Abstract
In an effort to expand human islets and enhance allogeneic islet transplant for the treatment of type 1 diabetes, identifying signaling pathways that stimulate human β-cell proliferation is paramount. TGF-β superfamily members, in particular activin-A, are likely involved in islet development and may contribute to β-cell proliferation. Nodal, another TGF-β member, is present in both embryonic and adult rodent islets. Nodal, along with its coreceptor, Cripto, are pro-proliferative factors in certain cell types. Although Nodal stimulates apoptosis of rat insulinoma cells (INS-1), Nodal and Cripto signaling have not been studied in the context of human islets. The current study investigated the effects of Nodal and Cripto on human β-cell proliferation, differentiation, and viability. In the human pancreas and isolated human islets, we observed Nodal mRNA and protein expression, with protein expression observed in β and α-cells. Cripto expression was absent from human islets. Furthermore, in cultured human islets, exogenous Nodal stimulated modest β-cell proliferation and inhibited α-cell proliferation with no effect on cellular viability, apoptosis, or differentiation. Nodal stimulated the phosphorylation of mothers against decapentaplegic (SMAD)-2, with no effect on AKT or MAPK signaling, suggesting phosphorylated SMAD signaling was involved in β-cell proliferation. Cripto had no effect on human islet cell proliferation, differentiation, or viability. In conclusion, Nodal stimulates human β-cell proliferation while maintaining cellular viability. Nodal signaling warrants further exploration to better understand and enhance human β-cell proliferative capacity.
Type 1 diabetes mellitus remains a disease associated with significant morbidity and mortality despite medical and technological advances. Prolonged hyperglycemia, variable blood glucoses, and development of hypoglycemia unawareness and hypoglycemic events are all associated with secondary complications in type 1 diabetes. Pancreas and allogeneic islet transplantation are β-cell replacement therapies available for patients with type 1 diabetes to improve glycemic control while minimizing hypoglycemia. Although both require immunosuppression, pancreas and islet transplant have been associated with improved patient outcomes and quality of life (1–3).
Although allogeneic islet transplantation remains an experimental and infrequently used form of therapy, rates of insulin independence after transplantation are improving, and islet transplant recipients who have only partial graft function still report less hypoglycemia and may have better diabetes control and better quality of life (2). For these reasons, pursuit of allogeneic islet transplantation continues. A major limitation to islet transplantation is the limited amount of islet tissue available for transplant. Typical allogeneic islet transplant infusions require one to three human donor pancreases, and patients frequently require more than one infusion to maintain islet function (2). Identifying methods to expand human islets would remove a significant roadblock preventing more routine use of allogeneic islet transplantation.
Efforts to expand human islet tissue have included attempts at expansion of primary human islets, differentiation of stem cell populations, and transdifferentiation of alternate cell types into β-cells, as reviewed (4). Ideally, protocols for β-cell proliferation would involve the production of functional, differentiated β-cells without uncontrolled growth or dedifferentiation. Modest proliferation of primary human β-cells has been obtained with growth factor exposure or directed gene therapy but not enough to substantially expand functional human islets (5–9). The first step to expansion is to formulate a better understanding of the mechanisms and cell signaling events involved in adult human islet cell proliferation.
Nodal is a TGF-β superfamily member critical for endomesodermal induction, specification of left-right asymmetry during embryonic development and maintenance of embryonic stem cell pluripotency (10–12). Nodal may also modulate growth and development of certain cancers (13). Although Nodal expression is rarely detected in normal adult human tissues, microarray data suggest adult human islets do express low levels of the Nodal gene (14, 15). Additionally, we have previously shown that pronounced Nodal expression is present in embryonic and regenerating adult mouse islets with less intense expression observed in control adult mice, suggesting Nodal may function in both embryonic and adult islets (16).
The effects of Nodal are cell type specific, and, in certain cell types, particularly cancer cells, Nodal has been shown to promote proliferation (17, 18). In contrast, treatment of a pancreatic cell line with Nodal inhibited cellular proliferation and induced apoptosis via mothers against decapentaplegic (SMAD)-2- and SMAD3-dependent processes (16). Similarly, Zhao et al (19) recently elucidated that exposure to Nodal increased apoptosis in a rat insulinoma cell line, INS-1, through the up-regulation of the Nodal receptor ALK7. Although data from pancreatic cell lines would suggest Nodal to be antiproliferative and proapoptotic, the effects of Nodal on human islet proliferation and viability are currently unknown.
Cripto, an epidermal growth factor-Cripto-FRL-Criptic protein, is a coreceptor for Nodal that modulates Nodal signaling but can also independently activate the MAPK and AKT pathways, thus stimulating a pro-proliferative program (20, 21). Like Nodal, Cripto is expressed in regenerating mouse islets and in this setting may serve to inhibit activin activity, thereby allowing proliferation of β-cell progenitors (22). Cripto has also been identified as an oncogene and likely contributes to multiple properties of tumorigenesis including cell survival, proliferation, migration, and invasion (23).
To better understand the proliferative capacity of adult human β-cells, different avenues and cell signaling pathways must be investigated. The known influence of the TGF-β superfamily on islet development and function coupled with the potential pro-proliferative capacity of Nodal and Cripto make exploring these factors as mitogens for human β-cells an intriguing concept. On the other hand, if Nodal is expressed in human islets and serves as a proapoptotic factor for human β-cells, this would represent an avenue to explore and potentially improve β-cell survival in human allogeneic islet transplants. We sought to determine human islet responsiveness to Nodal and its coreceptor, Cripto, to better understand the role of these factors in human β-cell proliferation, differentiation, and survival. Our results suggest that Nodal is expressed in adult human islets and that exogenous Nodal promotes human β-cell proliferation while maintaining differentiation status and viability of these cells.
