Abstract
Maternal care experienced during postnatal development has enduring effects on neuroendocrine function and behavior. Previous studies in rats have illustrated the effect of maternal licking/grooming (LG) on hormone receptors and maternal behavior of adult female offspring associated with altered DNA methylation. However, the developmental timing of these effects, which provide insight into the cellular and molecular pathways through which early experience alters later behavior, had not been explored. Here, we demonstrate the developmental emergence of these outcomes and use cross-fostering to identify sensitive periods for these effects. Estrogen receptor (ER)α and ERβ mRNA levels within the medial preoptic area (MPOA) of the hypothalamus were increased by postnatal day (PN)21 in female offspring of high LG dams; LG-associated increases in oxytocin receptor mRNA levels were observed beyond the weaning period. Quantification of ERα-immunoreactivity indicated a high degree of neuroanatomical specificity of LG effects within the MPOA that were observed by PN6. Reduced DNA methylation and histone 3 lysine 9 tri-methylation and increased histone 3 lysine 4 tri-methylation at the ERα gene promoter (Esr1) were detected at PN21 in high LG female offspring. Latency to engage in maternal behavior toward donor pups was significantly shorter among high LG females. Cross-fostering revealed that maternal sensitization and MPOA ERα levels are sensitive to maternal care experienced before but not after PN10. Differential windows of plasticity were identified for ERβ and oxytocin receptor mRNA levels. These studies contribute significantly to our understanding of the molecular, neurobiological, and behavioral pathways through which variation in maternal behavior is transmitted from one generation to the next.
Individual differences in hypothalamic hormone receptor levels are implicated in variation in complex social behaviors, including maternal care (1–3), pair bonding (4), sexual receptivity (5, 6), and aggression (7). Within the hypothalamus, the medial preoptic area (MPOA) is critical for maternal behavior (8–11), and studies in rodents have demonstrated that activation of oxytocin receptors (OTRs) and estrogen receptor (ER)α in the MPOA are necessary for nest building, pup retrieval, and pup licking/grooming (LG) (1, 11–14). Variation in ERα expression contributes to individual differences in maternal behavior and may also mediate variation in OTR levels (1, 15–17). Rat dams that engage in high frequencies of pup LG (high LG) have higher OTR density and ERα mRNA in the MPOA compared with low LG dams (1, 15). In light of these findings, a critical question is regarding the mechanistic pathways through which these individual differences emerge.
Postnatal mother-infant interactions contribute to variation in offspring hormone receptor levels and maternal behavior in adulthood (1, 15–18). Adult female offspring of high LG dams have elevated ERα mRNA and OTR density in the MPOA and exhibit increased maternal LG, in comparison with offspring of low LG dams (1, 19, 20). Cross-fostering studies (where fostering is conducted on the day of birth) suggest that it is the experience of LG during the postnatal period, rather than genetic or prenatal factors, that shapes these outcomes. Analyses of the promoter region of the ERα gene (Esr1) indicate that adult offspring of high LG dams have reduced DNA methylation levels, suggesting an epigenetic effect of maternal care on ERα (2). However, the developmental emergence of this LG-associated epigenetic variation has yet to be established.
We hypothesized that variation in ER levels and epigenetic regulation of Esr1 among female offspring of low and high LG dams would emerge in the early postnatal period when group differences in maternal LG are most pronounced. Thus, we proposed these molecular changes to be primary responses to maternal LG rather than downstream consequences that emerge in later life (which we predicted would be the case for OTR). Our experiments examined ERα, ERβ, and OTR across postnatal development in the MPOA of females reared by high and low LG dams. To determine the contribution of epigenetic mechanisms to this developmental effect, we analyzed DNA methylation and chromatin modifications associated with the Esr1 regulatory region. Finally, based on our finding of postnatal emergence of variation within the developing MPOA, we hypothesized that offspring would display sensitivity to the maternal environment experienced early rather than later in development. To assess these sensitive periods, female offspring were cross-fostered between low and high LG dams at 2 postnatal time points and assessed on measures of maternal sensitization and hypothalamic hormone receptor expression. Our findings suggest that there are windows of sensitivity to the effects of maternal care and that manipulation of the rearing environment during a critical window of the postnatal period can shift these molecular and behavioral outcomes.
Materials and Methods
Animals
Long-Evans rats (Charles River) were maintained on a 12-hour light, 12-hour dark schedule (lights on at 8 am) with food and water provided ad libitum. Adult virgin females were mated for 1 week and singly housed 1–2 days before parturition. Weaned offspring were pair-housed by sex. All procedures were performed in accordance with guidelines of the National Institutes of Health regarding the Guide for the Care and Use of Laboratory Animals and with the approval of the Columbia University Institutional Animal Care and Use Committee.
Maternal behavior
Home cage maternal behavior was scored as previously described (19, 21). Maternal behavior was observed for 5 60-minute observation periods daily during postnatal day (PN)1–PN6. Behavioral observations were made every 3 minutes during each observation period for a total of 600 observations per litter. Frequency of LG behavior was calculated as the number of observations of LG divided by the total number of observations. Low and high LG dams were defined as engaging in LG frequencies that were either 1 SD below (low LG) or above (high LG) the mean LG of the cohort. In one cohort, low (n = 4) and high (n = 4) LG dams were observed on PN10 for 1 hour, and the number and duration of LG bouts (discrete, continuous periods of LG) were continuously recorded (for methodological description, please see Ref. 22).
Tissue collection
Female offspring of low and high LG dams were killed at the day of birth (PN0), PN6, PN21 at weaning, as young adults at PN66, or as lactating dams on postpartum day 6 (n = 6–8 animals/time point per group). Dissected brain tissue was from female siblings sampled at multiple developmental time points (ie, 1 female per litter per time point). Whole brains were removed and immediately frozen at −80°C. The anterior ventral medial region of the hypothalamus (bregma 0.12 to −0.72), containing the MPOA, was dissected from one half of each brain in a cryostat cooled to −20°C. The other half of each brain was dissected and used for chromatin immunoprecipitation (ChIP) assay.
RNA and DNA extraction
Dissected MPOA samples were weighed and homogenized in 700-μL lysis buffer RLTplus (QIAGEN) with 1% β-mercaptoethanol using a tissue homogenizer (Omni) for 15 seconds. RNA and DNA were extracted using a dual RNA-DNA extraction kit (QIAGEN). cDNA was created from RNA using a reverse transcription kit (Applied Biosystems). Samples were stored at −20°C.
Gene expression
Relative gene expression was measured by real-time semiquantitative PCR (qPCR) on a 96-well 7500Fast qPCR machine (Applied Biosystems) using SybrFast. All primers were designed to span exons and had 87%–110% efficiency in a standard curve, with single melt peaks. Primer pairs are included in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. Calculations of relative gene expression of ERα (Esr1), ERβ (Esr2), OTR (Otr), and DNA methyltransferases (Dnmt1 and Dnmt3) were conducted using the 2−ΔΔCt method (23, 24) with cyclophilin-A (CypA) and β-actin (Actb) as control genes and normalizing to PN0 low LG offspring.
Immunohistochemistry
At PN6, female offspring (n = 5–6/group) were decapitated and whole brains placed into 4% paraformaldehyde in PBS for 48-hour fixing (2 h at room temperature and then at 4°C). Adult lactating females (n = 6/group) were anesthetized and transcardially perfused with PBS followed by 4% paraformaldehyde, and brains were postfixed overnight in 4% paraformaldehyde at 4°C. Brains were then dehydrated in 30% sucrose at 4°C until isotonic and stored at −80°C. Coronal sections (40 μm) were sliced on a cryostat and floated into PBS. Hypothalamic sections were washed in PBS and blocked with normal goat serum before incubating in primary antibody (rabbit-anti-ERα, 1:3000; Santa Cruz Biotechnology) at 4°C overnight. Signal was amplified with biotinylated goat-antirabbit (Vectastain) and visualized by horseradish peroxidase (Vector SG), and slices were mounted on gelatin-coated slides. Slides were dehydrated in a series of ethanol washes before clearing with xylene and coverslipping.
