Abstract
GnRH neurons form the final common pathway for the central control of reproduction. GnRH release occurs from terminals in the external layer of the median eminence (ME) for neuroendocrine control of the pituitary, and near GnRH-GnRH fiber appositions within the preoptic area (POA). Whether or not control of GnRH secretion by neuromodulators is different in these 2 areas is unknown. Mutations in neurokinin B (NKB) or the neurokinin-3 receptor (NK3R) are linked to hypogonadotropic hypogonadism in humans, suggesting that NKB may regulate GnRH secretion. Using fast scan cyclic voltammetry through carbon-fiber microelectrodes, we examined real-time GnRH release in response to the NK3R agonist senktide in the ME and POA. Coronal brain slices were acutely prepared from adult gonad-intact GnRH-green fluorescent protein male mice, and carbon-fiber microelectrodes were placed either within green fluorescent protein-positive terminal fields of the ME or near GnRH-GnRH fiber appositions in the POA. Senktide induced GnRH release consistently in the ME but not the POA, indicating that GnRH release is differentially regulated by NKB in a location-dependent manner. Senktide also induced GnRH secretion in the ME of kisspeptin-knockout (Kiss1 knockout) mice. Interestingly, release amplitude was lower compared with wild-type mice. These data indicate regulation of GnRH release by NK3R agonists is site specific and suggest that kisspeptin is not a required mediator between NK3R activation and GnRH secretion in the ME. This information will be useful for informing future models of afferent regulation of GnRH release.
GnRH neurons play a critical role in the central regulation of fertility. The somata of these neurons, primarily located in the preoptic area (POA), extend their processes into the median eminence (ME) where GnRH release occurs to stimulate pituitary gonadotropin secretion (1, 2). GnRH release also occurs perisomatically near GnRH-GnRH fiber appositions within the POA (3, 4). POA GnRH release may have neuromodulatory functions, as opposed to the neuroendocrine function of ME GnRH secretion. Whether control of GnRH release is different between these 2 areas is unknown.
Neuromodulators provide one type of input to regulate GnRH neuronal output. Recently, neurokinin B (NKB) was identified as an important modulator of reproductive neuroendocrine function and a potential regulator of GnRH neurons. Mutations in NKB or the neurokinin-3 receptor (NK3R) are linked to hypogonadotropic hypogonadism (5, 6), and central administration of NKB or the NK3R agonist senktide alters serum LH levels (7, 8), an indirect marker of GnRH release.
The effect of NKB or NK3R on GnRH neuron activity may be direct or indirect. NK3R immunoreactivity localized to GnRH terminals in the rat (9) suggests that direct action is possible. However, neither a consistent change in GnRH neuron firing frequency in response to NK3R agonists nor receptor immunoreactivity has been observed at the soma (9–12). With regard to indirect action on GnRH neurons, NKB and NK3R are expressed by a population of arcuate neurons that also coexpress kisspeptin and dynorphin (KNDy neurons) (13). KNDy neurons are an important upstream regulator of GnRH neurons (14, 15). One prevailing model suggests that NKB primarily serves to stimulate kisspeptin release from KNDy neurons and that kisspeptin, in turn, directly activates GnRH neurons (16–18).
Using fast scan cyclic voltammetry (FSCV) (3), we examined GnRH release in response to activation of NK3R. We demonstrate that activation of NK3R stimulates GnRH release in a location-dependent, but kisspeptin-independent, manner.
Materials and Methods
Animals
Adult (60–90 d) gonad-intact male transgenic GnRH-green fluorescent protein (GFP) mice (19) and male and female kisspeptin-knockout (Kiss1 KO) mice (generously provided by Dr Seminara and Dr Chan; Massachusetts General Hospital) were used (20, 21). Animals were fed Harlan 2916 chow (Harlan) and water ad libitum and were housed on a 14-hour light, 10-hour dark photoperiod (lights on 4 am eastern standard time). All procedures were approved by the University of Michigan University Committee on Use and Care of Animals.
Experimental methods
Brain slices (22, 23) and carbon-fiber microelectrodes (CFMEs) (3) were prepared as described. For FSCV measurements, CFMEs were placed either near GnRH neuron terminals within the ME or near GnRH-GnRH fiber appositions in the POA of brain slices. CFME potential was scanned from 0.5–1.45 V at 400 V/s every 100 ms. Potential was applied, and changes in current (I) were monitored using an extended-range (±2 V) EPC10 patch-clamp running PatchMaster (HEKA Elektronic). CFMEs were stabilized more than or equal to 15 minutes before recordings.