Materials and Methods
Human islet procurement and culture
Human islets from donors without a history of diabetes (Table 1) were obtained from Prodo Laboratories and upon arrival, washed and cultured in CMRL (5.5 mM glucose; GIBCO) + 10% fetal bovine serum (FBS) + penicillin/streptomycin and glutamine in 6-well, ultralow adherence plates (Corning) at a concentration of 1 islet equivalent (IEQ) per 1 μL media. After 24 hours, islets were harvested, washed in PBS, and resuspended in serum-free CMRL containing insulin-transferrin-selenium (Invitrogen), glutamine, and penicillin/streptomycin. The islets were cultured (37°C in 5% CO2) in 24-well, ultralow adherence plates (Corning) at a concentration of 1 IEQ per 1 μL media. After 24 hours in serum-free media, recombinant human Nodal (R&D Systems) and recombinant human Cripto (R&D Systems) were added to the media. Simultaneously, the nucleotide analog, 5-bromo-2-deoxyuridine (BrdU) was added to a final 10 μM concentration (Sigma Aldrich). The media were changed after 72 hours.
Table 1.
Demographic Data of Donors Whose Islets Were Used in the current Study
| Gender | Ethnicity | Age, y | BMI, kg/m2 | Cause of Death | HbA1c | Diabetes? |
|---|---|---|---|---|---|---|
| Female | Caucasian | 39 | 21.9 | Head trauma | 5.5% | No |
| Male | Asian | 26 | 22.7 | ICH | 5.2% | No |
| Female | Hispanic | 24 | 34.9 | Head trauma | NP | No |
| Male | African American | 28 | 21.0 | Head trauma | 5.4% | No |
| Female | Hispanic | 24 | 19.6 | CVA | 5.8% | No |
| Male | Asian | 62 | 26.0 | CVA | NP | No |
| Male | Caucasian | 45 | 31.3 | CVA | NP | No |
| Female | Hispanic | 60 | 26.5 | CVA | NP | No |
| Male | Hispanic | 46 | 26.7 | Head trauma | NP | No |
| Female | Asian | 48 | 30.6 | CVA | 5.8% | No |
| Male | Hispanic | 45 | 24.0 | ICH | NP | No |
| Male | Hispanic | 25 | 29.3 | Head trauma | 5.5% | No |
| Male | Hispanic | 23 | 27.3 | Head trauma | 5.6% | No |
Abbreviations: BMI, body mass index; CVA, cerebrovascular accident; HbA1c, glycosylated hemoglobin; ICH, intracranial hemorrhage; NP, not performed.
Cell culture
PANC-1, TCF7, and human pancreatic nestin-expressing (HPNE) cells were cultured to confluence in DMEM (25 mM glucose) + 10% FBS + penicillin/streptomycin and glutamine at 37°C in 5% CO2. Cells were harvested, pelleted, washed, and frozen at −80°C for later protein lysate or RNA extraction. MIN6 cells were cultured to near confluence in DMEM (25 mM glucose) + 10% FBS + penicillin/streptomycin and glutamine and 50 μM β-mercaptoethanol. Media were changed to low-glucose DMEM (5.5 mM glucose) + insulin-transferrin-selenium for 18 hours incubation after which a subset of the flasks were then exposed to high glucose (25 mM) for 30 minutes. The cells were harvested, washed, and frozen at −80°C for later protein lysate extraction.
Flow cytometry
At the end of the culture period, islets were harvested, washed in PBS, and resuspended in 0.025% trypsin + EDTA (GIBCO) at 500 IEQ per 750 μL trypsin. Islets were incubated in trypsin at 37°C for 15–20 minutes followed by gentle pipetting with 1000 μL pipette tip near the end of the dissociation period. Trypsin treatment was monitored under a tissue microscope and stopped with CMRL + 10% FBS. The resultant single cell suspension was washed with PBS and prepared for flow cytometry. Viability staining was performed with Live/Dead Fixable Stain (Invitrogen). Cells were then fixed and permeabilized (Fix/Perm; BD Biosciences) and stained for insulin, glucagon, and BrdU. Antibodies used included antihuman insulin allophycocyanin monoclonal antibody (R&D Systems) and anti-BrdU fluorescein isothiocyanate monoclonal antibody (BD Biosciences). The glucagon antibody was a mouse monoclonal purified and concentrated from ascites fluid (Sigma-Aldrich), followed by conjugation to R-phycoerythrin using a commercially available kit (Abcam). Flow cytometry was performed at the University of Nebraska Medical Center Flow Cytometry Core Facility using a Becton Dickinson FACStarPlus flow cytometer. Analysis of flow cytometry data was performed with FlowJo software (Tree Star, Inc).
Reverse transcription and nonquantitative and quantitative, real-time PCR
RNA was extracted from cells (HPNE and PANC-1) and freshly isolated cadaveric human pancreatic islets from two separate donors using an RNeasy minikit according to the manufacturer's instructions (QIAGEN Inc). RNA (0.8 μg) was reverse transcribed using the GoScript reverse transcription kit according to manufacturer's protocol (Promega Inc.). Primers used are listed in Table 2. Finally, the Phusion polymerase reaction kit (Fermentas) was used for PCR of reverse transcribed DNA. Samples were run for 35 cycles with an annealing temperature of 58°C. Products were then resolved on a 2% agarose gel and imaged using a Gel-Doc EZ system (Bio-Rad Laboratories Inc.). Images were cut and grouped into figure form using Photoshop CS5 (Adobe Systems Inc).
Table 2.