Imaging and cell count
Slides were imaged on an Olympus light microscope. The relative anterior-posterior position of the slice was noted. ERα-expressing cell counts were determined using MCID Core software (InterFocus Imaging Ltd). An observer blind to condition outlined each region of interest for nuclei-specific analysis. Statistics were performed on an animal's mean count across sections, except where noted.
Bisulfite conversion and pyrosequencing
Analysis of DNA methylation within cytosine-guanine nucleotide sequences (CpG sites) was conducted using DNA extracted from the MPOA of female offspring of low and high LG dams. DNA purified from the MPOA (1 μg) was bisulfite converted, and the Esr1 regulatory region “B/1b” (GenBank X98236.1; 25) (see Figure 3 below) was amplified with a biotinylated reverse primer (EpigenDX). The rat promoter B has 87% homology to human Esr1 promoter B and contains necessary elements for transcriptional regulation (2, 25, 26). Transcription initiated from promoter B includes a 5′-untranslated exon “1b” (25). This B/1b sequence includes the same 14 CpG sites previously assessed by bisulfite sequencing (2). Samples were pyrosequenced on a 96-well pyrosequencer (PSQ™96HS; QIAGEN) with 2 sets of sequencing primers (EpigenDX: ADS1511 and ADS1512). Percent methylation was determined by the ratio of C to T incorporation.
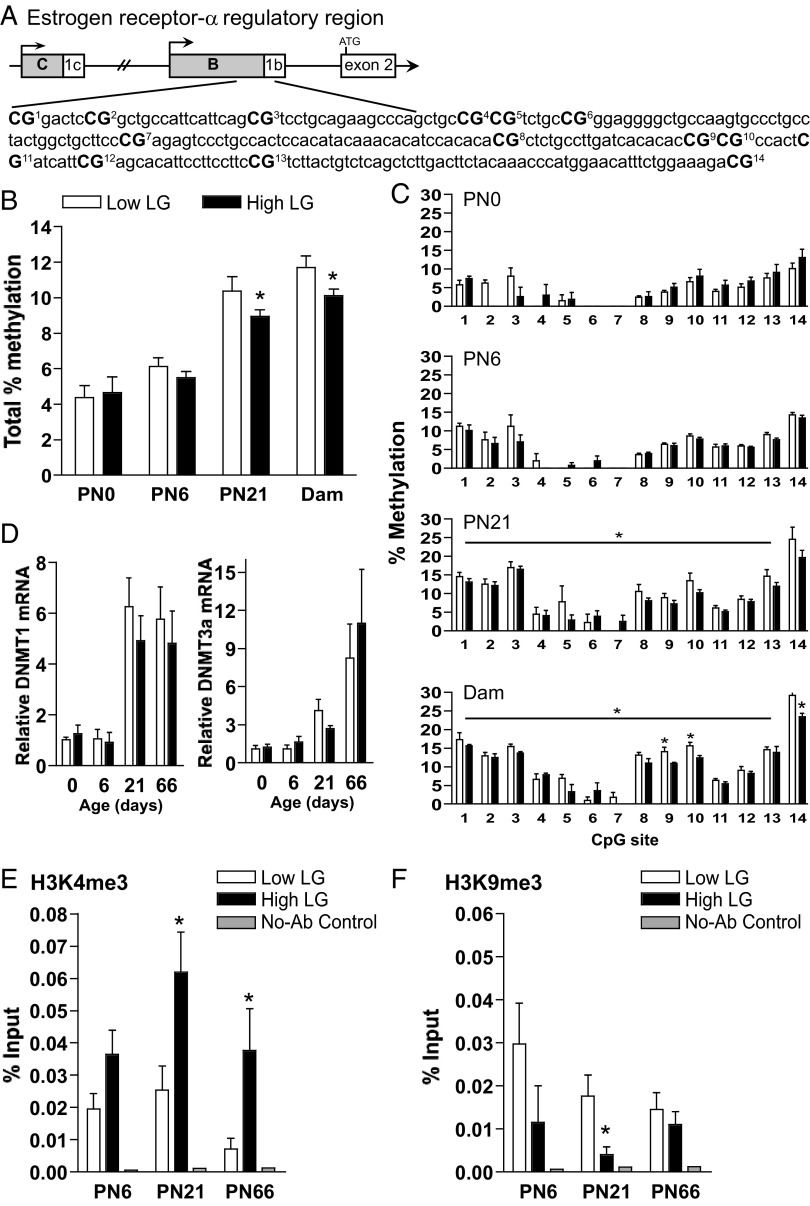
Figure 3.
Epigenetic regulation of Esr1 in female offspring of low and high LG dams. A, Schematic of the Esr1 gene regulatory region, the proximal B promoter and 5′UTR (untranslated region) 1b; the first translated exon is exon 2 (adapted from Freyschuss and Grandien [25]; Ensembl ENSRNOT00000026350). Fourteen CpG sites in the B/1b regulatory region were analyzed by bisulfite pyrosequencing and are numbered and indicated in bold. B, Mean ± SEM total percent methylation from all 14 CpG sites among low and high LG female offspring at PN0, PN6, and PN21 and among adult females at postpartum day 6 (Dam). C, Mean ± SEM percent methylation at each CpG site among low and high LG females at each developmental stage. D, Mean ± SEM Dnmt1 and Dnmt3a relative mRNA expression among low and high LG offspring across postnatal development. ChIP of the Esr1 B/1b regulatory region by antibodies against (E) H3K4me3 or (F) H3K9me3. The B/1b regulatory region amplified (+22 to +151) includes 6 of the 14 CpG sites analyzed for methylation. Mean ± SEM percent input among low and high LG female offspring at PN6, PN21, and PN66. *, P < .05.
Chromatin immunoprecipitation
DNA and protein from microdissected MPOA samples were cross-linked in 1-mL cold PBS with 1% formaldehyde and protease inhibitors for 15 minutes at room temperature, with rotation. Tissue was washed in PBS and protease inhibitors and then homogenized for 10 seconds. Samples were spun down at 2000 rpm for 5 minutes at 4°C and resuspended in ice-cold cell lysis buffer (Millipore) with protease inhibitors and incubated on ice for 15 minutes with occasional gentle vortexing. Samples were spun down again and resuspended in nuclear lysis buffer (Millipore) with protease inhibitors. Sonication was done in an ice bath using a Bioruptor (Diagenode) for 30 cycles of 30 seconds on and 30 seconds off. Temperatures were kept at 4°C–6°C. Gel analysis of sheared DNA confirmed DNA fragments between 200 and 700 bp. Samples were diluted 10-fold in ChIP dilution buffer (Millipore) with protease inhibitors: 20 μL from each was saved as “input,” and 50 μL were used for immunoprecipitation with antibody (antitrimethyl-histone H3 lys 4 [H3K4me3] or antitrimethyl-histone H3 lys9 [H3K9me3]; ChIP grade from Upstate/Millipore). Immunoprecipitation, washing, decross-linking, clean-up, and elution was conducted with Magna ChIP kit (Millipore). Elutant was stored at −20°C until analysis with qPCR. Input (5 μL) of samples was decross-linked by incubation with proteinase K at 62°C for more than 4 hours followed by column-based purification (QIAGEN PCR cleanup kit). Samples and input were analyzed by qPCR and “percent input” was calculated. Primers used in qPCR (see Supplemental Table 1) were designed to amplify the upstream Esr1 promoter region, the Esr1 B/1b regulatory region corresponding to the sequence analyzed by bisulfite pyrosequencing, and first translated Esr1 exon (Ensembl ENSRNOT00000026350 exon 2).