Experiment 1
FSCV was used to record GnRH release from the ME. We first tested whether air bubbles produced by solution changes generate a false positive signal. Spontaneous GnRH release was recorded for 8–10 minutes in artificial cerebrospinal fluid (ACSF), during which 2 vehicle solution changes were conducted. Next, the NK3R agonist senktide (10nM; Phoenix Pharmaceuticals) was bath applied for 2 minutes, followed by ACSF wash for 10–14 minutes. In some experiments, kisspeptin-10 (10nM; Phoenix Pharmaceuticals) was bath applied for 2 minutes, followed by an 8- to 10-minute washout, for positive control (data not shown). Ability to detect GnRH release was confirmed by application of 20mM potassium ACSF for 4 minutes. If potassium ACSF did not induce GnRH release, recordings were not included in analysis.
Experiment 2
FSCV recordings of GnRH release were made in the POA near GnRH-GnRH fiber appositions as above.
Experiment 3
FSCV recordings of GnRH secretion were made in the ME of Kiss1 KO mice. The ACSF control period was followed by senktide (10nM) for 2 minutes, then an 8- to 10-minute ACSF wash, then kisspeptin was applied as a positive control for 2 minutes, followed by ACSF, and finally a 4-minute application of 20mM potassium ACSF.
Data analysis
FSCV data were analyzed with Demon Voltammetry software (Wake Forest University). Cyclic voltammograms (CVs) were background subtracted using an average of 10 traces. A correlation was obtained between the background-subtracted CV and an average of CVs obtained after application of 5μM GnRH near a CFME placed in the cortex. An R2 of more than 0.9 was required to positively identify an event as GnRH release. Changes in GnRH concentration were estimated based on CFME calibration in 0.1μM–50μM GnRH (Figure 1). Data are expressed as mean ± SEM and were analyzed using 2-tailed Wilcoxon t tests in GraphPad (Prism); P < .05 was considered significant.
Figure 1.
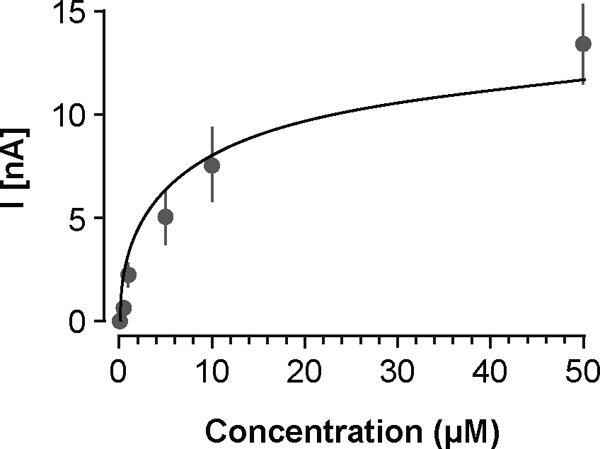
GnRH dose-response curve illustrating the relationship between GnRH concentration and voltammetric current. GnRH was applied at 0.1μM, 0.5μM, 1μM, 5μM, 10μM, and 50μM near CFMEs placed in the cortex. The data are fit with the semilogarithmic equation y = 5.0x + 3.1, R2 = 0.91.
