Abstract
Resistin is a circulating mediator of insulin resistance mainly expressed in human monocytes and responsive to inflammatory stimuli. Recent clinical studies have connected elevated resistin levels with the development and severity of heart failure. To further our understanding of the role of human resistin in heart failure, we studied a humanized mouse model lacking murine resistin but transgenic for the human Retn gene (Hum-Retn mice), which exhibits basal and inflammation-stimulated resistin levels similar to humans. Specifically, we explored whether resistin underlies acute anthracycline-induced cardiotoxicity. Remarkably, doxorubicin (25mg/kg ip) led to a 4-fold induction of serum resistin levels in Hum-Retn mice. Moreover, doxorubicin-induced cardiotoxicity was greater in the Hum-Retn mice than in littermate controls not expressing human resistin (Retn−/−). Hum-Retn mice showed increased cardiac mRNA levels of inflammatory and cell adhesion genes compared with Retn−/− mice. Macrophages, but not cardiomyocytes, from Hum-Retn mice treated with doxorubicin in vitro showed dramatic induction of hRetn (human resistin) mRNA and protein expression. We also examined resistin levels in anthracycline-treated breast cancer patients with and without cardiotoxicity. Intriguingly, serum resistin levels in women undergoing anthracycline-containing chemotherapy increased significantly at 3 months and remained elevated at 6 months in those with subsequent cardiotoxicity. Further, elevation in resistin correlated with decline in ejection fraction in these women. These results suggest that elevated resistin is a biomarker of anthracycline-induced cardiotoxicity and may contribute in the development of heart failure via its direct effects on macrophages. These results further implicate resistin as a link between inflammation, metabolism, and heart disease.
Resistin is a secreted factor and circulating hormone initially discovered as an adipocyte-derived mediator of obesity-related insulin resistance in rodents (1). In humans, however, resistin is predominantly expressed in monocytes and macrophages and is responsive to inflammatory stimuli (2–5). Resistin is elevated in a wide range of inflammatory conditions from autoimmune disease to sepsis (6). Recent large-scale clinical cohorts have connected elevated serum resistin levels with the subsequent development of heart failure (7–9). Additionally, several smaller studies have shown that resistin rises with acute decompensated heart failure and correlates with disease severity (10–14). Specifically, resistin has been shown to correlate with other serum markers of heart failure severity and, retrospectively, predicted heart failure mortality even after adjusting for known risk factors (3, 15). The association between resistin and heart failure is likely due to multiple factors (6). Preclinical studies have shown that resistin directly impairs cardiomyocyte glucose handling and contractility and, when overexpressed, drives cardiac hypertrophy (16, 17). However, whether human resistin is a biomarker or a true etiologic factor in heart failure remains unanswered. The role of resistin in the pathophysiology of inflammatory cardiomyopathy has begun to be explored, with higher inflammation in an animal overexpression model and increased resistin seen in human inflammatory cardiomyopathy (13, 18). However, the role of resistin in the development of acute cardiotoxicity has not been explored.
To investigate the role of human resistin in heart failure, we studied a humanized mouse model lacking murine resistin but transgenic for a bacterial artificial chromosome containing the human Retn gene (Hum-Retn mice). These mice exhibit basal and inflammation-stimulated resistin levels similar to those in humans and provide a model that closely mimics human physiology (19). The differences between murine and human resistin have been recently described (6), but several studies have explored the negative impact of human resistin in mouse models, indicating a conserved functionality across species (16, 20). Anthracycline-induced cardiotoxicity has long been recognized as a common and serious adverse effect of treatment for many malignancies (21), leading to cardiac dysfunction underpinned by inflammatory infiltration of neutrophils and macrophages (22, 23). Here, we report that doxorubicin dramatically induced human resistin in the Hum-Retn mice, and Hum-Retn mice exhibited worse cardiotoxicity with doxorubicin than mice lacking resistin. Moreover, serum resistin levels were induced in women who received doxorubicin-containing chemotherapy for breast cancer, and resistin elevation correlated with decline in ejection fraction in these women. These data suggest that resistin is induced by doxorubicin and may contribute to doxorubicin-induced cardiotoxicity.
Materials and Methods
Animal derivation
C57Bl/6 mice expressing human resistin under control of human resistin regulatory elements have been previously described (19). Animals used in all experiments were age-matched (10- to 12-wk-old) male mice. All protocols for animal use and euthanasia were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania Perelman School of Medicine in accordance with National Institutes of Health guidelines.
Doxorubicin administration
A 1-time dose of 25-mg/kg doxorubicin (Sigma-Aldrich) or an equivalent volume of normal saline (0.9%) was injected ip to wild-type (WT), resistin knockout (RKO), or Hum-Retn mice (24–26). Mice were killed at day 5 after doxorubicin administration, after elimination from heart and plasma and in accordance with previous reports showing both cardiotoxicity and possible cardioprotection at that time point (27, 28). Whole blood samples were taken, and hearts were excised at the time of killing.
Echocardiography
Echocardiography was performed using a 30-mHz transducer on a Vevo 770, VisualSonics under light sedation with 1%–2% inhaled isoflurane. Left ventricular (LV) chamber dimensions were measured, and ejection fraction (EF) was calculated using M-Mode images in the parasternal short-axis view at the level of the papillary muscles (D3 formula). Stroke volume was calculated using two-dimensional measurements of LV outflow tract diameter in the parasternal long-axis view and pulsed wave Doppler measurements of LV outflow tract flow in the apical view. All measurements were performed by one reader blinded to animal and treatment types.
RNA extraction and quantitative PCR
At killing, LV heart tissue was isolated and immediately frozen in liquid nitrogen. Total RNA from tissues was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA integrity was determined with UV spectrophotometry. Reverse transcription was performed with MultiScribe Reverse Transcriptase (Applied Biosystems) according to the manufacturer's instructions. Expression of genes was analyzed using real-time (SYBR) quantitative PCR (ABI Prism; Applied Biosystems) as previously described (20). The level of mRNA expression was normalized to 36B4.