Cross-fostering
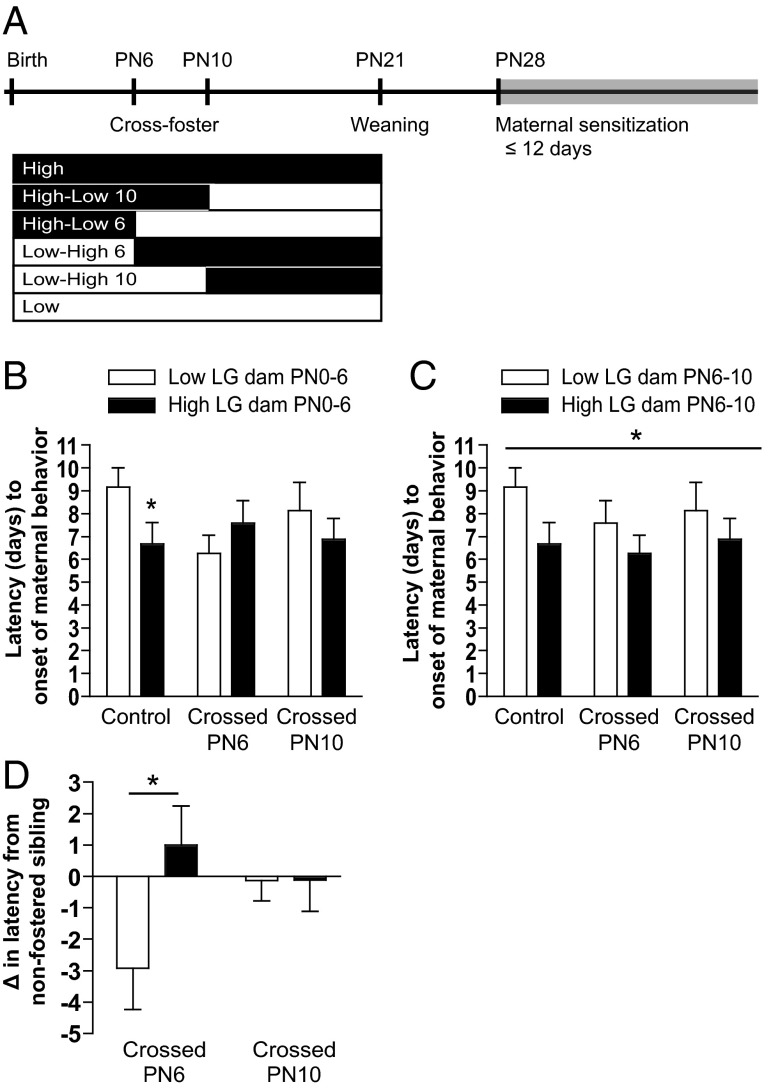
In order to assess sensitive periods for the effect of maternal care on offspring gene expression and behavior, offspring of low LG and high LG litters were divided into 3 conditions: 1) reared by birth dam from birth until weaning, 2) cross-fostered at PN6 between low LG and high LG dams (“low-high 6” and “high-low 6”), or 3) cross-fostered at PN10 between low LG and high LG dams (“low-high 10” and “high-low 10”). Each low LG litter was paired to a high LG litter, and offspring were switched between them on cross-fostering days. Consistent with cross-fostering performed at birth in previous studies (2), multiple pups were cross-fostered at each time point to minimize potential differential treatment of a single new pup added to the litter. At PN6, 1 female and 2 male offspring were cross-fostered together. At PN10, 1 female and 1 male sibling were cross-fostered together. This methodological approach was used to maintain litters at 50% biological offspring. Pups were cross-fostered between litters born on the same day, and all pups were observed to be successfully fostered. Pictures of the unique back pattern of each pup were taken to identify cross-fostered offspring. Offspring were singly housing at weaning in preparation for maternal sensitization testing.
Juvenile maternal sensitization
Juvenile (PN28) virgin female offspring of low and high LG dams (n = 12 animals/group) and female offspring cross-fostered between low and high LG dams at PN6 (n = 12 animals/group) and PN10 (n = 7–8 animals/group) were assessed for maternal sensitization. Maternal sensitization involves exposing test subjects to neonates daily until they display maternal behavior toward the neonates; continual pup exposure or treatment with hormones is known to induce maternal responsivity in otherwise nonmaternal adults (27, 28). Maternal sensitization was conducted as previously described (1, 29). Animals were tested daily between 3 and 5 pm beginning on PN28. Each day, 3 recently fed, 2- to 5-day-old pups from a donor litter were placed in 3 quadrants in the cage. Test animals were observed continuously for 1 hour. Donor pups were left in the cage for 23 hours. On test days 2–12, pups from the previous session were removed, and 30 minutes later, a new set of recently fed pups of the same age were introduced into each test cage, thereby commencing another 1-hour test session. Testing continued for 12 consecutive days or until a female displayed full maternal behavior. Females were scored as fully maternal if they retrieved all 3 test pups to the nest and crouched over them within the 60-minute test session. Juveniles failing to respond in this way were assigned a score of 13. After induction of maternal behavior or on day 12, juveniles were killed and brains extracted for analysis of gene expression.
Statistics
All statistics were performed using SPSS (PASW, IBM, version 18.0). Litters were not standardized in size or male/female ratio, and these variables were used as covariates. Two-tailed Student's t test was used for single comparisons among high and low LG animals. Main effects and interactions were determined using ANOVA. Repeated-measures ANOVA was used to determine effects with multiple time points sampled (behavior and gene expression) where described within the results. Generalized Wilcoxon test was used for Kaplan-Meier survival analysis, as indicated. All significance thresholds were set at P < .05. Outliers were defined as more than 2 SDs from the group mean and were removed from analysis as stated in the results to avoid type II error.
Results
Postpartum maternal LG behavior in low LG vs high LG dams
LG frequency (averaged across PN1–PN6) was significantly elevated among high compared with low LG dams (t1,14 = 8.23, P < .001) (Supplemental Figure 1A), and repeated-measures analysis indicated a significant effect of day (F5,14 = 6.54, P < .001) (Supplemental Figure 1B) and maternal LG (F1,14 = 50.74, P < .001). Additional observations were conducted on PN10 to determine the number and length of LG bouts among low and high LG dams at this later time point. No group differences were found in the number of LG bouts on PN10 (P > .2) (Supplemental Figure 1C). However, average LG bout length was found to be significantly longer in duration in high compared with low LG dams (t1,8 = 2.70, P < .05) (Supplemental Figure 1D). This analysis suggests that behavioral differences between high and low LG dams extend beyond PN6.
