in oophorectomized women. J Clin Endocrinol Metab. 1985;60:178–183 [DOI] [PubMed] [Google Scholar]
- 54. Van Vugt DA, Bakst G, Dyrenfurth I, Ferin M. Naloxone stimulation of luteinizing hormone secretion in the female monkey: influence of endocrine and experimental conditions. Endocrinology. 1983;113:1858–1864 [DOI] [PubMed] [Google Scholar]
- 55. Yang K, Haynes NB, Lamming GE, Brooks AN. Ovarian steroid hormone involvement in endogenous opioid modulation of LH secretion in mature ewes during the breeding and non-breeding seasons. J Reprod Fertil. 1988;83:129–139 [DOI] [PubMed] [Google Scholar]
- 56. Wagner EJ, Rønnekleiv OK, Grandy DK, Kelly MJ. The peptide orphanin FQ inhibits β-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology. 1998;67:73–82 [DOI] [PubMed] [Google Scholar]
- 57. An XF, He M, Feng Y, Feng H, Yu JY. Central administration of Orphanin FQ inhibits GnRH secretion by ORL1 receptor in the median eminence of freely moving ovariectomized rats. Neurosci Bull. 2009;25:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nestor CC, Billings HJ, Hileman SM, et al. Orphanin FQ Acts Primarily at the Hypothalamus to Inhibit Pulsatile LH Secretion in Sheep. Chicago, Illinois: Society for Neuroscience; 2009 [Google Scholar]
- 59. Whisnant CS, Curto K, Goodman RL. Immunocytochemical localization of β endorphin and gonadal steroid regulation of proopiomelanocortin messenger ribonucleic acid in the ewe. Neuroendocrinology. 1992;56:812–821 [DOI] [PubMed] [Google Scholar]
- 60. Broad KD, Kendrick KM, Sirinathsinghji DJ, Keverne EB. Changes in pro-opiomelanocortin and pre-proenkephalin mRNA levels in the ovine brain during pregnancy, parturition and lactation and in response to oestrogen and progesterone. J Neuroendocrinol. 1993;5:711–719 [DOI] [PubMed] [Google Scholar]
- 61. Wetsel WC, Valença MM, Merchenthaler I, et al. Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proc Natl Acad Sci USA. 1992;89:4149–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Terasawa E, Keen KL, Mogi K, Claude P. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology. 1999;140:1432–1441 [DOI] [PubMed] [Google Scholar]
- 63. Olszewski PK, Grace MK, Sanders JB, Billington CJ, Levine AS. Effect of nociceptin/orphanin FQ on food intake in rats that differ in diet preference. Pharmacol Biochem Behav. 2002;73:529–535 [DOI] [PubMed] [Google Scholar]
- 64. Polidori C, de Caro G, Massi M. The hyperphagic effect of nociceptin/orphanin FQ in rats. Peptides. 2000;21:1051–1062 [DOI] [PubMed] [Google Scholar]
- 65. Pomonis JD, Billington CJ, Levine AS. Orphanin FQ, agonist of orphan opioid receptor ORL1, stimulates feeding in rats. Neuroreport. 1996;8:369–371 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.