Serum and cell culture supernatant assays
Whole blood was collected by cardiac puncture at the time of killing, clotted at room temperature for 30 minutes, centrifuged at 4°C for 15 minutes, and stored at −80°C. Cell culture supernatant was collected and stored at −80°C. Resistin levels were measured by ELISA (Millipore Corp). Lactate dehydrogenase (LDH) activity was measured by colorimetric assay (Abcam). TNFα levels were measured by ELISA (R&D Systems).
Neonatal cardiomyocyte isolation
Neonatal cardiomyocytes were isolated from 1- to 2-day-old pups of RKO and Hum-Retn mice using a protocol adapted as follows from Wang and Kang (29). Briefly, pups were killed, and hearts were removed aseptically into cold Hanks' balanced salt solution. Ventricles were minced and cells dissociated at 37°C for 30 minutes with an enzyme solution (0.5% wt/vol trypsin in Hanks' balanced salt solution without Ca++ and Mg++ [pH 7.4]). Cells were centrifuged for 5 minutes at 500 rpm, then resuspended in DMEM (Invitrogen) with 10% horse serum, 5% fetal bovine serum, 100-U/mL penicillin, and 100-μg/mL streptomycin (Invitrogen). This step was repeated. Suspended cells were then collected and plated at 1.0 × 105 cells/cm2 and incubated at 37°C with 5% CO2. After 1–2 days, cells were treated with doxorubicin (1μM) or saline vehicle for 24 hours. Cells were then collected, and was RNA isolated using a commercially available kit (QIAGEN). Culture supernatant was collected and stored at −80°C.
Peritoneal macrophage isolation
Macrophages were elicited as previously described (30). Briefly, 10- to 12-week-old Hum-Retn and RKO mice were injected once with 3-mL thioglycollate ip. At day 4, mice were killed and macrophages harvested via peritoneal lavage. After 24 hours of adherence purification in culture, cells were treated for 24 hours with vehicle or doxorubicin (10μM). All cells were cultured in high-glucose DMEM (Invitrogen) with 10% fetal bovine serum (U.S. Biotechnologies), 100-U/mL penicillin, and 100-μg/mL streptomycin (Invitrogen), incubated at 37°C with 5% CO2. RNA isolation was performed using a commercially available kit (QIAGEN). Culture supernatant was collected and stored at −80°C.
Human study design
Subjects were enrolled as previously described (31). In brief, women age 18 or older diagnosed with human epidermal growth factor receptor 2-overexpressing breast cancer and scheduled to receive treatment, including anthracycline and trastuzumab, or scheduled to receive trastuzumab after previous anthracycline treatment were eligible. Those with LV EF less than 50% were excluded. Subjects were enrolled at 2 institutions, and all subjects signed informed consent forms approved by the institutional review board of the participating institutions. This investigation conformed to the principles outlined in the Declaration of Helsinki. Subjects were studied before chemotherapy and at 3 and 6 months of treatment, using questionnaires, echocardiography, and blood samples. A subset of 50 subjects was randomly selected for analysis in the present study. Subjects who received trastuzumab before anthracycline were excluded for this analysis. Cardiotoxicity was defined according to recent guidelines (Cardiac Review and Evaluation Committee of trastuzumab-associated cardiotoxicity) (32).
Statistical analysis
All data are presented as mean ± SEM. Comparisons of means were performed using Student's t tests for normally distributed data and Wilcoxon Signed-Rank or Wilcoxon Rank-Sum tests for nonparametric data. Correlation between continuous variables was performed using Spearman's correlation. Statistical analysis was performed using STATA/IC statistics software version 12.1 (StataCorp). P < .05 was defined as significant.
Results
Human resistin is dramatically increased by doxorubicin treatment of humanized mice
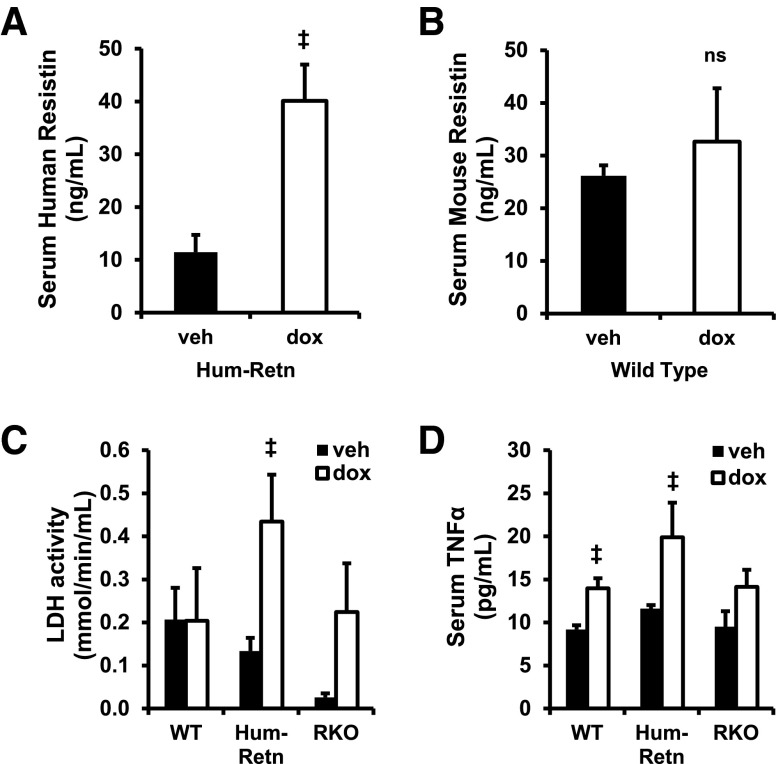
Transgenic mice lacking mouse resistin and expressing human resistin from a bacterial artificial chromosome containing the entire human resistin gene, as well as 21 300 bp upstream and 4248 bp downstream of the transcription start site (Hum-Retn mice), had serum resistin levels similar to humans as previously described (Figure 1A) (19). To study the effects of doxorubicin, WT and Hum-Retn mice, both on a C57Bl/6 background, were treated with a 1-time ip dose of doxorubicin 25 mg/kg, an established protocol for studying anthracycline toxicity (24–26, 33, 34). The vast majority of published studies use male mice (20, 33, 34), which appear to be more susceptible to anthracycline cardiotoxicity (35–37), and therefore, males were studied here. Treatment with doxorubicin led to a marked increase in serum resistin levels in the Hum-Retn mice 5 days after injection (Figure 1A). By contrast, doxorubicin had little effect on serum levels of mouse resistin in WT C57Bl6 mice (Figure 1B).