Developmental timing of the effects of LG on gene expression in the MPOA
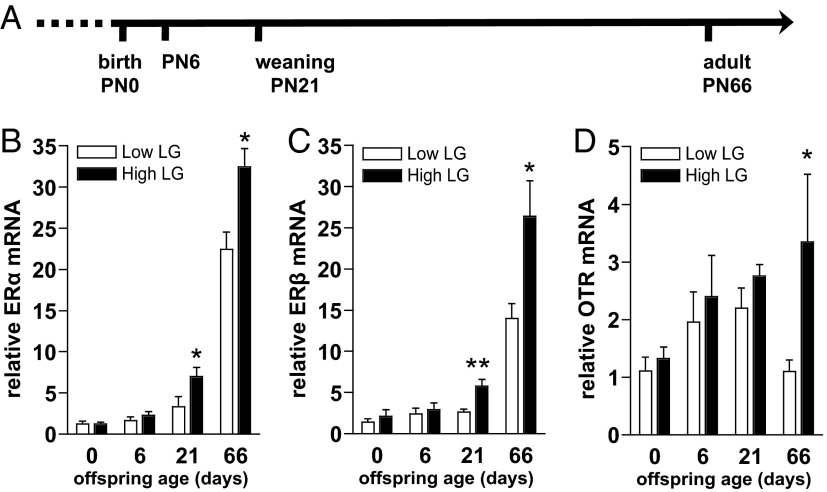
To determine whether neuroendocrine changes apparent in adult female offspring that experience varying levels of maternal care (2, 15) can be observed at the level of transcription during postnatal development, brains were collected from female offspring of low and high LG dams at PN0, PN6, PN21, and PN66 (Figure 1A). Levels of ERα, ERβ, and OTR mRNA were analyzed in the MPOA at each developmental age. We found a main effect of maternal LG (F1,54 = 7.69, P < .01) and age (F3,54 = 83.79, P < .001) and an LG by age interaction (F3,54 = 3.41, P < .05) on relative ERα mRNA (Figure 1B). ERα mRNA was elevated among high compared with low LG female offspring at PN21 (P < .01) and in adulthood (P < .05), whereas no group differences were found at birth or PN6 (P > .13). Three animals were determined to be outliers in ERβ expression and were removed from analysis (n = 1 PN21 low, n = 1 PN66 low, n = 1 PN66 high; removal was specific to ERβ analyses). ERβ mRNA varied as a function of maternal LG and age (maternal LG: F1,51 = 13.28, P < .001; age: F3,51 = 54.59, P < .001), with a significant interaction between LG and age (F3,51 = 5.61, P < .01) (Figure 1C). Similar to ERα, ERβ mRNA was elevated among high compared with low LG female offspring at PN21 (P < .01) and in adulthood (P < .05), whereas no differences were found at birth or PN6 (P > .45). Additionally, we examined levels of OTR mRNA, because OTR signaling is likewise critical for maternal behavior (16), downstream of estrogen-induced ER activation (17), and previously found to be elevated among adult female offspring of high LG dams (1). We found a main effect of maternal LG (F1,54 = 4.64, P < .05) with a trend for an effect of age (F3,54 = 2.13, P = .11), on relative OTR mRNA (Figure 1D). OTR mRNA was elevated among high compared with low LG female offspring in adulthood (F1,13 = 6.61, P < .05), with no significant effects of LG at other developmental time points.
Figure 1.
Developmental effects of LG on offspring gene expression in the MPOA. A, Timeline indicating ages at which brains of female offspring of low and high LG dams were compared for gene expression. Mean ± SEM (B) ERα, (C) ERβ, and (D) OTR relative mRNA among low and high LG offspring across postnatal development. *, P < .05; **, P < .01.
ERα levels in the MPOA of low and high LG female offspring at PN6
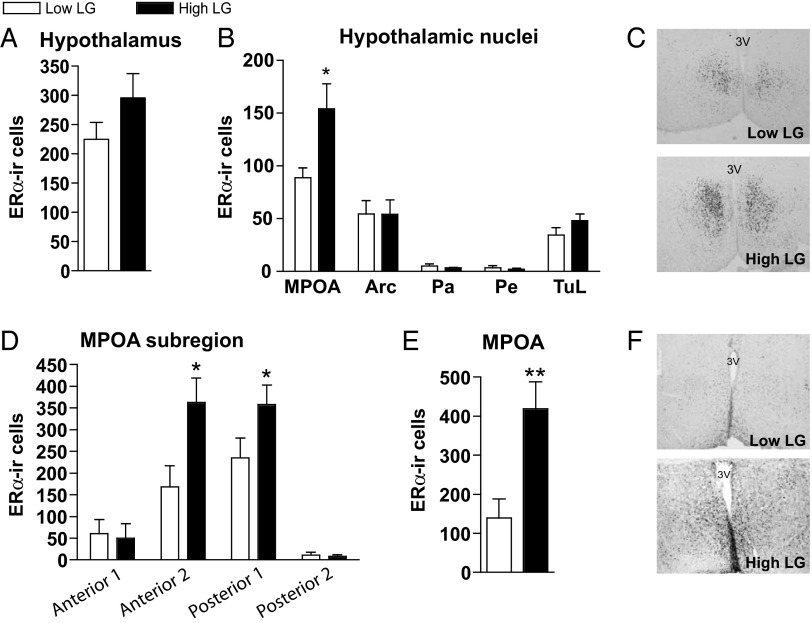
Although group differences in Esr1 (ERα) expression were not evident until PN21, we hypothesized that LG-associated effects on ERα levels would emerge earlier in development (<PN7), when group differences in LG are most pronounced, and be specific to neuroanatomical locations within the hypothalamus. Brains of PN6 female offspring of low LG (n = 6) and high LG dams (n = 5) were immunostained for ERα. ERα-immunoreactivity (ERα-ir) was observed in the MPOA, paraventricular nucleus, periventricular nucleus, arcuate nucleus, and tubercle. When all nuclei were combined in the analysis, there was no effect of maternal LG on ERα cell count or cell density (P > .2) (Figure 2A), a finding consistent with the mRNA data at this age. However, a significant effect of maternal LG was found within the MPOA on the average number of ERα-ir cells (t1,9 = 2.75, P < .05) (Figure 2B) and on cell density (t1,9 = 2.90, P < .05) when this brain region was analyzed separately. This effect was not observed in any other nucleus expressing ERα (Figure 2B). There were no group differences in the average size (square millimeters) of any nucleus within the hypothalamus (P > .2). We then subdivided the MPOA into 4 anterior-posterior sections corresponding to adult relative locations to bregma: anterior 1 (0.24 to −0.12), anterior 2 (−0.12 to −0.48), posterior 1 (−0.48 to −0.84), and posterior 2 (−0.84 to −1.32). Repeated-measures ANOVA indicated a significant effect of LG (F1,9 = 11.79, P < .01), section (F3,9 = 25.53, P < .001), and interaction between LG and section (F3,9 = 3.00, P < .05, sphericity assumed). Maternal LG had a significant effect (high LG > low LG) on the number of ERα-ir cells in the middle 2 portions of the MPOA (t1,9 < 2.63, P < .05) but not the most anterior or posterior regions (P > .75) (Figure 2D). These results confirm that variation in offspring hypothalamic ERα emerges proximal to the timing of differential maternal care rather than as a consequence of increased gonadal hormones in later life (occurring during puberty or gestation).
Figure 2.
LG effects on ERα-expressing cells in PN6 and adult hypothalamus. Comparison of ERα-ir cells (mean ± SEM) in PN6 female offspring of low and high LG dams within (A) total hypothalamus and (B) all hypothalamic regions showing any ERα expression: MPOA, arcuate nucleus (Arc), paraventricular nucleus of the hypothalamus (Pa), periventricular nucleus of the hypothalamus (Pe), and lateral tubercle (TuL). C, Representative images of ERα-ir cells within the MPOA of low and high LG females at PN6. D, ERα-ir cells in the PN6 MPOA, subdivided into 4 anterior-posterior sections. E, ERα-ir cells in the MPOA of adult lactating (postpartum d 6) female offspring of low and high LG dams. F, Representative images of ERα-ir cells within the MPOA of low and high LG lactating females. *, P < .05, **, P < .01.
Effects of maternal LG on ERα levels in the MPOA area are sustained into adulthood
The maintenance of group differences in ERα-ir cells in the MPOA through adulthood was determined in adult lactating dams. The average number of ERα-ir cells within the MPOA was significantly elevated in high LG compared with low LG lactating female offspring (t1,10 = 3.28, P < .01) (Figure 2, E and F). Repeated-measures ANOVA indicated a significant main effect of section (F3,14 = 7.44, P < .01) and of maternal LG (F1,7 = 12.84, P < .01). Consistent with PN6 findings, ERα-ir cells were elevated within the 2 middle portions of the MPOA among high compared with low LG females (P < .05).