Table of Sequences for Forward and Reverse Primers for Genes Analyzed by PCR
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Insulin | cagctggagaactactgcaactaga | gctggttcaagggctttattcc |
| Nodal | agacatcatccgcagcctaca | gacctgggacaaagtgacagtgaa |
| Cripto | gatacagcacagtaaggagc | tagttctggagtcctggaag |
| Alk4 | tcctccttcttcccccttgttg | tgctccatcccatccagattg |
| Alk7 | tcgccaggactgaagtgtgtatg | gaagcagcattcggttttggtaac |
| ActR2B | tcagcacacctggcatgaag | agttcgttccatgtgatgatgttc |
| β-Actin | tggcaccacaccttctacaatgagc | gcacagcttctccttaatgtcacgc |
Quantitative real-time PCR was performed using the Fermentas Maxima SYBR Green quantitative PCR master mix (Thermo Scientific) according to the manufacturer's instructions. Samples were run as described above for the nonquantitative RT-PCR for a total of 40 cycles using a Step-One Plus real-time PCR machine (Applied Biosystems). All results are normalized for the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primer sequences for real-time PCR are as follows: MafA forward, AGCGAGAAGTGCCAACTCC, MafA reverse, TTGTACAGGTCCCGCTCTTT; GAPDH forward, AGCCACATCGCTCAGACAC, GAPDH reverse, GCCCAATACGACCAAATCC; Pdx1 forward, AAGCTCACGCGTGGAAAG; and Pdx1 reverse, GCCGTGAGATGTACTTGTTGAA. Nodal sequences are those used for nonquantitative RT-PCR.
Western blot
Islets were harvested, washed with PBS, and frozen at −80°C. To prepare protein lysates, thawed islets were lysed for 30 minutes at 4°C in cell lysis buffer with the addition of phosphatase and protease inhibitors and the resultant lysate centrifuged at 20 000 × g for 20 minutes. Protein concentrations were obtained using a standard Bradford method (Bio-Rad Laboratories). Protein concentration-normalized cell extracts were diluted in sodium dodecyl sulfate-loading dye and separated on a 10% sodium dodecyl sulfate-polyacrylamide gel (Bio-Rad Laboratories) followed by transfer to a nitrocellulose membrane. Membranes were blocked in Tris-buffered saline-0.1% Tween 20 containing 5% nonfat milk for 30 minutes and then incubated with the antibody of choice at 4°C. After overnight incubation, the membranes were washed, and a horseradish peroxidase-coupled secondary antibody was applied and incubated for 1 hour (Cell Signaling, Jackson ImmunoResearch). Enhanced chemiluminescence (SuperSignal; West Pico; and Femto; Pierce) was used to develop the membranes. Antibodies utilized included antiphospho-SMAD2 (Cell Signaling), antiphospho-AKT (Cell Signaling), antiphospho-ERK 1/2 (Cell Signaling), anticytokeratin 19 (Cell Signaling), anti-Sry-type high-mobility-group box transcription factor (SOX)-9 (Millipore), anticleaved caspase-3 (Cell Signaling), anti-Cripto1 (Abcam), and anti-Nodal (Abcam). Horseradish peroxidase-conjugated anti-GAPDH antibody (Abcam) was used as a loading control. Developed images were captured using a Gel-Doc EZ system (Bio-Rad Laboratories Inc).
Immunofluorescence
Islets were harvested, washed, and fixed for 24 hours in 4% paraformaldehyde in 1.5-mL microcentrifuge tubes. After fixation, the islets were precipitated (200 g for 3 min), aspirated, and embedded in 1.5% agarose. The agarose plugs were subsequently embedded in paraffin and sectioned at 4 μm. For immunofluorescence, slides were deparaffinized, rehydrated, and antigens retrieved by microwaving in sodium citrate (0.01 M, pH 6.0) for 20 minutes. Sections were blocked with 5% donkey serum for 1 hour. Primary antibodies to insulin (rabbit antiinsulin; Cell Signaling), BrdU (mouse anti-BrdU; NeoMarkers), Ki67 (mouse antihuman Ki67; Dako), glucagon (rabbit antiglucagon; Cell Signaling), Nodal (mouse antinodal; Abcam), and cytokeratin-19 (mouse biotin anticytokeratin-19; Abcam) were applied and incubated at 4°C overnight in a humidified chamber. After a wash, fluorochrome-conjugated secondary antibodies and donkey-Alexa Fluor-488 and -594 conjugates (Life Technologies) were added and incubated for 1 hour. Coverslips were mounted with VectaShield HardSet mounting medium containing 4′,6-diaminido-2-phenylindole (DAPI; Vector Laboratories) and slides visualized with an Axio Imager (Carl Zeiss). Overlay was performed with Photoshop CS5 software (Adobe Systems, Inc). Cell counting was performed with ImageJ software (available at http://rsb.info.nih.gov/ij/).
TUNEL Staining
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining was performed on islet sections using an in situ death detection kit, fluorescein (Roche) as per the kit instructions. Coverslip mounting with DAPI was performed and images obtained as above. Overlay and counting was performed with ImageJ software (National Institutes of Health).
Statistical analysis
All values are reported as mean ± SEM. Calculations and statistics were performed with Prism 4 (GraphPad). One-sample t test, unpaired 2-tail Student's t test, and ANOVA with Bonferroni's posttest were used to compare differences between treatment groups. Statistical significance was considered to be P < .05.