Figure 1.
Doxorubicin induces resistin in humanized resistin mice (Hum-Retn) not in WT. A, Doxorubicin at (dox) 25 mg/kg ip induced serum human resistin in 10- to 12-week-old male Hum-Retn mice compared with vehicle (veh) at day 5. B, Murine resistin was not induced by doxorubicin in WT C57Bl6 mice. C, Serum LDH activity was induced significantly by doxorubicin in Hum-Retn mice only. D, Serum TNFα was elevated significantly in Hum-Retn and WT but not RKO. Mean + SEM, n = 5–12 for all groups; ‡, P < .05 doxorubicin vs vehicle of same animal type. ns, not significant.
Doxorubicin cardiotoxicity is potentiated in humanized resistin mice, whereas lack of resistin appears protective
At 5 days after doxorubicin administration, Hum-Retn mice exhibited a nearly 4-fold induction in serum LDH activity, a marker of myocardial injury known to stay robustly elevated several days after doxorubicin in mice (Figure 1C) (38). WT mice had no increase in LDH, and RKO mice had a nonsignificant elevation. LDH, however, is a relatively nonspecific marker of cardiac damage and can indicate generalized inflammation (39). TNFα serum levels after doxorubicin were induced significantly in Hum-Retn and WT, but not RKO, mice (Figure 1D). Although the elevation in LDH levels was greater in the Hum-Retn than in WT and RKO, the inflammatory response after doxorubicin, as detected by TNFα, was similar between Hum-Retn and WT, suggesting worsened cardiotoxicity in the Hum-Retn mice.
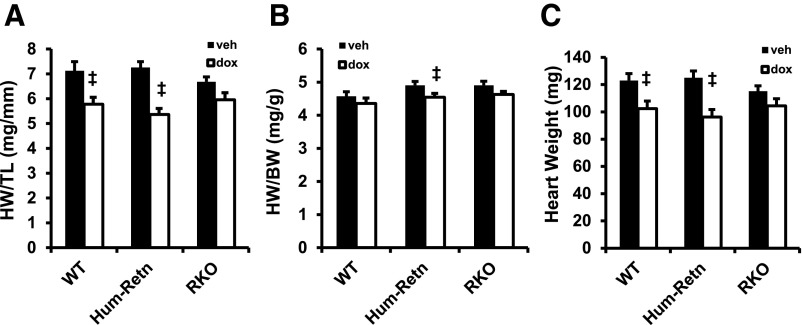
At day 5 after doxorubicin, Hum-Retn mice also showed a decrease in gross heart weight normalized to tibia length, another measure of doxorubicin cardiotoxicity (26, 33) (7.3 vs 5.4 mg/mm, P < .05) (Figure 2A). This effect was attenuated in the RKO mice lacking resistin (6.7 vs 6.0 mg/mm, P = not significant) (Figure 2A). The greater doxorubicin cardiotoxicity in the Hum-Retn mice was also observed when heart weight was normalized to body weight (4.9 vs 4.5 mg/g, P < .05 and 4.9 vs 4.6 mg/g, P = ns for Hum-Retn and RKO, respectively) (Figure 2B) or measured absolutely (125 vs 96 mg, P < .05 and 115 vs 104 mg, P = ns for Hum-Retn and RKO, respectively) (Figure 2C). Thus, doxorubicin-induced cardiotoxicity was exacerbated by human resistin. Importantly, doxorubicin led to cardiotoxic changes in WT mice as well (Figures 2, A–C), and to a greater degree than in the RKO mice lacking resistin.
Figure 2.
Effect of doxorubicin on cardiotoxicity attenuated in mice lacking resistin. A, Heart weight normalized to tibia length was decreased at day 5 after doxorubicin in Hum-Retn and WT but not RKO. B, Heart weight normalized to body weight was decreased by doxorubicin only in Hum-Retn. C, Gross heart weight was decreased at day 5 after doxorubicin in Hum-Retn and WT but not RKO. Mean + SEM, n = 5–12 for all groups; ‡, P < .05 doxorubicin vs vehicle of same animal type. veh, vehicle; dox, doxorubicin.
Echocardiography corroborated the gross findings. In terms of LV function, a significant decrease in stroke volume was observed in Hum-Retn mice after doxorubicin (Table 1). Structurally, this was due to a decrease in LV diastolic internal diameter in Hum-Retn mice after doxorubicin, whereas the LV size did not change in the RKO mice (Table 1). In addition, doxorubicin-treated Hum-Retn mice exhibited higher heart rates, potentially in compensation for the decreased stroke volume (Table 1).
Table 1.
Echocardiographic Evidence of Exacerbated Doxorubicin Cardiotoxicity in Hum-Retn Mice
| RKO Vehicle (n = 7) | Hum-Retn Vehicle (n = 8) | RKO Doxorubicin (n = 9) | Hum-Retn Doxorubicin (n = 5) | |
|---|---|---|---|---|
| Heart rate (bpm) | 470 ± 4 | 434 ± 10b | 451 ± 13 | 477 ± 18a |
| Septal wall thickness (mm) | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 |
| LVIDd (mm) | 4.0 ± 0.0 | 4.3 ± 0.1b | 4.0 ± 0.1 | 3.7 ± 0.2a |
| LVIDs (mm) | 3.1 ± 0.1 | 3.2 ± 0.1 | 3.1 ± 0.1 | 2.7 ± 0.2a |
| Stroke volume (μL) | 38 ± 2 | 38 ± 3 | 33 ± 2 | 29 ± 3a |
| Ejection fraction (%) | 47 ± 2 | 48 ± 1 | 48 ± 2 | 51 ± 1a |
| FS (%) | 24 ± 1 | 24 ± 0 | 24 ± 1 | 28 ± 3a |
bpm, beats per minute; LVIDd, LV diastolic internal diameter; LVIDs, LV systolic internal diameter. Mean ± SEM, n = 5–12 for all groups.