Maternal LG alters DNA methylation within the Esr1 regulatory region
Variation in postnatal maternal LG has been associated with altered DNA methylation within the Esr1 B/1b sequence in the hypothalamus of adult female offspring (2). We examined the developmental onset of differences in DNA methylation associated with the experience of low vs high maternal LG across 14 CpG sites in the Esr1 B/1b regulatory region (Figure 3A). Analysis of average percent methylation for all CpG sites, with litter as a covariate, revealed a main effect of age (F3,43 = 51.41, P < .001) and maternal LG (F1,43 = 5.86, P < .05). Increased total Esr1 promoter 1b methylation was found among low compared with high LG offspring at PN21 (P < .05) (Figure 3B) and in adult lactating females at postpartum day 6 (P < .05). Significant differences in Esr1 DNA methylation were not detected at birth or at PN6. These results were confirmed by repeated-measures analysis accounting for percent methylation at each individual CpG site with a significant effect of maternal LG at PN21 (F1,9 = 5.55, P < .05) (Figure 3C) and at postpartum day 6 (F1,9 = 8.80, P < .05). Analyses examining methylation levels at individual CpG sites found significant group differences (P < .05) at CpG9, CpG10, and CpG14 among adult lactating females. We have previously observed region-specific differences in DNA methylation to correspond to differences in the expression of DNA methyltransferases (DNMTs) (30). Comparison of DNMT1 and DNMT3a mRNA levels of low and high LG female offspring across development revealed a significant effect of age but not of maternal LG (age: F3,54 > 9.48, P < .001) (Figure 3D).
Developmental timing of maternal LG effects on chromatin remodeling at Esr1
Trimethylation at 2 lysine residues on the histone 3 tail is associated with active (H3K4me3) and repressed (H3K9me3) gene transcription (31–34). We found a significant effect of maternal LG (F1,35 = 13.97, P < .001) (Figure 3E) and a trend for a main effect of age (F2,35 = 2.90, P = .07) on the association of H3K4me3 with the Esr1 B/1b region. In offspring of high compared with low LG dams, there was a significant increase in Esr1 B/1b association with H3K4me3 at PN21 (P < .05) and PN66 (P < .05). We also found an effect of maternal LG on the association of H3K9me3 with the Esr1 B/1b region (F1,35 = 5.84, P < .05) (Figure 3F) and with the first translated exon (F1,35 = 4.38, P < .05) (Supplemental Figure 2B). At PN21, H3K9me3-Esr1 B/1b was significantly increased in low compared with high LG female offspring (P < .05) (Figure 3F). Differences in chromatin remodeling associated with LG were not detected within a more upstream region of the promoter (Supplemental Figure 2, A and B).
Postnatal maternal LG predicts maternal sensitization in juvenile female offspring
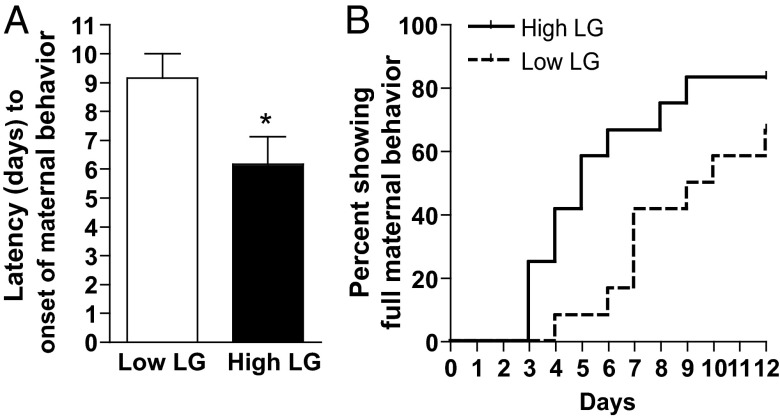
Based on the findings of maternal LG-associated effects on ERα-ir, gene expression and Esr1 epigenetic regulation occurring by PN21, we predicted that offspring maternal behavior would likewise emerge before adulthood. Indeed, we found that latency to maternal sensitization was significantly shorter in juvenile female offspring reared by high compared with low LG dams (t1,22 = 2.36, P < .05) (Figure 4A). This finding was confirmed using Kaplan-Meier survival analysis (Generalized Wilcoxon test, X21,22 = 4.88, P < .05) (Figure 4B).
Figure 4.
Maternal sensitization latency among low and high LG female offspring. A, Mean ± SEM latency of low or high LG juvenile female offspring to engage in maternal behavior toward donor pups. B, Survival analysis showing percent of fully maternal low and high LG female offspring each day until the end of testing at 12 days. *, P < .05.
Sensitive periods for maternal LG-associated variation in maternal sensitization
To determine whether maternal sensitization is sensitive to maternal LG experienced at different time points in the preweaning period, female offspring were cross-fostered between low LG and high LG dams at PN6 or at PN10 (Figure 5A) and tested for juvenile maternal sensitization latency. ANOVA with initial rearing condition (low/high LG), whether the pup was cross-fostered at PN6, and whether cross-fostered at PN10 as independent factors revealed that the effect of initial rearing condition (PN1–PN6) on offspring maternal sensitization latency is significantly altered by cross-fostering at PN6 (initial rearing condition by PN6 fostering interaction: F1,62 = 6.60, P < .05) (Figure 5B) but not by cross-fostering at PN10 (P > .8). Because cross-fostering at PN6 produced an altered behavioral phenotype, but cross-fostering at PN10 did not, we subsequently determined whether there was an effect of the maternal LG experienced between PN6 and PN10 on offspring maternal sensitization latency. ANOVA with PN6–PN10 rearing condition (low/high LG), whether the pup was cross-fostered at PN6, and whether cross-fostered at PN10 as independent factors, revealed a main effect of PN6–PN10 maternal care on maternal sensitization latency (F1,62 = 4.15, P < .05) (Figure 5C). Regardless of what rearing condition preceded or followed, rearing by a high LG dam from PN6–PN10 significantly reduced maternal sensitization latency (main effect of rearing condition PN6–PN10: F1,62 = 7.07, P < .01) (Figure 5C). We also compared the change in maternal sensitization behavior in fostered vs nonfostered siblings (Figure 5D) and found an interaction between initial dam and cross-fostering at PN6 (F1,62 = 5.22, P < .05) but no interaction with cross-fostering at PN10. Taken together, these analyses indicate that cross-fostering female offspring at PN10 has no significant effect on maternal sensitivity, whereas cross-fostering females at PN6 produces a shift in maternal sensitivity corresponding to the rearing condition provided by the adoptive dam.
Figure 5.
Maternal sensitization latency among offspring cross-fostered between low and high LG dams during the postnatal period. A, Timeline of cross-fostering and maternal sensitization testing and rearing conditions created. Offspring were cross-fostered between low LG and high LG dams at PN6 and PN10 or were nonfostered controls. Six groups were created by cross-fostering: control low, control high, cross-fostered at PN6 (low-high 6 and high-low 6), and cross-fostered at PN10 (low-high 10 and high-low 10). B, Mean ± SEM latency to maternal sensitization, based on initial dam (PN0–PN6), and age of cross-fostering or control. C, As in B, with group based on LG status of the dam experienced between PN6 and PN10. D, Maternal sensitization latency of females that were cross-fostered at PN6 or PN10 as a difference in latency compared with their nonfostered sibling. *, P < .05.