Results
Senktide induces GnRH release in the ME (experiment 1)
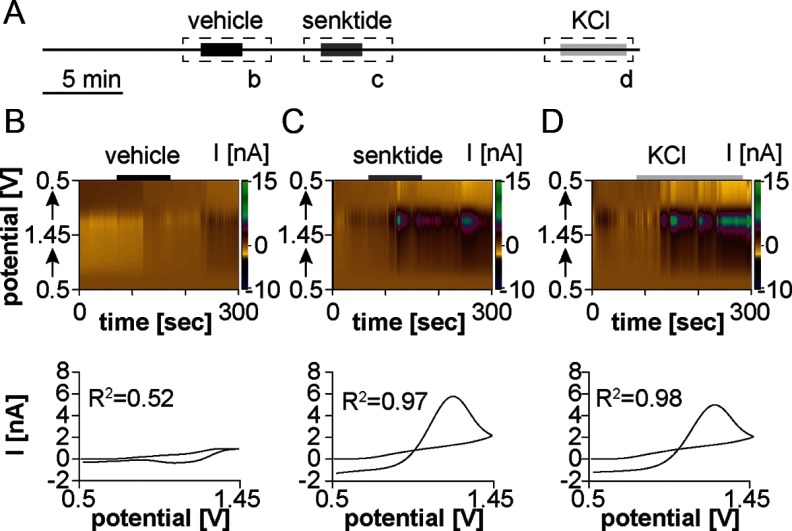
We first examined the effect of senktide (10nM) on GnRH release in the ME. Before treatments, spontaneous GnRH release was observed in 2 of 13 recordings (data not shown). This low frequency of spontaneous release is what may be expected over these relatively short-duration recordings based on the frequency of GnRH release (2). Observation of spontaneous GnRH release did not affect response to treatment. Figure 2A shows the experimental design. Changes in electrochemical current as a function of applied voltage and background-subtracted CVs are shown in Figure 2, B and C. Vehicle solution changes did not induce GnRH release, indicated by a lack of change in current during this transition and a flat CV (Figure 2B). In contrast, senktide consistently induced GnRH release from the ME (n = 11/13 slices from 7 mice [Figure 2C]; concentration change, 7.0 ± 0.6μM). GnRH release induced by 20mM potassium ACSF was of similar magnitude to that induced by senktide (concentration change, 10.2 ± 1.4μM; P > .1, Figure 2D).
Figure 2.
Senktide induces GnRH release from the ME of the adult male mouse. (A) Experimental design illustrating timing of vehicle solution change to control ACSF, senktide treatment, and KCl treatment. Dashed boxes with lower case letters depict the 5-minute periods shown in the top portion of B–D. Solid boxes within the dashed box indicate time of treatment. (B–D) Top, a color heat plot of electrochemical current as a function of voltage and time in response to either vehicle solution change (B), bath application of 10nM senktide application (C), or 20-mm high-potassium ACSF (D). Bottom, peak background-subtracted CVs after vehicle solution change (B), senktide treatment (C), or high-potassium ACSF (D).
Senktide fails to consistently induce GnRH release in the POA (experiment 2)
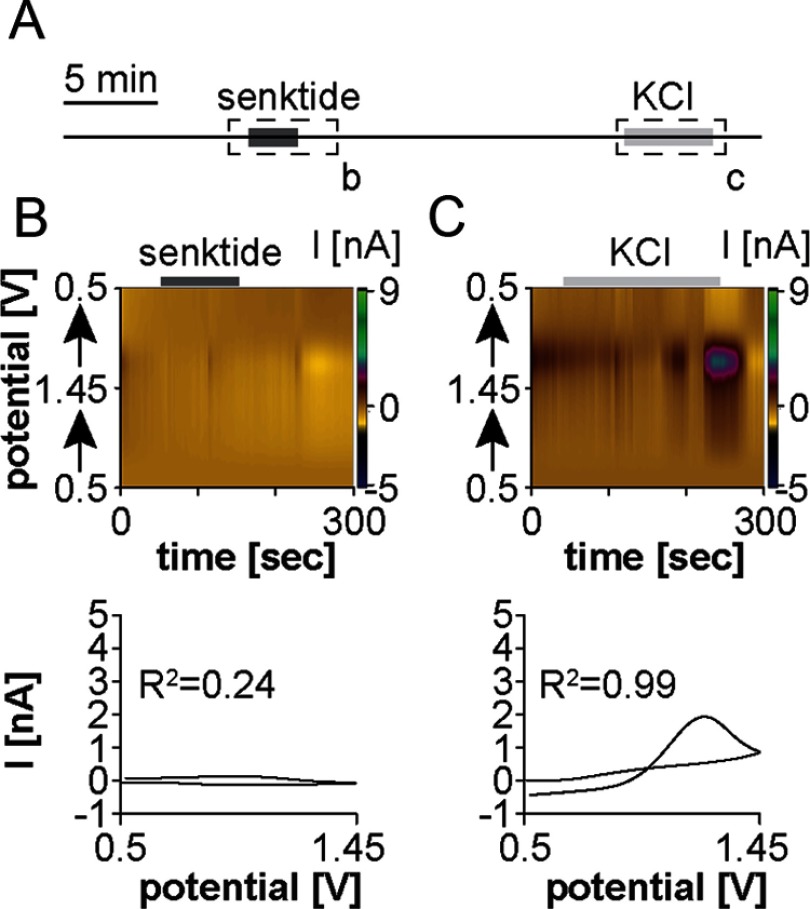
We next tested whether senktide induces GnRH release in the POA (Figure 3A). CFMEs were positioned near GnRH-GnRH fiber appositions visualized by green fluorescent protein. In contrast to the ME, 10nM senktide rarely induced GnRH release within the POA (n = 1/6 slices from 5 mice; concentration change, 2.6μM) (Figure 3B). Application of 20mM potassium ACSF generated GnRH release in all six recordings (Figure 3C), but the magnitude of release was lower (concentration change, 1.7 ± 0.3μM) than recordings from the ME (10.2 ± 1.4μM, P < .01). This is likely due to the high density of fibers within the ME in comparison with, at most, 2 GnRH-releasing elements being within range of the CFME in the POA.