P < .05 doxorubicin vs vehicle of same animal type.
P < .05 Hum-Retn vs RKO of same treatment.
Cardiac gene expression is altered after doxorubicin treatment of humanized resistin mice
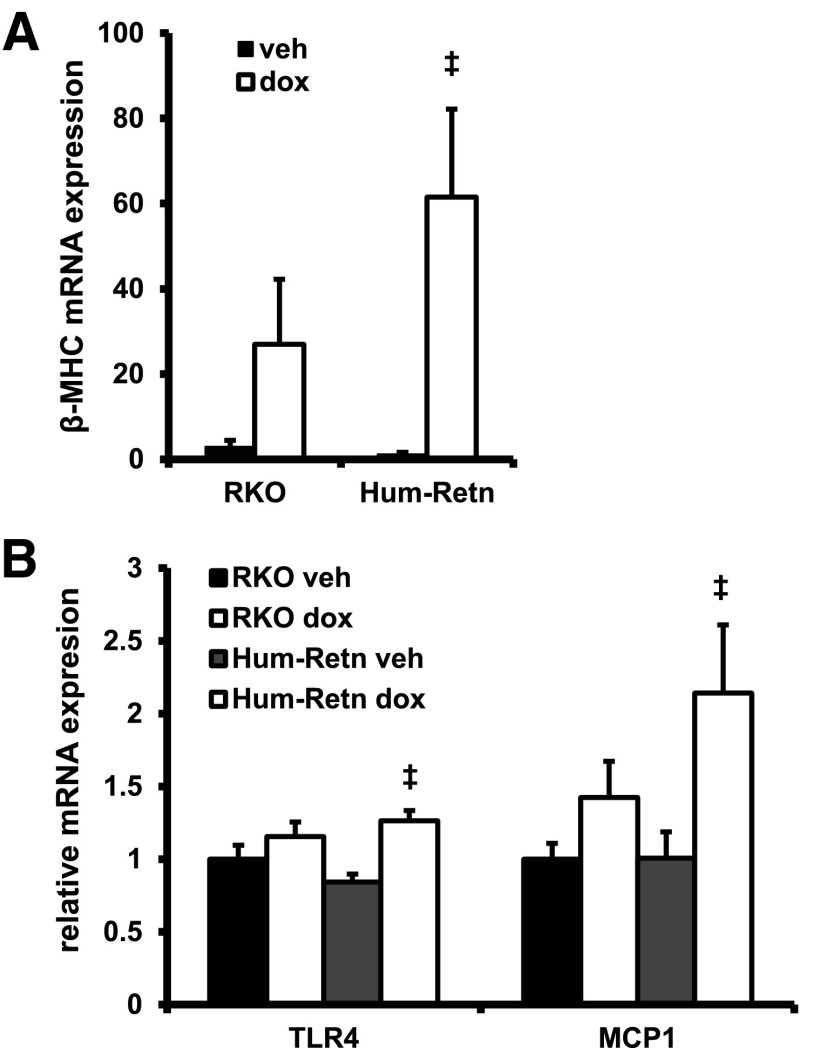
Consistent with the exacerbation of doxorubicin toxicity in the hearts of Hum-Retn mice, the gene expression of a marker of heart failure, β-myosin heavy chain (β-mhc), was induced to a greater extent in the hearts of Hum-Retn mice than the RKO mice (Figure 3A). Additionally, doxorubicin significantly induced cardiac expression of inflammatory markers toll-like receptor-4 (tlr4) and monocyte chemoattractant protein-1 (mcp-1) in Hum-Retn only, whereas this effect was blunted in and RKO mice (Figure 3B). Further, doxorubicin did induce expression of anp mRNA, but this did not reach statistical significance and did not differ across animal types (data not shown), likely representative of the modest gross cardiac changes seen.
Figure 3.
Heart tissue gene expression profiles are distinct between Hum-Retn and RKO mice after exposure to doxorubicin. A, The heart failure gene marker β-MHC was induced significantly only in Hum-Retn mice. B, Doxorubicin induced the inflammatory marker TLR4 and the chemokine MCP-1 significantly only in Hum-Retn. All gene expression data are normalized to 36B4 expression. Mean + SEM, n = 5 per group; ‡, P < .05 doxorubicin vs vehicle of same animal type. veh, vehicle; dox, doxorubicin.
Doxorubicin cardiotoxicity is likely mediated through effects on macrophages not cardiomyocytes in humanized mice
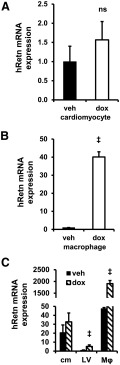
To begin to understand the mechanism of human resistin in the development of cardiotoxicity, primary cardiomyocytes and macrophages were isolated from Hum-Retn mice and studied in cell culture. Importantly, expression of resistin from cardiomyocytes remained unchanged after 24 hours of treatment with doxorubicin (1μM) (Figure 4A). Because human resistin is mainly expressed in monocytes and macrophages (2, 40), we turned our attention to the effect of doxorubicin on macrophages. Notably, in stark contrast to its effect on cardiomyocytes, doxorubicin induced a nearly 40-fold increase in resistin mRNA expression in thioglycollate-elicited peritoneal macrophages from Hum-Retn mice (Figure 4B). Further, we found that human resistin mRNA expression levels were greatest in peritoneal macrophages compared with neonatal cardiomyocytes or LV tissue. Expression of hRetn (human resistin) was 40-fold greater in untreated macrophages than in LV tissue from vehicle-treated mice and 2000 times greater in doxorubicin-treated macrophages than LV tissue from doxorubicin-treated mice (Figure 4C). Most expressed resistin in the mice was, therefore, felt to be due to monocyte and macrophage secretion.
Figure 4.