Sensitive periods for maternal LG-associated variation in gene expression
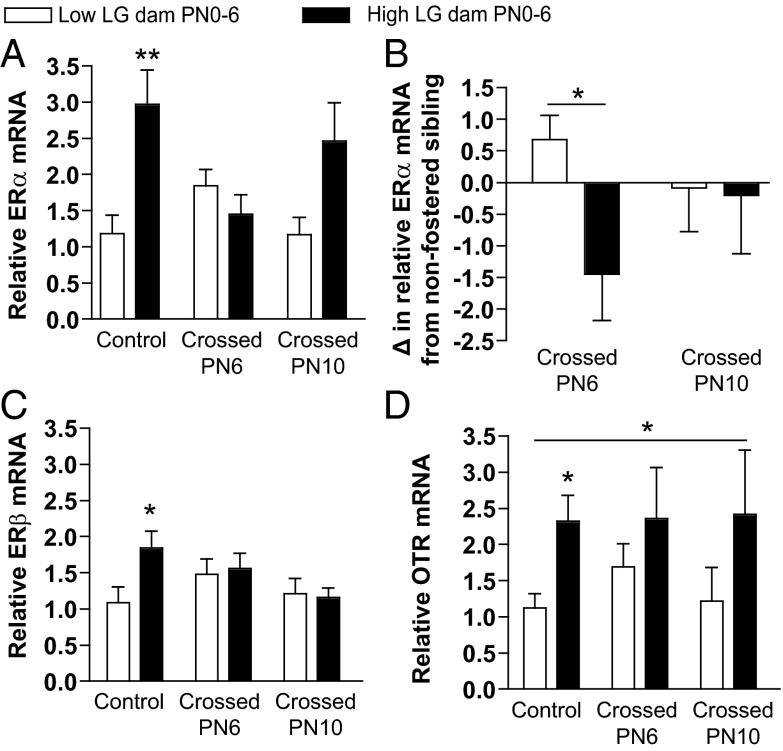
We next examined whether the changes in maternal sensitivity observed after cross-fostering were accompanied by changes in hormone receptor mRNA levels in the MPOA. Although a caveat of these analyses is the potential effects of maternal sensitization testing on mRNA levels, it should be noted that the group differences in gene expression observed in noncross-fostered offspring that underwent testing (see Figure 6) is consistent with those observed in nontested P21 offspring (see Figure 1), suggesting that the testing itself is unlikely to account for the observed effects. Within the control noncross-fostered group, a significant difference in ERα mRNA levels by initial rearing condition was confirmed (t1,10 = 3.53, P < .01). We found a significant effect of initial rearing condition on relative ERα mRNA (F1,36 = 5.44, P < .05) (Figure 6A) and a significant interaction between initial rearing condition and cross-fostering at PN6 (F1,36 = 8.27, P < .01) but no interaction when cross-fostering occurred at PN10 (P > .5). There was also a main effect of maternal LG from PN6 to PN10 on offspring ERα mRNA levels (F1,36 = 9.54, P < .01) and an interaction between maternal LG from PN6 to PN10 and being cross-fostered at PN6 (F1,36 = 4.15, P < .05). We also examined the change in relative ERα levels in fostered compared with nonfostered siblings (Figure 6B) and found a trend for an interaction between initial rearing condition and being cross-fostered at PN6 (F1,29 = 3.77, P = .06) but no interaction with being cross-fostered at PN10 (P > .9). Taken together, these results parallel the observed effects of cross-fostering on maternal sensitivity, with significant shifts in MPOA ERα mRNA after PN6, but not PN10, cross-fostering.
Figure 6.
Hormone receptor gene expression in the MPOA among offspring cross-fostered between low and high LG dams during the postnatal period. Mean ± SEM relative (A) ERα, (C) ERβ, and (D) OTR mRNA levels among females reared initially by a low LG or high LG dam from PN0 to PN6 and that were nonfostered controls, cross-fostered at PN6, or cross-fostered at PN10. B, The difference (mean ± SEM) in ERα mRNA levels among females cross-fostered at PN6 or PN10 compared with their nonfostered sibling. *, P < .05; **, P < .01.
There was a significant effect of initial rearing condition on relative ERβ mRNA levels among noncross-fostered controls (t1,10 = 2.35, P < .05) (Figure 6C), but no significant group differences were found after cross-fostering at either age (P > .4). This finding suggests that ERβ mRNA levels are sensitive to levels of maternal LG occurring beyond PN10. In addition to a significant effect of initial rearing condition among noncross-fostered controls on OTR mRNA levels (t1,10 = 2.96, P < .05) (Figure 6D), there was a trend for a main effect of initial rearing condition on OTR mRNA levels across all groups (F1,35 = 3.88, P = .06), and cross-fostering at either age did not significantly impact this initial rearing experience effect (P > .6) (Figure 6D). Collapsing by initial maternal LG group and controlling for cross-fostering condition revealed a significant effect of initial PN0–PN6 maternal LG on OTR levels (F1,35 = 4.69, P < .05). These results suggest that OTR mRNA levels are sensitive to maternal LG experienced before but not after PN6. The differential windows of sensitivity of ERα compared with ERβ and OTR further highlight the potential role of ERα as a critical mediator of maternal responsivity of offspring.
Discussion
Although maternal LG experienced during the postnatal period has previously been shown to predict adult ER mRNA and protein levels, CpG methylation in the Esr1 gene promoter, and postpartum maternal behavior in adult female offspring, the developmental timing of these effects had yet to be elucidated. Here, we demonstrate that maternal LG is associated with the emergence of changes in the expression of hormone receptors (ERα, ERβ, OTR) in the developing hypothalamus, and in the case of ERα, group differences in transcriptional activity are associated with variation in DNA methylation and histone modifications within the Esr1 gene regulatory region. These epigenetic and transcriptional effects are apparent by PN21, and in the current study, we identify a parallel behavioral outcome to these molecular changes: variation in the maternal sensitization of juveniles. Moreover, we provide evidence for a critical period for maternal LG-associated effects on juvenile maternal sensitivity and hypothalamic gene expression, which limits the plasticity of these outcomes beyond PN10. Overall, these studies illustrate the molecular and behavioral pathways that link the experience of maternal care during infancy to variation in maternal behavior exhibited in adulthood, thus demonstrating a mechanism of the behavioral transmission of maternal behavior and variation in the maternal brain across generations.
Estrogen sensitivity is a critical feature of the neuroendocrine regulation of maternal behavior. This sensitivity is mediated in large part through ligand-dependent interactions with ERs and consequent activation of transcription through estrogen-response elements in target genes (35). ERα knockout mice exhibit significant deficits in pup retrieval and increased rates of infanticide (13). Targeted reductions of ERα specifically within the MPOA by short hairpin RNA (14, 36) or RNA interference (37) severely disrupts maternal behaviors, including pup retrieval, LG, and nursing. The reduced estrogen sensitivity previously observed in the offspring of low LG dams (1) is likely mediated by reduced levels of hypothalamic ERα. However, our findings also suggest that LG effects on ER expression and behavioral indices of maternal responsivity emerge before puberty and the onset of elevated gonadal hormone release. In previous studies, ligand-independent activation of ERs by dopamine has been found to induce brain-region-specific neuronal activation and differences in the social behavior of juvenile female rats (38, 39). Thus, ligand-independent ER activation may account for the early emergence of ERα-dependent maternal responses that we observe in juvenile offspring of high LG dams, and we are currently exploring the development of dopaminergic pathways during this period in response to postnatal maternal care.
The time course of maternal care-associated changes in ERs and OTR determined in the current study is consistent with previous research investigating the levels of these hormone receptors across development. From embryonic day 18 to PN5, there is a critical period for estrogen to masculinize sexually dimorphic brain region morphology, including the MPOA (40–43). The distribution of hypothalamic ERα-ir has been found to be similar among neonates and adults (44), and ER levels increase significantly in the hypothalamus and amygdala during puberty (45). Although these studies explored the general developmental pattern of hormone receptor levels, the current study is the first investigation of the developmental time course of individual differences in these hormonal systems that underlie variation in maternal behavior. Notable within these analyses is the delayed increase in hypothalamic OTR mRNA in offspring of high compared with low LG dams, which is only evident beyond the time of weaning. Previous studies indicate that OTR expression significantly increases in the hypothalamus and olfactory tubercle during puberty (46), and this developmental period may be a critical time for ER-hormone interactions, OTR regulation, and adult maternal behavior.