Results
Nodal and Activin receptors are expressed in adult human pancreatic islets
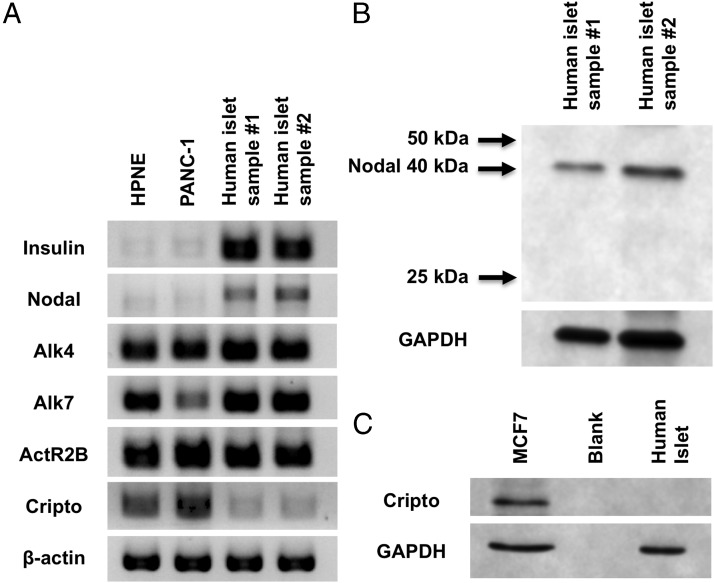
Expression of Nodal has been reported in rat islets, mouse β-cells, and some pancreatic β-cell lines (16, 19). Microarray studies of human islets have also revealed the expression of the Nodal gene (14, 15). Nodal expression is common in human pancreatic adenocarcinoma; however, Nodal was not identified in normal, control human pancreases (24). Protein analysis of whole human pancreas may not be sensitive enough to identify islet expression of Nodal because human islets comprise only 1% of pancreatic mass. Our experiments used isolated human islets of 95% or greater purity, thereby minimizing contamination from pancreatic exocrine and duct tissue. We confirmed the presence of Nodal gene expression in islets using RT-PCR (Figure 1A), with quantitative RT-PCR revealing modest Nodal expression compared with the β-cell genes, MafA and Pdx1 (Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). We also confirmed the expression of Nodal-specific activin receptors, activin receptor 1B (Alk4), activin receptor 2B, and Alk7 (Figure 1A). Using Western blot, we further identified Nodal protein expression (Figure 1B).
Figure 1.
Nodal, but not Cripto, is expressed in adult human islets. Expression of Nodal and Cripto mRNA and protein in isolated adult human islets are shown. A, PCR for Nodal, Cripto, and activin receptors using 0.8 μg of mRNA and specific primers (Table 1) resolved on a 2% agarose gel. HPNE and PANC-1 cells were used as controls. B, Western blot for Nodal protein in isolated human islets cultured for 24 hours before islets were harvested, washed with PBS, and frozen at −80°C. C, Western blot for Cripto in isolated human islets, with MCF7 cells as a positive control.
Cripto expression has not been identified in islets to date, and the Cripto gene was likewise not identified in microarray studies of human islets (14, 15). To further evaluate for Cripto expression, we performed RT-PCR and Western blot analysis in isolated human islets (Figure 1, A and C). Cripto expression was not detected in human islets, whereas PANC-1 cells and HPNE cells showed modest expression. Western blot analysis revealed Cripto protein expression in a breast cancer cell line known to express Cripto at low levels (MCF7) (25). However, no Cripto expression was appreciated in human islets. Our results confirm previous data that Cripto is not expressed in adult human islets.
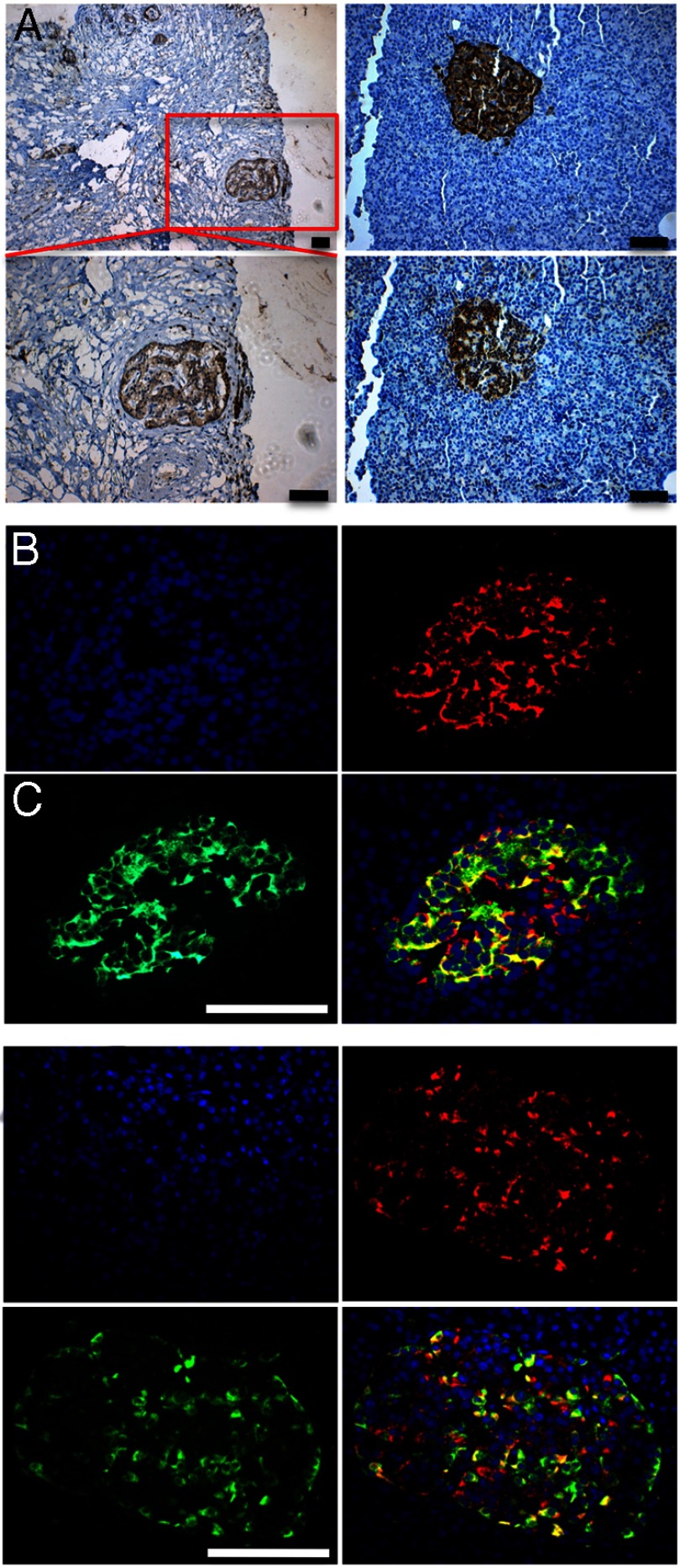
To further confirm Nodal expression in human islets, we performed immunohistochemistry on normal human pancreas sections with staining of human pancreatic adenocarcinoma sections as a positive control (Figure 2A). Nodal expression was found to be essentially limited to pancreatic islets, confirming our Western blot findings and further explaining the lack of Nodal protein expression seen in whole human pancreas protein lysates in a previous study (24). Further examination with immunofluorescence of normal human pancreas revealed coexpression of Nodal within scattered pancreatic β- and α-cells (Figure 2, B and C). These findings suggest that the manipulation of the Nodal signaling pathway is possible within human islets and may affect human islet physiology.