Resistin gene expression is not altered in cardiomyocytes but is induced dramatically by doxorubicin in macrophages. A, In neonatal cardiomyocytes isolated from Hum-Retn mice, gene expression of hRetn was not significantly induced by doxorubicin (1μM) in vitro. B, Peritoneal macrophages harvested from 10- to 12-week-old male Hum-Retn mice and then treated with doxorubicin (10μM) showed a 40-fold increase in resistin gene expression vs vehicle. C, Comparing multiple tissue and cell types revealed that human resistin mRNA expression levels were greatest in peritoneal macrophages (Mϕ) compared with neonatal cardiomyocytes (cm) or LV tissue. hRetn expression was 40-fold greater in untreated macrophages than LV tissue from vehicle-treated mice and 2000 times greater in doxorubicin-treated macrophages than LV tissue from doxorubicin-treated mice. All gene expression data are normalized to 36B4 expression. Mean + SEM, n = 5 per group; ‡, P < .05 doxorubicin vs vehicle. veh, vehicle; dox, doxorubicin.
Serum resistin levels are a biomarker of cardiotoxicity after doxorubicin-containing chemotherapy for women with breast cancer
Elevation in serum resistin in a variety of heart failure settings raised the question of whether similar changes would be observed in patients who developed cardiotoxicity from anthracyclines. Measurement of serum resistin levels in 50 women who underwent anthracycline-containing chemotherapy for breast cancer revealed a marked elevation of serum resistin levels at 3 months (11.5 ± 7.3 ng/mL at baseline to 19.6 ± 20.1 ng/mL at 3 mo, mean ± SD; P < .01) (Figure 5A). On average, resistin levels returned to baseline by 6 months. However, in those subjects developing subsequent cardiotoxicity, serum resistin remained elevated (13.5 vs 10.8 ng/mL in no cardiotoxicity, P = .073) (Figure 5B). Further, the percentage increase in resistin over the course of 6 months significantly correlated inversely with maximum change in EF measured by echocardiography (Spearman's rho = −0.29, P = .045) (Figure 5C). Although the change in resistin level by 6 months appeared predictive of cardiotoxicity, baseline resistin levels, follow-up absolute levels, or change in resistin at any other time points did not correlate with the diagnosis or degree of cardiotoxicity (data not shown).
Figure 5.
Serum resistin is a biomarker for anthracycline-induced cardiotoxicity in breast cancer patients. A, Across the entire cohort, serum resistin levels increased from preanthracycline (Pre) baseline (11.5 ± 1.0 ng/mL) to 3 months (19.6 ± 2.8 ng/mL) but not at 6 months (12.1 ± 0.7 ng/mL) after initiation of chemotherapy, P < .05 (mean ± SEM, n = 50). B, At 6 months, serum resistin levels remained elevated in those who developed cardiotoxicity compared with those without toxicity (13.5 vs 10.8 ng/mL, P = .073). C, Change in serum resistin level at 6 months significantly correlated inversely with maximum change in EF in all women (Spearman's rho = −0.29, P = .045); ‡, P < .05. Chemo, anthracycline chemotherapy; Tox, cardiotoxicity present; No Tox, cardiotoxicity absent.
Discussion
Resistin has been implicated in the development of heart failure and has direct effects on cardiomyocytes and vascular tissue (6), while being clearly linked to inflammation. Our findings place resistin at the intersection of inflammation and heart failure resulting from the widely used chemotoxic agent doxorubicin. Acute administration of doxorubicin to humanized resistin mice elicited a dramatic increase in circulating resistin and a more pronounced cardiotoxicity than in RKO mice. The nearly 4-fold induction of serum resistin was similar to that observed after acute inflammation due to lipopolysaccharide (19). Moreover, we have shown that serum levels of resistin are elevated in women treated with doxorubicin for breast cancer. These results have, for the first time, connected resistin to the cardiotoxic effects of doxorubicin in rodents and humans, shown that acute cardiotoxicity was blunted in mice by a lack of resistin, and suggested that these positive effects may be mediated by changes in macrophages rather than cardiomyocytes.
Acute administration of doxorubicin causes rapid cardiac changes and experimentally mimics the toxicity profile of chronic dosing (34). This well-studied acute model was used to elicit a robust cardiotoxic response while limiting variability, preventing significant mortality and yet allowing observation of differences between animals (24–26). Importantly, doxorubicin did not alter mouse resistin expression in WT mice, underscoring the differences in human and mouse resistin biology, and the value of the humanized model. Moreover, the humanized resistin mice offer the unique ability to study human resistin biology in a controlled laboratory setting (19).
In the humanized resistin mice only, doxorubicin induced a significant increase in serum LDH activity, indicating a worsened degree of myocardial damage than in WT or RKO mice (41). TNFα serum levels after doxorubicin were induced in Hum-Retn and WT mice. Therefore, despite an inflammatory response to doxorubicin, it did not directly correlate with LDH elevation, indicating greater cardiotoxicity in the Hum-Retn mice. The findings of smaller gross heart weight as well as echocardiographic decreases in stroke volume and ventricular size corroborated the cardiotoxicity findings in the Hum-Retn mice and were consistent with previous reports of acute experimental doxorubicin cardiotoxicity (26, 33, 42). Note that WT mice in our study, which lacked human resistin, developed changes in heart weight after doxorubicin as well, which speaks to the multifactorial nature of this process (21). Although there are many differences in mouse and human responses to anthracycline, the heart weight response is similar. Recent reports have shown that in humans, a decrease in LV mass after anthracycline is predictive of cardiovascular events (43). We acknowledge that the fractional shortening (FS) results we observed are in the lower range of values reported in the literature. However, FS values are known to vary widely as a function of mouse strain, genotype, loading conditions, and a variety of other factors (44, 45).
The molecular mechanisms of anthracycline cardiotoxicity are complex, involving myofibrillar loss, fibrosis, mitochondrial dysfunction, reactive oxygen species, and likely multiple other pathways (21). Consistent with the only modest changes in cardiac size and function observed after doxorubicin, we did not see significant histologic changes in fibrosis, apoptosis, or inflammatory infiltration (data not shown). From a molecular standpoint, our model recapitulated previous work showing elevated cardiac β-mhc, tlr4, and mcp-1 expression after acute doxorubicin dosing (46, 47). The increased expression of tlr4 in the Hum-Retn mice due to doxorubicin is especially intriguing, because tlr4 was shown to be crucial to anthracycline-induced cardiotoxicity, with its absence being protective (46). More recently, TLR signaling has been tied to directly to resistin function and its inflammatory effects in both leukocytes (48) and in vascular smooth muscle (49).