Figure 3.
Senktide does not consistently induce GnRH release near GnRH-GnRH fiber appositions in the POA of the adult male mouse. (A) Experimental design illustrating time of senktide and KCl treatment. Dashed boxes with lower case letters depict the 5-minute periods shown in the top portion of B and C, solid boxes within the dashed box indicate time of treatment. (B and C) Top, a color heat plot of electrochemical current as a function of voltage and time in response to either bath application of 10nM senktide (B) or of 20mM potassium application (C). Bottom, peak background-subtracted CVs after 10nM senktide (B) or 20mM potassium (C) treatment.
Senktide-induced GnRH release in the ME does not require kisspeptin (experiment 3)
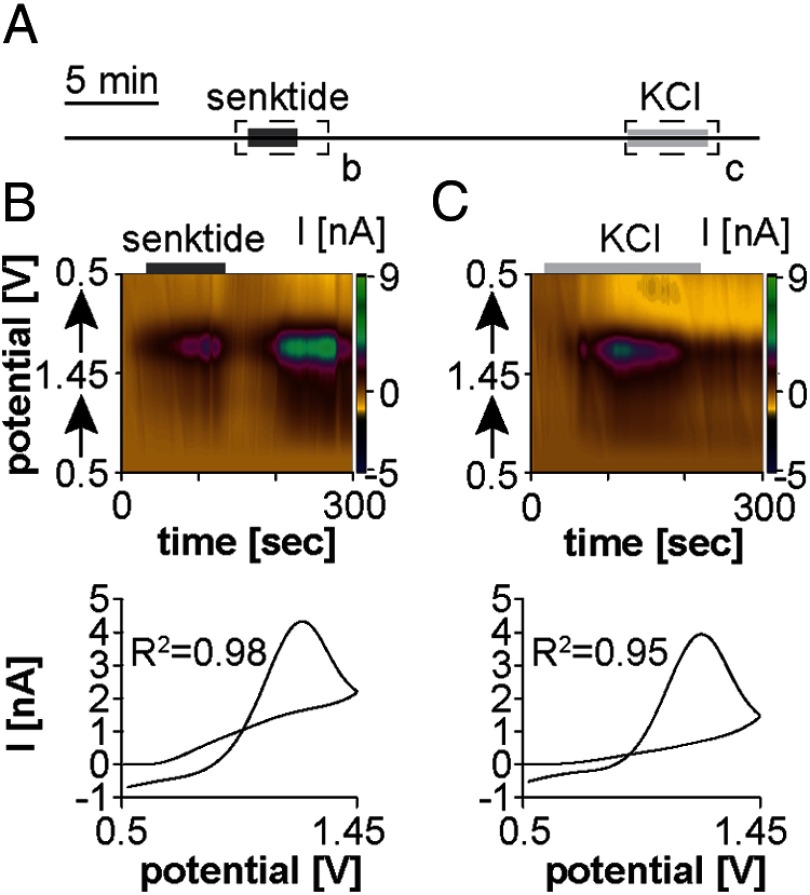
To examine whether senktide-induced GnRH release from the ME requires kisspeptin, recordings were performed in the ME of brain slices from Kiss1 KO mice (Figure 4A) (20, 21). Senktide induced GnRH release from the ME of all Kiss1 KO mice studied (n = 4/4 slices from 3 males; n = 3/3 slices from 2 females) (Figure 4B), indicating that kisspeptin is not required for GnRH release in this region. The changes in GnRH concentration in response to senktide were similar between males (2.6 ± 0.9μM) and females (3.7 ± 0.5μM; P > .15) and were combined for comparison with wild-type mice. Notably, the change in GnRH concentration after senktide application in the ME was lower in Kiss1 KO mice (3.1 ± 0.6μM) than in wild-type mice (7.0 ± 0.6μM, P ≤ .02), whereas the response to high-potassium ACSF was similar (6.3 ± 2.5μM, P ≥ .10, Figure 4C).