Figure 2.
Nodal is expressed in scattered human pancreatic β- and α-cells. A, Left panels, Human pancreatic cancer sections stained for Nodal as positive control (top ×10, bottom ×20). Right panels, Detection of insulin (top) or Nodal (bottom) in consecutive sections in normal human pancreas. B and C, Immunofluorescent staining of human pancreatic islets revealing coexpression of Nodal (red) and insulin (B) and glucagon (C), both green. Scale bar, 50 μM.
Exogenous Nodal stimulates human β-cell proliferation but inhibits alpha cell proliferation
Flow cytometry analysis was utilized to define the proportion of proliferating β- and α-cells, as measured by BrdU incorporation. Figure 3A illustrates the gating strategy used to gate out live, proliferating β- and α-cells. Human β-cells exposed to high-dose Nodal (10 μg/mL), with or without Cripto, underwent significant proliferation as measured by BrdU incorporation (Figure 3B). Cripto treatment alone did not stimulate β-cell proliferation, and the addition of Cripto to Nodal did not further enhance the β-cell proliferative capacity. Interestingly, α-cell proliferation was inhibited by treatment with Nodal, and this inhibition was not affected by the addition of Cripto (Figure 3C).
Figure 3.
Nodal stimulates human β-cell proliferation while inhibiting α-cell proliferation. Flow cytometry analysis of human islets trypisinized to single cells after 120 hours of exposure to Cripto (250 ng/mL) and/or Nodal (10 μg/mL). Cells were stained for insulin, glucagon, and BrdU to evaluate for proliferating β- and α-cells in Cripto- and/or Nodal-treated groups compared with controls. A, Gating strategy used for flow cytometry analysis. Viable cells, cells that did not take up Live/Dead stain, were gated for insulin and glucagon positive cells (red arrow). Endocrine cells were further gated for double-positive insulin +/BrdU+ (blue arrow) or glucagon+/BrdU+ (green arrow), indicating the proliferating subset of β and α-cells. (BrdU isotype is shown.) B, Comparison of proliferating β-cells in Cripto- and/or Nodal-treated islets compared with control. A total of 3773 ± 577 β-cells were counted per treatment group per experiment (n = 5). C, Comparison of proliferating α-cells in Cripto- and/or Nodal-treated islets compared with control. A total of 5707 ± 961 α-cells were counted per treatment group per experiment (n = 4). Bars indicate mean ± SEM. *, P < .05.
To validate our flow cytometry analysis of human islet cell proliferation, we embedded untreated (control) and Nodal/Cripto-treated islets in paraffin and processed for immunofluorescent analysis. Costaining for insulin and BrdU was performed, and nonconsecutive sections were counted for insulin and BrdU copositive cells (Figure 4A). As was seen with flow cytometry analysis, our immunofluorescence analysis confirmed that proliferation in β-cells exposed to Nodal treatment alone and combination Nodal and Cripto was significantly greater than that observed in controls (Figure 4B). Cripto was again shown to have no effect on proliferation. To confirm the positive effect of Nodal on β-cell proliferation (as measured by β-cell incorporation of BrdU), Ki67 staining of control and Nodal treated islets was performed (Figure 4, C and D). Nodal treatment did stimulate a significant increase in Ki67+ β-cells, confirming the positive effect of Nodal on β-cell proliferation observed by BrdU staining with flow cytometry and immunofluorescence. By flow cytometry, Nodal treatment stimulated β-cell proliferation at 145.2% ± 15.1% of control (P < .05). This compared well with the increase in β-cell proliferation as measured by immunofluorescence of β-cell BrdU incorporation and Ki67+ β-cells (137.5% ± 11.1% and 143.3% ± 4.4% of control, respectively, both P < .05). Our findings of Nodal-driven proliferation of human β-cells reveal a distinct effect of these TGF-β factors on freshly isolated human islet cells compared with tumor-derived β-cell lines.
Figure 4.
Measurement of β-cell proliferation by immunofluorescence confirms robustness of flow cytometry proliferation assay. A, Example of control and Nodal-treated (10 μg/mL) human islets embedded in paraffin, sectioned, and stained for insulin (green), BrdU (red), and DAPI (blue). β-Cells were considered BrdU+ if BrdU and DAPI colocalized and insulin staining surrounded the nucleus (white arrow). B, Quantification of BrdU-positive β-cells in islets after 120 hours exposure to Cripto 250 ng/mL, Nodal 10 μg/ml, or Nodal (10 μg/mL) + Cripto (250 ng/mL) compared with control. Insulin+/BrdU+ cells were quantified per total number of insulin+ cells and results are shown as a percentage of control [n = 4 separate experiments (mean number of β-cells counted per group per experiment: control, 3359 ± 265; Cripto, 3074 ± 253; Nodal, 3137 ± 414; Nodal + Cripto, 3628 ± 280]. C, Quantification of Ki67-positive β-cells in islets after 120 hours exposure to Nodal 10 μg/mL compared with control. Insulin+/Ki67+ cells were quantified per total number of insulin+ cells and results are shown as a percentage of control [n = 3 separate experiments (mean number of β-cells counted per group per experiment: control, 4245 ± 73; Nodal, 4247 ± 175]. D, Example of control and Nodal-treated (10 μg/ml) human islets stained for insulin (green), Ki67 (red), and DAPI (blue). β-Cells were considered Ki67+ if Ki67 and DAPI colocalized and insulin staining surrounded the nucleus (white arrow). Bars indicate mean ± SEM. Scale bar, 50 μM. *, P < .05.