The lack of induction of human resistin in isolated cardiomyocytes strongly suggested macrophages as mediators of the exacerbated doxorubicin-induced cardiotoxicity in the Hum-Retn mice. Previous work has detailed the production and secretion of human resistin by macrophages and its impact on systemic inflammatory response (2–5, 40, 50). The role of cardiac inflammation after various insults from ischemia to infection has also been well described (51). Macrophages are integral to this process, secreting inflammatory cytokines, which drive the inflammatory cascade, and altering contractile function acutely (51). Macrophages are also responsible for reparative functions, including extracellular matrix deposition, fibrosis, and angiogenesis.
Anthracycline-induced cardiotoxicity involves inflammatory infiltration with monocytes and macrophages as well (46, 52). We found that in vitro doxorubicin treatment of primary macrophages from humanized resistin mice elicited a robust increase in resistin gene expression. A comparison of cell and tissue gene expression also corroborated that the vast majority of resistin secretion was macrophage derived. Further, in heart tissue, we showed that elevated resistin after systemic doxorubicin drove an increased signal for activation and recruitment of macrophages in the heart as evidenced by an increase in expression of chemoattractant and cell adhesion molecules in the Hum-Retn mice. We did not detect a significant inflammatory infiltrate by histology or immunohistochemistry. However, the doxorubicin-induced elevation in human resistin in both serum and heart and the concomitant rise in inflammatory markers in the heart support a role for resistin in driving inflammation and subsequent toxicity. Circulating resistin produced by peripheral monocytes might act via an endocrine effect on cardiomyocytes (16, 17). Alternatively, resistin produced either by infiltrating macrophages or by other nonmacrophage cells in the heart might act in a paracrine fashion to promote inflammation and toxicity (53–55).
Lastly, we sought to connect resistin to human cardiotoxicity by exploring its role in a human cohort. We found that serum resistin levels were strikingly increased 3 months after administration of anthracycline to women with breast cancer. The resistin level at 6 months remained elevated in those subjects with subsequent cardiotoxicity, although this did not quite meet statistical significance. Importantly, the degree of resistin elevation by 6 months was inversely related to the decline in cardiac function across the entire cohort. That this later time point was most strongly correlated with cardiac functional decline suggested that although a transient resistin response after anthracycline may be expected, the persistence of resistin is the most influential risk factor.
Of note, the subset of women included for this analysis was small and heterogeneous, which may account for the less distinct correlation between resistin and cardiotoxicity. These women also received a regimen containing trastuzumab, after the anthracycline, which is known to induce cardiomyopathy through a separate mechanism (56). Additionally, the levels of resistin in these women may have been confounded given evidence that breast cancer itself may induce resistin (15, 57). A growing body of work has described the association of elevated systemic resistin in women with breast cancer, with recent reports revealing resistin levels in treatment naïve patients similar to ours (58, 59). Indeed, the resistin levels in our cohort appear higher than those in recently published reports of acute and inflammatory heart failure (13, 60). Despite the potential confounding of the malignancy itself, resistin remained an indicator of the development of cardiac dysfunction.
Thus, although we have shown resistin to correlate with subsequent cardiotoxicity, it is certainly not the only mechanism and may prove to be an even more robust and specific biomarker for anthracycline-induced cardiotoxicity in a more highly selected group. The findings of our pilot study in humans support an important and intriguing relationship between resistin and anthracycline-induced cardiotoxicity. We acknowledge that the molecular etiologies for this injury are multiple and wide ranging (21). Therefore, some variability in the results presented here is not surprising. Our data do not allow us to assert whether there is 1 dominant mechanism or multiple mechanisms leading to additive effects underlying our results. Understanding the role of resistin in the mechanism of anthracycline-induced cardiotoxicity will be important in the design of future therapies and treatment protocols.
In sum, our preclinical studies suggest that resistin may be a biomarker as well as a contributor to anthracycline-induced cardiotoxicity by its effects on macrophage biology. This further implicates resistin as a link between inflammation and heart disease. The elevation of serum resistin in humans treated with cardiotoxic agents supports the possibility of diagnostic and therapeutic potential of resistin across a range of diseases. Because resistin continues to be discussed as a biomarker for diagnosis and prognosis in heart failure (61), these findings suggest a need to more thoroughly and frequently screen for resistin levels to better understand the natural history of resistin change and its relationship to cardiotoxicity.
Acknowledgments
We thank Xi Liu, MD, of the Echocardiography Core of the Cardiovascular Institute at University of Pennsylvania Perelman School of Medicine for work with mouse echocardiograms; Anbin Mu of the Cardiovascular Institute for help with cardiomyocyte isolation; Daniel Martinez of Pathology Core Laboratory at the Children's Hospital of Philadelphia and Beamon Agarwal, MD, of the Institute for Diabetes and Metabolism for help with immunohistochemistry and histology; and Jean Richa, PhD, and the Transgenic Mouse Core of the Penn Diabetes Research Center for help in generating the Hum-Retn mice. We also thank Michael Parmacek, MD, for critically reading the manuscript and for helpful discussions.
Present address for M.Q.: Division of Diabetes and Endocrinology, Merck Research Laboratories, Rahway, New Jersey 07065.
This work was supported by National Institutes of Health Grants NIK P01 DK49210 and P30 DK19525 (to M.A.L.) and 5-T32 HLO007843 (to D.R.S.) and by an investigator-initiated grant from the Susan G. Komen for the Cure Foundation (M.S.-C.).
Disclosure Summary: M.A.L. and University of Pennsylvania have filed patents related to resistin. M.A.L. has consulted for Eli Lilly and Co, Merck, Novartis, Lycera Corp, Melior Discovery, and Madrigal Pharmaceuticals. D.R.S., E.R.B., M.Q., H.S., I.A.S., M.H.P., and M.S.-C. have nothing to disclose.
Footnotes
- EF
- ejection fraction
- FS
- fractional shortening
- hRETN
- human resistin
- LDH
- lactate dehydrogenase
- LV
- left ventricular
- MCP-1
- monocyte chemoattractant protein-1
- β-MHC
- β-myosin heavy chain
- RKO
- resistin knockout
- TLR4
- toll-like receptor-4
- WT
- wild type.