Figure 4.
Senktide induces GnRH release from the ME in kisspeptin knockout mice. (A) Experimental design illustrating time of senktide and KCl treatment. Dashed boxes with lower case letters depict the 5-minute periods shown in the top portions of B and C. Solid boxes indicate time of treatment. Top, a color heat plot of electrochemical current as a function of voltage and time in response to either bath application of 10nM senktide (B) or of 20mM potassium application (C). Bottom, peak background-subtracted CVs after 10nM senktide (B) or 20mM potassium (C) treatment.
Discussion
GnRH neurons integrate a number of inputs to regulate their secretory output. Studies investigating genetic mutations in infertile patients (6) identified NKB as an important modulator of reproductive neuroendocrine function. Here, we demonstrate the NK3R agonist senktide consistently induces GnRH release from the ME, but not the POA, of adult male mice, indicating that GnRH release is differentially regulated by NKB in a location-dependent manner. Further, senktide induced GnRH release from the ME of Kiss1 KO mice (20, 21), demonstrating that kisspeptin is not a required mediator between NK3R activation and GnRH secretion.
Recent studies examining the effect of NKB or the NK3R agonist senktide on LH release have produced conflicting results. Intracerebroventricular (icv) senktide treatment decreased serum LH in ovariectomized mice and rats (15, 24, 25) and in ovariectomized rats treated with estradiol in a model of steroid negative feedback (24, 26, 27), suggesting that senktide inhibits GnRH neural activity. In contrast, icv senktide increased serum LH in intact female rats (25) but had no effect (28) or stimulated LH secretion (11) in intact male rats. Disparities in the effect of senktide on LH levels among these experiments may be attributable to experimental paradigm, steroid milieu, and/or time of day. Additionally, the use of LH as a marker of GnRH may contribute to varying results, because the pituitary response to GnRH may be modulated by steroid milieu (29, 30).
Examination of GnRH release using FSCV in coronal brain slices from adult male intact mice revealed that senktide consistently induces GnRH release from the ME but not the POA. Although it is possible that release below the limit of detection occurs in the POA, arguing against this, the magnitude of GnRH release induced by the positive control (20mM potassium) was similar to that induced by senktide in both the POA and ME. Further, the change in GnRH concentration during both senktide- and potassium-induced release in the POA was also similar to that during spontaneous release in this region (3). Stimulation of GnRH release by senktide in the ME corroborates earlier studies showing that senktide treatment increased LH levels in intact male and female rats (11, 25). The region-dependent stimulation of GnRH release by senktide suggests site-specific trafficking of NK3R in GnRH neurons or in region-specific upstream elements. In this regard, GnRH terminal fibers exhibit NK3R immunoreactivity, whereas NK3R immunoreactivity at the soma is limited (9, 10). The present data and NK3R distribution are also consistent with the failure of senktide to consistently modulate GnRH neuron action potential firing rate measured at the soma (11, 12).
Senktide-induced GnRH release in the ME was not blocked in Kiss1 KO mice. This observation is likely to influence current models of afferent regulation of GnRH release, particularly with regard to regulation of GnRH release by KNDy neurons (13, 14). KNDy neurons make close appositions with GnRH cell bodies and terminals (14) and are thought to stimulate GnRH neurons primarily through kisspeptin output, rather than via NKB (13, 16–18). This postulate is supported by previous experiments demonstrating that icv or iv injection of senktide initiates an LH pulse (31–34) but fails to do so if a kisspeptin receptor antagonist is present (31), the animal is desensitized to kisspeptin receptor activation (35), or the kisspeptin receptor Kiss1r is knocked out (36).