Nodal activates SMAD signaling in human islets, whereas MAPK and AKT signaling remain unaffected by Nodal or Cripto exposure
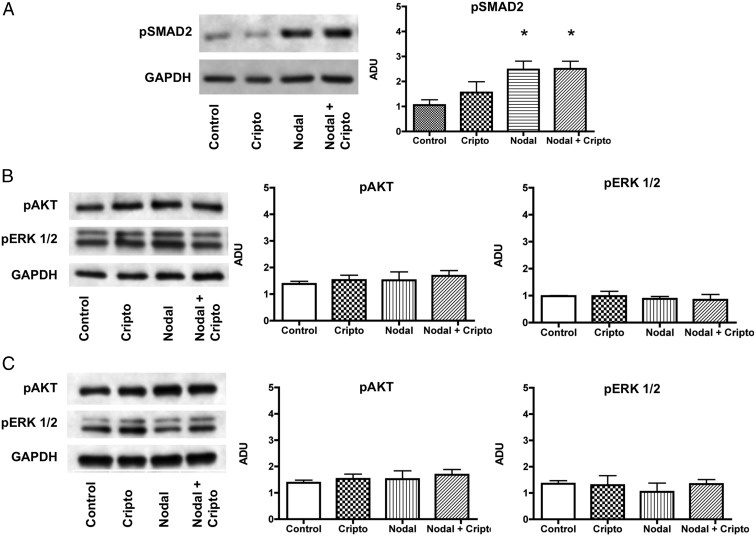
Given the evidence that Nodal treatment was effective in stimulating human islet β-cell proliferation, we sought to identify the cell signaling pathways involved. Upon interacting with an activin receptor complex, Nodal signals through the phosphorylation of SMAD proteins, primarily SMAD2, -3, and -4 to regulate gene transcription. Using Western blot analysis, we confirmed that Nodal stimulated phosphorylation of SMAD2 in human islets, although the addition of exogenous Cripto to Nodal did not further amplify this signal (Figure 5A).
Figure 5.
Nodal signals through SMAD proteins in adult human islets, whereas Nodal and Cripto have no effect on acute or chronic AKT or MAPK activity. Western blot analysis of cell signaling pathways in human islets exposed to acute (30 min) treatment with Nodal (10 μg/mL) and/or Cripto (250 ng/mL). A, Phosphorylated-SMAD2 (n = 5). B, Phosphorylated AKT (n = 3) and phosphorylated ERK 1/2 (n = 4). Western blot analysis of cell signaling pathways in human islets exposed to chronic (120 h) treatment with Nodal (10 μg/mL) and/or Cripto (250 ng/mL). C, Phosphorylated-AKT and ERK 1/2 (n = 3–4). Bars indicate mean ± SEM. *, P < .05.
Cripto is capable of signaling independently of Nodal, primarily through MAPK and AKT, stimulating cellular proliferation in some cell types (26). In human islets, however, Cripto by itself did not stimulate proliferation of β- or α-cells. Accordingly, an acute 30-minute exposure to Cripto also did not up-regulate the phosphorylation of MAPK or AKT (Figure 5B). Similarly, Nodal showed no effect on acute MAPK or AKT signaling. To rule out significant effects on AKT signaling by the insulin/transferrin/selenium (ITS) media supplement, we incubated MIN6 cells for 18 hours in 5.5 mM glucose + ITS and then challenged these cells with high glucose (25 mM) to stimulate AKT signaling (Supplemental Figure 2). Basal AKT phosphorylation was observed in low glucose + ITS, but AKT signaling was significantly up-regulated with a high-glucose challenge, indicating β-cells grown in the presence of chronic, exogenous insulin have intact AKT signaling that responds to known stimuli.
Islets exposed to chronic doses of Nodal and/or Cripto were also studied. Once again, we observed no significant increase in AKT or MAPK phosphorylation at the end of 120 hours of treatment (Figure 5C). Unlike other cell types, we found that Cripto is unable to independently stimulate these specific pathways in human islets. Treatment of human islets with Nodal maintained but did not stimulate MAPK or AKT activity. These results suggest that Nodal treatment does not adversely affect these prosurvival pathways in human β-cells as compared with β-cell lines, which, upon exposure to Nodal, undergo apoptosis associated with a decrease in AKT activity (19).
Chronic Nodal and Cripto exposure do not affect cultured human islet cell differentiation or viability
Under standard culture conditions, human islets undergo cellular dedifferentiation as noted by the loss of cellular endocrine markers and gain of markers consistent with pancreatic progenitor cells (27–29). To evaluate the role of Nodal and Cripto on cellular dedifferentiation, we measured expression of cell markers in treated islets and compared with control: cytokeratin 19, for dedifferentiated β-cells; and SOX9, a known progenitor cell marker that is up-regulated over time in cultured human islets. Isolated human islet preparations are typically not 100% pure and contain measurable amounts of ductal tissue. Therefore, fresh, uncultured islets were used as a baseline measurement of cytokeratin 19 (CK19) and SOX9 expression. By immunofluorescence, CK 19 expression was noted at baseline as well as control and Nodal/Cripto-treated islets (Figure 6A), confirming the presence of ductal tissue contamination in our baseline, uncultured islets. Similarly, by Western blot analysis, CK19 and SOX9 expression were clearly evident in baseline, uncultured islets, again suggesting some level of ductal tissue contamination (Figure 6B). Interestingly, under our culture conditions, CK19 and SOX9 expression did not increase significantly during the culture period, independent of the treatment group, indicating that minimal overt dedifferentiation occurred. Our results suggest that neither Nodal nor Cripto accelerates the islet dedifferentiation in this setting.