References
- 1. Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312 [DOI] [PubMed] [Google Scholar]
- 2. Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPAR γ activators. Biochem Biophys Res Commun. 2003;300:472–476 [DOI] [PubMed] [Google Scholar]
- 3. Lu SC, Shieh WY, Chen CY, Hsu SC, Chen HL. Lipopolysaccharide increases resistin gene expression in vivo and in vitro. FEBS Lett. 2002;530:158–162 [DOI] [PubMed] [Google Scholar]
- 4. Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309:286–290 [DOI] [PubMed] [Google Scholar]
- 5. Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz DR, Lazar MA. Human resistin: found in translation from mouse to man. Trends Endocrinol Metab. 2011;22:259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Serum resistin concentrations and risk of new onset heart failure in older persons: the health, aging, and body composition (Health ABC) study. Arterioscler Thromb Vasc Biol. 2009;29:1144–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frankel DS, Vasan RS, D'Agostino RB, Sr, et al. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol. 2009;53:754–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang MH, Na B, Schiller NB, Whooley MA. Association of resistin with heart failure and mortality in patients with stable coronary heart disease: data from the heart and soul study. J Card Fail. 2011;17:24–30 [DOI] [PubMed] [Google Scholar]
- 10. Takeishi Y, Niizeki T, Arimoto T, et al. Serum resistin is associated with high risk in patients with congestive heart failure–a novel link between metabolic signals and heart failure. Circ J. 2007;71:460–464 [DOI] [PubMed] [Google Scholar]
- 11. Ho YL, Lin YH, Lee CM, et al. Prognostic significance of adipocytokines and extracellular matrix activity in heart failure patients with high B-type natriuretic peptide. Clin Biochem. 2009;42:1407–1412 [DOI] [PubMed] [Google Scholar]
- 12. Baldasseroni S, Mannucci E, Di Serio C, et al. Resistin level in coronary artery disease and heart failure: the central role of kidney function. J Cardiovasc Med (Hagerstown). 2013;14:150–157 [DOI] [PubMed] [Google Scholar]
- 13. Bobbert P, Jenke A, Bobbert T, et al. High leptin and resistin expression in chronic heart failure: adverse outcome in patients with dilated and inflammatory cardiomyopathy. Eur J Heart Fail. 2012;14:1265–1275 [DOI] [PubMed] [Google Scholar]
- 14. Wu XM, Lin YH, Chen A, et al. Prognostic significance of adipocytokines in systolic heart failure patients. Eur J Clin Invest. 2012;42:1079–1086 [DOI] [PubMed] [Google Scholar]
- 15. Lee YC, Chen YJ, Wu CC, Lo S, Hou MF, Yuan SS. Resistin expression in breast cancer tissue as a marker of prognosis and hormone therapy stratification. Gynecol Oncol. 2012;125:742–750 [DOI] [PubMed] [Google Scholar]
- 16. Graveleau C, Zaha VG, Mohajer A, et al. Mouse and human resistins impair glucose transport in primary mouse cardiomyocytes, and oligomerization is required for this biological action. J Biol Chem. 2005;280:31679–31685 [DOI] [PubMed] [Google Scholar]
- 17. Kim M, Oh JK, Sakata S, et al. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol. 2008;45:270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chemaly ER, Hadri L, Zhang S, et al. Long-term in vivo resistin overexpression induces myocardial dysfunction and remodeling in rats. J Mol Cell Cardiol. 2011;51:144–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park HK, Qatanani M, Briggs ER, Ahima RS, Lazar MA. Inflammatory induction of human resistin causes insulin resistance in endotoxemic mice. Diabetes. 2011;60:775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest. 2009;119:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905 [DOI] [PubMed] [Google Scholar]
- 22. Fujihira S, Yamamoto T, Matsumoto M, et al. The high incidence of atrial thrombosis in mice given doxorubicin. Toxicol Pathol. 1993;21:362–368 [DOI] [PubMed] [Google Scholar]
- 23. Mukherjee S, Banerjee SK, Maulik M, Dinda AK, Talwar KK, Maulik SK. Protection against acute adriamycin-induced cardiotoxicity by garlic: role of endogenous antioxidants and inhibition of TNF-α expression. BMC Pharmacol. 2003;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lynch DH, Rubin AS, Miller RE, Williams DE. Protective effects of recombinant human interleukin-1 α in doxorubicin-treated normal and tumor-bearing mice. Cancer Res. 1993;53:1565–1570 [PubMed] [Google Scholar]
- 25. Pacher P, Liaudet L, Bai P, et al. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904 [DOI] [PubMed] [Google Scholar]
- 26. Liu L, Zhang X, Qian B, et al. Over-expression of heat shock protein 27 attenuates doxorubicin-induced cardiac dysfunction in mice. Eur J Heart Fail. 2007;9:762–769 [DOI] [PubMed] [Google Scholar]
- 27. van der Vijgh WJ, Maessen PA, Pinedo HM. Comparative metabolism and pharmacokinetics of doxorubicin and 4′-epidoxorubicin in plasma, heart and tumor of tumor-bearing mice. Cancer Chemother Pharmacol. 1990;26:9–12 [DOI] [PubMed] [Google Scholar]
- 28. Horie T, Ono K, Nishi H, et al. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc Res. 2010;87:656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang GW, Kang YJ. Inhibition of doxorubicin toxicity in cultured neonatal mouse cardiomyocytes with elevated metallothionein levels. J Pharmacol Exp Ther. 1999;288:938–944 [PubMed] [Google Scholar]
- 30. Lefterova MI, Steger DJ, Zhuo D, et al. Cell-specific determinants of peroxisome proliferator-activated receptor γ function in adipocytes and macrophages. Mol Cell Biol. 2010;30:2078–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107:1375–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martín M, Esteva FJ, Alba E, et al. Minimizing cardiotoxicity while optimizing treatment efficacy with trastuzumab: review and expert recommendations. Oncologist. 2009;14:1–11 [DOI] [PubMed] [Google Scholar]