In contrast, the present results indicate that senktide can activate GnRH release independent of kisspeptin and, when coupled with evidence demonstrating NK3R expression on GnRH terminals (9), suggest that this effect could be directly on GnRH neurons. Although it is premature to exclude any action via other intermediaries, these data challenge the dogma that kisspeptin is a mandatory intermediate for NK3R receptor activation of GnRH release. There are several possible explanations for this apparent discrepancy in requirement for kisspeptin. First, in studies examining the LH response to senktide after desensitization to kisspeptin (35), continuous exposure to kisspeptin over several days could reduce pituitary sensitivity to GnRH, preventing senktide-induced GnRH release from initiating an LH pulse. GnRH-induced LH release observed in these experiments, however, indicates that pituitary sensitivity to GnRH was maintained in both primates and Kiss1r KO mice (35, 36). It is important to point out that information on the regulation of NK3R expression, sensitivity, signaling, and subcellular localization by kisspeptin and other factors is limited. Thus, a similar desensitization to action at NK3R could occur under these various treatment regimens. Of interest, either prolonged intermittent senktide injection after GnRH priming or an iv bolus of senktide after continuous 99-hour treatment with the drug appears to blunt LH release (34, 35). Second, the magnitude of GnRH release from the ME in response to NK3R stimulation may not be enough to initiate an LH pulse under conditions in which the response to kisspeptin is reduced. Consistent with this postulate, in Kiss1 KO mice, the GnRH release in response to senktide was approximately half that of wild-type mice. If in vivo GnRH secretion in response to senktide when kisspeptin action is blocked is similarly reduced, this could account for a failure to observe a downstream LH response. Third, there are species-specific differences in kisspeptin/NKB systems. In rodents, the arcuate KNDy neural population is postulated to be involved in steroid negative feedback, whereas as kisspeptin neurons in the anteroventral periventricular play a more prominent role in steroid positive feedback (37). In contrast, in monkeys and ewes, kisspeptin neurons in the arcuate nucleus may be involved in both positive and negative steroid feedback (38–42), whereas the role of the anteroventral periventricular kisspeptin population is not fully understood. Additionally, NK3R has been detected on GnRH terminals in the ME of the rat (9) but has not yet been examined in mice or sheep. Thus, differences in NK3R expression, anatomy of kisspeptin and NKB afferent networks, and/or function of these networks could cause differences in response to NK3R activation.
It is also important to point out caveats of the experimental preparation used in the present study. The brain slice preparation allows for focal measurement of GnRH release, and although synaptic interactions are likely well maintained within the slice, other potentially crucial afferents to both GnRH and kisspeptin/NKB neurons may be removed, thereby affecting GnRH output. For example, if NK3R agonists activate systems in vivo that are both inhibitory and stimulatory to GnRH neuron activity (11), this may block GnRH release. In our slice preparation, stimulatory NKB/kisspeptin systems are more proximal to the ME and thus more likely to remain intact (43) than some inhibitory systems such as gonadotropin-inhibitory hormone (44). Nonetheless, the present results regarding kisspeptin-independent GnRH release stimulated by NK3R activation are of interest in helping to define operative nodes in the upstream regulatory network.
Finally, the genetic removal of the Kiss1 gene results in its absence from conception. Thus, compensatory mechanisms such as increased NK3R expression by GnRH neurons cannot be ruled out. The reduced GnRH release observed in response to senktide from Kiss1 KOs compared with wild-type mice might seem to argue against this, but it is important to bear in mind that, in the wild-type mice, the GnRH response likely reflects secretion in response to senktide itself as well as secretion as a result of senktide-induced kisspeptin release.
The present data demonstrate that activation of NK3R differentially regulates GnRH release depending on location and can occur independent of kisspeptin. This information will inform future models of the afferent regulation of GnRH release.
Acknowledgments
We thank Dr Stephanie Seminara and Dr Yee-Ming Chan for the kisspeptin knockout mice, as well as Laura Burger, Tony DeFazio, and Elizabeth Wagenmaker for editorial and/or technical assistance.
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01 HD34860.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACSF
- artificial cerebrospinal fluid
- CFME
- carbon-fiber microelectrode
- CV
- cyclic voltammogram
- FSCV
- fast scan cyclic voltammetry
- GFP
- green fluorescent protein
- icv
- intracerebroventricular
- Kiss1 KO
- kisspeptin-knockout
- KNDy
- kisspeptin, neurokinin B, dynorphin co-expressing neurons
- ME
- median eminence
- NKB
- neurokinin B
- NK3R
- neurokinin-3 receptor
- POA
- preoptic area.