Figure 6.

Chronic Nodal and Cripto treatment does not enhance dedifferentiation of human islet cells. Western blot and immunofluorescent analysis of markers of differentiation and cell signaling pathways in human islets were conducted at baseline compared with untreated cultured islets (control) and islets exposed to chronic (120 h) treatment with Nodal (10 μg/mL) and/or Cripto (250 ng/mL). A, Representative images of CK19 expression in baseline, control, and treated islets after 120 hours of culture. CK19 (red), insulin (green), and DAPI (blue) are shown. B, CK19 and SOX9 expression in baseline islets compared with control and treated islets after 120 hours culture (n = 3 for both). Bars indicate mean ± SEM.
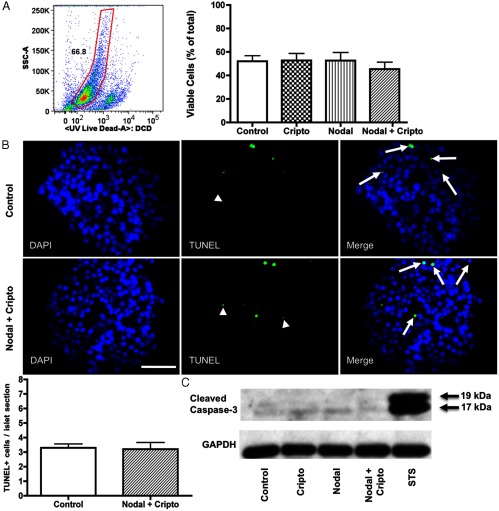
Evidence suggests that Nodal stimulates apoptosis in pancreatic cell lines, both non-β- and β-cell lines (16, 19). To investigate the effects of Nodal and Cripto exposure on human islet apoptosis, we analyzed viability in several ways. First, the proportion of viable cells was measured by flow cytometry analysis using an intracellular staining of dead cells. No difference was seen between the control and Nodal/Cripto-treated groups (Figure 7A). The process of dispersing human islets to single cells with trypsin can adversely affect viability (30). Although islets within each experiment were exposed to an identical trypsinization protocol, we analyzed apoptosis to confirm the viability results obtained from flow cytometry. TUNEL staining was performed on islets treated with and without Nodal and Cripto. The level of apoptosis at the end of the culture period was not different between control islets and those exposed to combination Nodal and Cripto treatment (Figure 7B). Finally, evidence of apoptosis was assessed by Western blot for cleaved caspase-3. Islets exposed for 18 hours to staurosporine were used as a positive apoptotic control to confirm the cleaved caspase-3 antibody specificity. After 120 hours, control islets and islets exposed to Cripto, Nodal, or a combination Nodal and Cripto all showed the same weak level of cleaved caspase-3 (Figure 7C).
Figure 7.
Nodal and Cripto do not adversely affect viability or stimulate apoptosis in human islets. A, Viability of human islet cells by flow cytometry assay (Live/Dead) after 120 hours of exposure to Nodal (10 μg/mL) and/or Cripto (250 ng/mL) compared with control. Live cells are shown within the gate (n = 5). B, TUNEL staining of human islets exposed to Nodal (10 μg/mL) and Cripto (250 ng/mL) for 120 hours compared with control (n = 3). Arrows indicate apoptotic cells (as indicated by TUNEL+/DAPI+). Arrowheads indicate examples of nonspecific fluorescence (DAPI−) that were not counted as apoptotic cells. Then 49 ± 1.4 islets were counted per treatment group per experiment. Bars indicate mean ± SEM. Scale bar, 50 μM. C, Western blot for cleaved caspase-3 expression in islets cultured for 120 hours exposed to Cripto (250 ng/mL) and/or Nodal (10 μg/mL) compared with control. Human islets exposed to staurosporine (STS) 0.5 μM for 18 hours were used as positive control. Image shown is representative of three separate experiments.
Discussion
In this report, we have revealed the presence of Nodal mRNA and protein in human islets, with protein expression identified both in β- and α-cells. We also offered the first evidence of Nodal as a pro-proliferative factor for β-cells in human islet cell culture. The effect on proliferation, albeit modest, is likely modulated by SMAD signaling. Interestingly, Nodal treatment also diminished α-cell proliferation. In contrast to findings from β-cell lines, exogenous Nodal, even at high concentrations, does not cause increased apoptosis in cultured human islet cells. Finally, our study revealed that Nodal and Cripto do not overtly induce the dedifferentiation of human islet cells.
The TGF-β superfamily consists of several proteins that coordinate diverse cellular processes including differentiation, proliferation, survival, and apoptosis in multiple cell and tissue types, including the pancreas (31). Members of the superfamily of TGF-β factors include TGF-β, activin, myostatin, growth differentiation factor, and Nodal. To date, activins are the most widely studied of these factors in pancreatic islets. Activin A and associated activin receptors are expressed in islets, including human islets, and activin A has been demonstrated to play a role in islet development and function (32). Knockdown of the activin receptor and partial knockdown of SMAD2 signaling both lead to reduced islet area in mouse models, suggesting a role for activin signaling in islet expansion (33, 34). Treatment of islets in vitro with activin A is associated with enhanced insulin secretion in response to glucose stimulation (35). Activin A also stimulates proliferation of a β-cell line (MIN6) and primary rat islets (36, 37). In human islets, TGF-β1 exposure may stabilize the endocrine phenotype and protect against apoptosis (38).