Although LG-associated epigenetic regulation and transcription of hypothalamic Esr1 are not observed until weaning, changes in ERα-ir are detected at PN6. One possible explanation for this lack of correspondence is the regional specificity of LG-associated effects within the MPOA. Our analyses of nuclei- and subregion-specific changes in ERα-ir at PN6 and the lack of group differences in ERα-ir determined when including multiple hypothalamic nuclei argues for the importance of this regional specificity. Previous studies using in situ hybridization indicate that ERα mRNA levels within the MPOA can be detected as early as PN6 (2). Thus, epigenetic and transcriptional effects of LG on Esr1 may be highly specific to hypothalamic subregions before weaning, and elucidating how this localized epigenetic effect may spread within the MPOA during development will be important in furthering our understanding of the neuroendocrine and behavioral phenotypes that emerge. However, there are previous reports of elevated immunoreactivity corresponding to low mRNA levels (47, 48), and thus, it may be the case that, although the number of ERα-ir cells is elevated among high LG offspring, the overall levels of mRNA produced by the cells is not different at PN6.
There is increasing evidence for epigenetic regulation of ERα levels in the brain through variation in DNA methylation of the regulatory region of Esr1 associated with developmental experiences. Treatment of PN0 pups with estradiol induces changes in MPOA Esr1 intron methylation over development at PN1, PN20, and PN60, and these alterations are restricted to specific CpG sites (49). Prenatal exposure to the endocrine-disrupting compound bisphenol A induces sex-specific and dose-dependent effects on ERα mRNA levels and sex- and brain region-specific effects on CpG methylation of the exon A Esr1 promoter in mice (50). The critical role of LG as opposed to other aspects of maternal behavior in modulating Esr1 methylation is suggested by studies manipulating neonatal tactile stimulation. Simulated maternal grooming (paintbrush stimulation of the anogential region from PN5 to PN7) increases rat Esr1 promoter B/1b methylation in females at 2 CpG sites, concurrent with decreases in relative ERα mRNA (51). Methylation within this promoter region may interfere with Stat5b (signal transducer and activator of transcription 5b) binding to inhibit gene transcription (2). Increased CpG methylation and decreased Stat5b binding within the Esr1 B/1b regulatory region has also been observed in adult low LG female offspring (2). The current studies indicate that this LG-associated effect on Esr1 B/1b methylation emerges by PN21, consistent with the developmental timing of group differences in mRNA expression, and provide a critical missing link in understanding mechanisms leading from experience of maternal LG to variation in ERα expression and adult maternal behavior.
Chromatin remodeling in the form of posttranslational histone modifications, and in particular H3K4 and H3K9 methylation, has been found to be sensitive to environmental modulation. Exposure of mice to several weeks of enriched social and physical environments increases hippocampal brain-derived neurotrophic factor (BDNF) mRNA and H3K4me3 at the BDNF III and VI promoters, while simultaneously decreasing H3K9me3 at the BDNF III and IV promoters (52). Chronic early life stress induced by maternal separation from PN2–PN9 decreases H3K9 mono- and trimethylation in the frontal cortex of mice, although association of these epigenetic marks with specific gene targets has not been explored (53). Here, we report increased H3K4me3 and decreased H3K9me3 association with the Esr1 B/1b regulatory region of high LG females at PN21. This epigenetic facilitation of Esr1 transcription by H3K4me3 appears to emerge during the preweaning period and is stable through adulthood, whereas transcriptional repression by H3K9me3 may be specific to the early juvenile period. These findings support the hypothesis that multiple forms of epigenetic regulation may work in concert to regulate ERα levels in the MPOA consequent to the experience of variation in LG.
Establishing the mediating role of maternal LG on development and regulation of hypothalamic hormone receptor systems is a critical issue. Although significant correlations between maternal LG and various outcomes (19, 54, 55) are suggestive of this mediation, cross-fostering studies have offered a more direct assessment of LG-associated effects. Cross-fostering female pups between low and high LG dams on the day of birth indicates that adult ERα levels and maternal behavior are associated with the quality of maternal LG experienced during postnatal development (2). Cross-fostering at PN6 or PN10 in the current studies demonstrates that both MPOA ERα mRNA levels and maternal sensitization behavior have continued sensitivity to maternal LG through PN10, despite diminishing variation in maternal LG frequency among high and low LG dams. This sensitive period is consistent with the finding that neonatal handling, which induces increased maternal LG (54, 56), is effective in attenuating stress reactivity if experienced during the first postnatal week but not when experienced exclusively during the second or third postnatal week (57, 58). These studies argue for a critical period of sensitivity to maternal LG, with limited plasticity in neuroendocrine systems beyond PN10.
The discovery of periods of sensitivity to variation in maternal care may account for the developmental outcomes associated with the experience of early life adversity in children. Children with a history of maltreatment are more likely to have long-term behavioral and psychological difficulties compared with nonabused children, with increased risk associated with older age at adoption (59, 60). However, children who are adopted after experiencing abuse or neglect have improved behavioral, psychological, and cognitive outcomes compared with children who remain in abusive homes (61, 62). Maternal depression, a significant predictor of reduced maternal care (63), is associated with increased behavioral problems in children that are exposed to this form of early life adversity during the first year of life, a sensitive period for emotional development (64). Our findings suggest that if interventions occur early enough in development, there may be the potential to improve neurobiological and behavioral outcomes. However, if adversity is experienced throughout the sensitive period of neuroendocrine development, there may be epigenetic reprogramming of gene expression with implications for maternal behavior and the transmission of adversity-associated reductions in maternal care across generations (65).
Supplementary Material
Acknowledgments
This work was supported by the Office of the Director National Institutes of Health Grant DP2OD001674–01 (F.A.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BDNF
- brain-derived neurotrophic factor
- ChIP
- chromatin immunoprecipitation
- CpG
- cytosine-guanine sequence
- DNMT
- DNA methyltransferase
- ER
- estrogen receptor
- ERα-ir
- ERα-immunoreactivity
- H3K4me3
- antitrimethyl-histone H3 lys 4
- H3K9me3
- antitrimethyl-histone H3 lys9
- LG
- licking/grooming
- MPOA
- medial preoptic area
- OTR
- oxytocin receptor
- PN
- postnatal day
- qPCR
- quantitative PCR.