- 33. Doroshow JH, Locker GY, Ifrim I, Myers CE. Prevention of doxorubicin cardiac toxicity in the mouse by N-acetylcysteine. J Clin Invest. 1981;68:1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robert J. Long-term and short-term models for studying anthracycline cardiotoxicity and protectors. Cardiovasc Toxicol. 2007;7:135–139 [DOI] [PubMed] [Google Scholar]
- 35. van Almen GC, Swinnen M, Carai P, et al. Absence of thrombospondin-2 increases cardiomyocyte damage and matrix disruption in doxorubicin-induced cardiomyopathy. J Mol Cell Cardiol. 2011;51:318–328 [DOI] [PubMed] [Google Scholar]
- 36. Ali M, Kamjoo M, Thomas HD, et al. The clinically active PARP inhibitor AG014699 ameliorates cardiotoxicity but does not enhance the efficacy of doxorubicin, despite improving tumor perfusion and radiation response in mice. Mol Cancer Ther. 2011;10:2320–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kratz F, Ehling G, Kauffmann HM, Unger C. Acute and repeat-dose toxicity studies of the (6-maleimidocaproyl)hydrazone derivative of doxorubicin (DOXO-EMCH), an albumin-binding prodrug of the anticancer agent doxorubicin. Hum Exp Toxicol. 2007;26:19–35 [DOI] [PubMed] [Google Scholar]
- 38. Anwar MJ, Pillai KK, Khanam R, Akhtar M, Vohora D. Effect of alprazolam on anxiety and cardiomyopathy induced by doxorubicin in mice. Fundam Clin Pharmacol. 2012;26:356–362 [DOI] [PubMed] [Google Scholar]
- 39. Walker DB. Serum chemical biomarkers of cardiac injury for nonclinical safety testing. Toxicol Pathol. 2006;34:94–104 [DOI] [PubMed] [Google Scholar]
- 40. Jung HS, Park KH, Cho YM, et al. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc Res. 2006;69:76–85 [DOI] [PubMed] [Google Scholar]
- 41. Saad SY, Najjar TA, Al-Rikabi AC. The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res. 2001;43:211–218 [DOI] [PubMed] [Google Scholar]
- 42. Li K, Sung RY, Huang WZ, et al. Thrombopoietin protects against in vitro and in vivo cardiotoxicity induced by doxorubicin. Circulation. 2006;113:2211–2220 [DOI] [PubMed] [Google Scholar]
- 43. Neilan TG, Coelho-Filho OR, Pena-Herrera D, et al. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. 2012;110:1679–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vinhas M, Araujo AC, Ribeiro S, Rosario LB, Belo JA. Transthoracic echocardiography reference values in juvenile and adult 129/Sv mice. Cardiovasc Ultrasound. 2013;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hinton RB, Jr, Alfieri CM, Witt SA, et al. Mouse heart valve structure and function: echocardiographic and morphometric analyses from the fetus through the aged adult. Am J Physiol Heart Circ Physiol. 2008;294:H2480–H2488 [DOI] [PubMed] [Google Scholar]
- 46. Riad A, Bien S, Gratz M, et al. Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. Eur J Heart Fail. 2008;10:233–243 [DOI] [PubMed] [Google Scholar]
- 47. Miyata S, Takemura G, Kosai K, et al. Anti-Fas gene therapy prevents doxorubicin-induced acute cardiotoxicity through mechanisms independent of apoptosis. Am J Pathol. 2010;176:687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med. 2010;14:1419–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gan AM, Butoi ED, Manea A, et al. Inflammatory effects of resistin on human smooth muscle cells: up-regulation of fractalkine and its receptor, CX3CR1 expression by TLR4 and Gi-protein pathways. Cell Tissue Res. 2013;351:161–174 [DOI] [PubMed] [Google Scholar]
- 50. Savage DB, Sewter CP, Klenk ES, et al. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-γ action in humans. Diabetes. 2001;50:2199–2202 [DOI] [PubMed] [Google Scholar]
- 51. Wrigley BJ, Lip GY, Shantsila E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail. 2011;13:1161–1171 [DOI] [PubMed] [Google Scholar]
- 52. Zhang J, Clark JR, Jr, Herman EH, Ferrans VJ. Doxorubicin-induced apoptosis in spontaneously hypertensive rats: differential effects in heart, kidney and intestine, and inhibition by ICRF-187. J Mol Cell Cardiol. 1996;28:1931–1943 [DOI] [PubMed] [Google Scholar]
- 53. Cho Y, Lee SE, Lee HC, et al. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J Am Coll Cardiol. 2011;57:99–109 [DOI] [PubMed] [Google Scholar]
- 54. Wang BW, Hung HF, Chang H, Kuan P, Shyu KG. Mechanical stretch enhances the expression of resistin gene in cultured cardiomyocytes via tumor necrosis factor-α. Am J Physiol Heart Circ Physiol. 2007;293:H2305–H2312 [DOI] [PubMed] [Google Scholar]
- 55. Verma S, Li SH, Wang CH, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–740 [DOI] [PubMed] [Google Scholar]
- 56. Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol. 2010;7:564–575 [DOI] [PubMed] [Google Scholar]
- 57. Kang JH, Yu BY, Youn DS. Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci. 2007;22:117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dalamaga M, Sotiropoulos G, Karmaniolas K, Pelekanos N, Papadavid E, Lekka A. Serum resistin: a biomarker of breast cancer in postmenopausal women? Association with clinicopathological characteristics, tumor markers, inflammatory and metabolic parameters. Clin Biochem. 2013;46(7–8):584–590 [DOI] [PubMed] [Google Scholar]
- 59. Alokail MS, Al-Daghri N, Abdulkareem A, et al. Metabolic syndrome biomarkers and early breast cancer in Saudi women: evidence for the presence of a systemic stress response and/or a pre-existing metabolic syndrome-related neoplasia risk? BMC Cancer. 2013;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schulze PC, Biolo A, Gopal D, et al. Dynamics in insulin resistance and plasma levels of adipokines in patients with acute decompensated and chronic stable heart failure. J Card Fail. 2011;17:1004–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cheng JM, Akkerhuis KM, Battes LC, et al. Biomarkers of heart failure with normal ejection fraction: a systematic review [published online July 11, 2013]. Eur J Heart Fail. doi:10.1093/eurjhf/hft106 [DOI] [PubMed] [Google Scholar]