References
- 1. Silverman AJ. The gonadotropin-releasing hormone (GnRH) neuron. In: Knobil E, Neill JD, ed. The Physiology of Reproduction. New York: Raven Press; 1994;1683–1709 [Google Scholar]
- 2. Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology. 1992;130:503–510 [DOI] [PubMed] [Google Scholar]
- 3. Glanowska KM, Venton BJ, Moenter SM. Fast scan cyclic voltammetry as a novel method for detection of real-time gonadotropin-releasing hormone release in mouse brain slices. J Neurosci. 2012;32:14664–14669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuenzalida LC, Keen KL, Terasawa E. Colocalization of FM1–43, Bassoon, and GnRH-1: GnRH-1 release from cell bodies and their neuroprocesses. Endocrinology. 2011;152:4310–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guran T, Tolhurst G, Bereket A, et al. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab. 2009;94:3633–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Navarro VM, Tena-Sempere M. Kisspeptins and the neuroendocrine control of reproduction. Front Biosci. 2011;3:267–275 [DOI] [PubMed] [Google Scholar]
- 8. Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489:372–386 [DOI] [PubMed] [Google Scholar]
- 10. Lucas LR, Hurley DL, Krause JE, Harlan RE. Localization of the tachykinin neurokinin B precursor peptide in rat brain by immunocytochemistry and in situ hybridization. Neuroscience. 1992;51:317–345 [DOI] [PubMed] [Google Scholar]
- 11. Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology. 2013;154:2761–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suter KJ, Song WJ, Sampson TL, et al. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419 [DOI] [PubMed] [Google Scholar]
- 20. Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol. 2009;21:1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 22. Chu Z, Moenter SM. Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci. 2005;25:5740–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online. 2003;5:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kinsey-Jones JS, Grachev P, Li XF, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153:307–315 [DOI] [PubMed] [Google Scholar]
- 25. Navarro VM, Castellano JM, McConkey SM, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sandoval-Guzmán T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026:307–312 [DOI] [PubMed] [Google Scholar]
- 27. Grachev P, Li XF, Kinsey-Jones JS, et al. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153:4894–4904 [DOI] [PubMed] [Google Scholar]
- 28. Corander MP, Challis BG, Thompson EL, et al. The effects of neurokinin B upon gonadotrophin release in male rodents. J Neuroendocrinol. 2010;22:181–187 [DOI] [PubMed] [Google Scholar]
- 29. Caraty A, Locatelli A, Martin GB. Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J Endocrinol. 1989;123:375–382 [DOI] [PubMed] [Google Scholar]
- 30. Clarke IJ, Cummins JT. Direct pituitary effects of estrogen and progesterone on gonadotropin secretion in the ovariectomized ewe. Neuroendocrinology. 1984;39:267–274 [DOI] [PubMed] [Google Scholar]
- 31. Grachev P, Li XF, Lin YS, et al. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PLoS One. 2012;7:e44344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Billings HJ, Connors JM, Altman SN, et al. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151:3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32:2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. García-Galiano D, van Ingen Schenau D, Leon S, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153:316–328 [DOI] [PubMed] [Google Scholar]
- 37. Smith JT. Sex steroid regulation of kisspeptin circuits. Adv Exp Med Biol. 2013;784:275–295 [DOI] [PubMed] [Google Scholar]
- 38. Blache D, Fabre-Nys CJ, Venier G. Ventromedial hypothalamus as a target for oestradiol action on proceptivity, receptivity and luteinizing hormone surge of the ewe. Brain Res. 1991;546:241–249 [DOI] [PubMed] [Google Scholar]
- 39. Caraty A, Fabre-Nys C, Delaleu B, et al. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology. 1998;139:1752–1760 [DOI] [PubMed] [Google Scholar]
- 40. Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides. 2009;30:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2007;19:432–438 [DOI] [PubMed] [Google Scholar]
- 42. Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod. 2010;83:568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsutsui K, Ubuka T, Bentley GE, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH): discovery, progress and prospect. Gen Comp Endocrinol. 2012;177:305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]