References
- 1. Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA. 2001;98(22):12736–12741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-α1b promoter and estrogen receptor-α expression in the medial preoptic area of female offspring. Endocrinology. 2006;147(6):2909–2915 [DOI] [PubMed] [Google Scholar]
- 3. Curley JP, Jensen CL, Franks B, Champagne FA. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm Behav. 2012;61(3):454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048–1054 [DOI] [PubMed] [Google Scholar]
- 5. Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83(2):329–346 [DOI] [PubMed] [Google Scholar]
- 6. Cameron N, Del Corpo A, Diorio J, et al. Programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS One. 2008;3(5):e2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robinson S, Penatti CA, Clark AS. The role of the androgen receptor in anabolic androgenic steroid-induced aggressive behavior in C57BL/6J and Tfm mice. Horm Behav. 2012;61(1):67–75 [DOI] [PubMed] [Google Scholar]
- 8. Kalinichev M, Rosenblatt JS, Morrell JI. The medial preoptic area, necessary for adult maternal behavior in rats, is only partially established as a component of the neural circuit that supports maternal behavior in juvenile rats. Behav Neurosci. 2000;114(1):196–210 [DOI] [PubMed] [Google Scholar]
- 9. Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cog Neurosci Rev. 2006;5(4):163–190 [DOI] [PubMed] [Google Scholar]
- 10. Numan M, Insel TR. The Neurobiology of Parental Behavior. New York: Springer-Verlag; 2003 [Google Scholar]
- 11. Febo M. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25(50):11637–11644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fahrbach SE, Pfaff DW. Effect of preoptic region implants of dilute estradiol on the maternal behavior of ovariectomized, nulliparous rats. Horm Behav. 1986;20(3):354–363 [DOI] [PubMed] [Google Scholar]
- 13. Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139(12):5070–5081 [DOI] [PubMed] [Google Scholar]
- 14. Ribeiro AC, Musatov S, Shteyler A, et al. siRNA silencing of estrogen receptor expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proc Natl Acad Sci USA. 2012;109(40):16324–16329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor α expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144(11):4720–4724 [DOI] [PubMed] [Google Scholar]
- 16. Fahrbach SE, Morrell JI, Pfaff DW. Oxytocin induction of short-latency maternal behavior in nulliparous, estrogen-primed female rats. Horm Behav. 1984;18(3):267–286 [DOI] [PubMed] [Google Scholar]
- 17. Young LJ, Wang Z, Donaldson R, Rissman EF. Estrogen receptor α is essential for induction of oxytocin receptor by estrogen. Neuroreport. 1998;9(5):933–936 [DOI] [PubMed] [Google Scholar]
- 18. Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121(6):1353–1363 [DOI] [PubMed] [Google Scholar]
- 19. Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79(3):359–371 [DOI] [PubMed] [Google Scholar]
- 20. Francis D, Diorio J, Liu D, Meaney M. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–1158 [DOI] [PubMed] [Google Scholar]
- 21. Jensen Peña C, Champagne FA. Implications of temporal variation in maternal care for the prediction of neurobiological and behavioral outcomes in offspring. Behav Neurosci. 2013;127(1):33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franks B, Curley JP, Champagne FA. Measuring Variations in Maternal Behavior: Relevance for Studies of Mood and Anxiety. New York: Springer; 2011 [Google Scholar]
- 23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 24. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–1108 [DOI] [PubMed] [Google Scholar]
- 25. Freyschuss B, Grandien K. The 5′ flank of the rat estrogen receptor gene: structural characterization and evidence for tissue- and species-specific promoter utilization. J Mol Endocrinol. 1996;17(3):197–206 [DOI] [PubMed] [Google Scholar]
- 26. Kos M. Minireview: genomic organization of the human ER gene promoter region. Mol Endocrinol. 2001;15(12):2057–2063 [DOI] [PubMed] [Google Scholar]
- 27. Fleming AS, Rosenblatt JS. Maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol. 1974;86(5):957–972 [DOI] [PubMed] [Google Scholar]
- 28. Rosenblatt JS, Wagner CK, Morrell JI. Hormonal priming and triggering of maternal behavior in the rat with special reference to the relations between estrogen receptor binding and ER mRNA in specific brain regions. Psychoneuroendocrinology. 1994;19(5–7):543–552 [DOI] [PubMed] [Google Scholar]
- 29. Bridges RS, Ronsheim PM. Prolactin (PRL) regulation of maternal behavior in rats: bromocriptine treatment delays and PRL promotes the rapid onset of behavior. Endocrinology. 1990;126(2):837–848 [DOI] [PubMed] [Google Scholar]
- 30. Jensen Peña C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS One. 2012;7(6):e39791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6(11):838–849 [DOI] [PubMed] [Google Scholar]
- 32. Stewart MD, Li J, Wong J. Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Mol Cell Biol. 2005;25(7):2525–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schotta G, Ebert A, Krauss V, et al. Central role of Drosophila SU(VAR)3–9 in histone H3–K9 methylation and heterochromatic gene silencing. EMBO J. 2002;21(5):1121–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santos-Rosa H, Schneider R, Bannister AJ, et al. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419(6905):407–411 [DOI] [PubMed] [Google Scholar]
- 35. Bale TL, Dorsa DM. Cloning, novel promoter sequence, and estrogen regulation of a rat oxytocin receptor gene. Endocrinology. 1997;138(3):1151–1158 [DOI] [PubMed] [Google Scholar]
- 36. Spiteri T, Ogawa S, Musatov S, Pfaff DW, Agmo A. The role of the estrogen receptor α in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behav Brain Res. 2012;230(1):11–20 [DOI] [PubMed] [Google Scholar]
- 37. Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci USA. 2006;103(27):10456–10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Olesen KM. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146(9):3705–3712 [DOI] [PubMed] [Google Scholar]
- 39. Olesen KM, Auger AP, Baune B. Dopaminergic activation of estrogen receptors induces fos expression within restricted regions of the neonatal female rat brain. PLoS One. 2008;3(5):e2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwarz JM, Mccarthy MM. Cellular mechanisms of estradiol-mediated masculinization of the brain. J Steroid Biochem Mol Biol. 2008;109(3–5):300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990;21(5):781–786 [DOI] [PubMed] [Google Scholar]
- 42. Rhees RW, Shryne JE, Gorski RA. Termination of the hormone-sensitive period for differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res Dev Brain Res. 1990;52(1–2):17–23 [DOI] [PubMed] [Google Scholar]
- 43. DonCarlos LL, Handa RJ. Developmental profile of estrogen receptor mRNA in the preoptic area of male and female neonatal rats. Brain Res Dev Brain Res. 1994;79(2):283–289 [DOI] [PubMed] [Google Scholar]
- 44. Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-α immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389(1):81–93 [DOI] [PubMed] [Google Scholar]
- 45. Brown TJ, Hochberg RB, Naftolin F, MacLusky NJ. Pubertal development of estrogen receptors in the rat brain. Mol Cell Neurosci. 1994;5(5):475–483 [DOI] [PubMed] [Google Scholar]
- 46. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683 [DOI] [PubMed] [Google Scholar]
- 47. Chen G, Gharib TG, Huang CC, et al. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002;1(4):304–313 [DOI] [PubMed] [Google Scholar]
- 48. Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4(9):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151(10):4871–4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kundakovic M, Gudsnuk K, Franks B, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci USA. 2013;110(24):9956–9961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-promoter within the developing preoptic area. Endocrinology. 2010;151(5):2297–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuzumaki N, Ikegami D, Tamura R, et al. Hippocampal epigenetic modification at the brain-derived neurotrophic factor gene induced by an enriched environment. Hippocampus. 2011;21(2):127–132 [DOI] [PubMed] [Google Scholar]
- 53. Kao GS, Cheng LY, Chen LH, et al. Neonatal isolation decreases cued fear conditioning and frontal cortical histone 3 lysine 9 methylation in adult female rats. Eur J Pharmacol. 2012;697(1–3):65–72 [DOI] [PubMed] [Google Scholar]
- 54. Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3(8):799–806 [DOI] [PubMed] [Google Scholar]
- 55. Liu D, Diorio J, Tannenbaum B, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662 [DOI] [PubMed] [Google Scholar]
- 56. Lee MHS, Williams DI. Changes in licking behaviour of rat mother following handling of young. Anim Behav. 1974;22:679–681 [Google Scholar]
- 57. Levine S, Lewis GW. Critical period for effects of infantile experience on maturation of stress response. Science. 1959;129(3340):42–43 [DOI] [PubMed] [Google Scholar]
- 58. Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res. 1985;354(2):301–304 [DOI] [PubMed] [Google Scholar]
- 59. Rutter M. Developmental catch-up, and deficit, following adoption after severe global early privation. English and Romanian Adoptees (ERA) Study Team. J Child Psychol Psychiatry. 1998;39(4):465–476 [PubMed] [Google Scholar]
- 60. Rushton A, Dance C. The adoption of children from public care: a prospective study of outcome in adolescence. J Am Acad Child Adolesc Psychiatry. 2006;45(7):877–883 [DOI] [PubMed] [Google Scholar]
- 61. Macmillan HL, Wathen CN, Barlow J, Fergusson DM, Leventhal JM, Taussig HN. Interventions to prevent child maltreatment and associated impairment. Lancet. 2009;373(9659):250–266 [DOI] [PubMed] [Google Scholar]
- 62. Johnson DE. Adoption and the effect on children's development. Early Hum Dev. 2002;68(1):39–54 [DOI] [PubMed] [Google Scholar]
- 63. Field T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav Dev. 2010;33(1):1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bagner DM, Pettit JW, Lewinsohn PM, Seeley JR. Effect of maternal depression on child behavior: a sensitive period? J Am Acad Child Adolesc Psychiatry. 2010;49(7):699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Champagne F. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29(3):386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.