Much less is known about Nodal in islet development and function. Nodal is involved in endomesodermal induction and primitive gut epithelial development from which the foregut, and eventually the pancreas, arises (39). Like activin A, Nodal most commonly signals through a complex of activin receptor chains 1B and 2A or 2B (11). This signaling pathway requires the presence of an essential coreceptor, Cripto, an epidermal growth factor-Cripto-FRL-Criptic protein that interacts with activin receptor IB (Alk4). However, Nodal can also signal independently of Cripto through the activin receptor 1C (Alk7) (40). To date, our understanding of Nodal's effects on β-cell biology is limited to studies of rodent islets and β-cell lines. We have previously shown that Nodal inhibits differentiation and stimulates apoptosis in a pancreatic cell line (16). Zhao et al (19) have shown that Nodal is expressed in rat islets and is up-regulated when these islets are exposed to proapoptotic agents, suggesting Nodal is involved in stimulating apoptosis. Zhao et al also revealed deleterious effects of Nodal on viability and proliferation of INS-1 cells, a rat insulinoma cell line, a process that was mediated through Alk7. These studies suggest that Nodal is a negative regulator of β-cell viability and proliferation. However, the up-regulation of Nodal expression seen in rat islets exposed to proapoptotic factors could represent a protective mechanism whereby Nodal acts as a prosurvival and pro-proliferative factor in primary islets. The identification of Nodal expression in human islets raises the intriguing possibility of similar autocrine effects of this hormone in response to human β-cell injury, as is seen in both type 1 and type 2 diabetes.
The effects of Nodal are cell, tissue, and context specific. Nodal, expressed in a number of normal and cancerous tissues, has variable biological effects. In normal human trophoblast cell lines, Nodal inhibits proliferation and stimulates apoptosis in an Alk7-dependent process (41). Similarly, Nodal inhibits proliferation of prostate cancer cell lines, although the inhibition is mediated through Alk4 (17). In contrast, Nodal expression in glioma cells correlated with cell proliferation, and knockdown of Nodal expression was associated with diminished glioma cell proliferation and invasiveness (18). Nodal also stimulates the proliferation and growth of pancreatic cancer stem cell spheres and spermatogonial stem cells (24, 42). Similarly, we have shown that Nodal exerts a pro-proliferative effect on human β-cells, with no overt effects on cellular differentiation or viability. Because Nodal has been reported to inhibit INS-1 cell proliferation, what we observed using freshly isolated human islets supports a cell- or tissue-specific effect for Nodal that could be related to species or mitogenic potential. In fact, human islets are known to have different responses to many exogenous stimuli compared with β-cell lines or even rodents (43, 44).
Activin A is also produced by human islets and activin A drives differentiation of human fetal islet cells; however, mature β-cells undergo proliferation upon exposure to activin A, similar to our results with Nodal (36). The exact mechanism for Nodal inhibition of human α-cell proliferation is not clear; however, Nodal does appear to have an opposite effect on α-cells compared with β-cells. This effect is similar to activin A, which has been shown to decrease proliferation of αTC1 and InR1G9 cells, both glucagon-secreting α-cell lines (45). Therefore, Nodal and activin may exert similar effects on pancreatic β- and α-cell proliferation.
The fact that Cripto is not expressed in human islets and Nodal still elicited SMAD2 phosphorylation suggests that Nodal works in a Cripto-independent manner in human islets, likely in an Alk7-dependent manner. This is further supported by our data showing no increase in SMAD signaling with combined Nodal and Cripto exogenous treatment. We hypothesized that Cripto may independently stimulate β-cell proliferation through MAPK and AKT signaling. However, in our study, Cripto did not affect proliferation or stimulate an acute or chronic up-regulation of either the MAPK or AKT pathways in human islets. Cripto exposure also did not affect viability or differentiation of human islets, suggesting, at doses used in this study, this protein alone has limited if any effect on islet growth.
We also showed that flow cytometry can efficiently measure human islet cell proliferation. Flow cytometry is used in human islet research for the measurement of apoptosis, viability, or cell sorting (46, 47). Flow cytometry has been used in the study of rodent islets, including measuring proportions of hormone-producing cells (48). Pechhold et al (49) used flow cytometric analysis of BrdU incorporation to investigate the effect of blood glucose levels on islet cell proliferation in a mouse model of autoimmune diabetes development.
In our study, we directly compared flow cytometry with direct visualization by immunofluorescence to study the effects of Nodal and Cripto treatment on β-cell proliferation. Overall, we found that the measurement of β-cell proliferation stimulated by Nodal and Cripto treatment was very comparable between flow cytometry and immunofluorescence. In fact, we have shown flow cytometry is a useful, high-throughput method for measuring cellular outcomes in human islet cells, including proliferation.
Our study is not without limitations. First, it is possible that with time-course studies, we may have seen acute activation of AKT or MAPK signaling at a time other than the one measured in our study (30 min). Additionally, AKT signaling in cultured human islets is stimulated by acute exogenous insulin exposure, suggesting in our study that basal AKT signaling may have been higher due to insulin in the ITS media supplement, interfering with our ability to recognize a change in AKT phosphorylation upon growth factor treatment (50). However, chronic insulin administration is not associated with significant, sustained AKT phosphorylation in islets (51). Additionally, in MIN6 cells, AKT signaling in response to high glucose was still robust in the face of chronic exogenous insulin exposure (Supplemental Figure 2). Therefore, our protocol of 24 hours of preculture in ITS-supplemented media prior to growth factor treatment should have significantly reduced interference in the AKT phosphorylation assay by exogenous insulin exposure. Finally, although we saw no significant increase in SOX9 or CK19 expression upon Nodal treatment, the possibility exists that Nodal may affect the maturity of β-cells as has been seen with activin treatment of β-cells (36).
Future work is needed to delineate the exact mechanism whereby Nodal stimulates human β-cell proliferation. Investigating cell cycle mechanisms will be of paramount interest, especially in light of recent data highlighting the importance of cytoplasmic-nuclear shuttling of cell cycle proteins in human β-cell proliferation (52). We also feel strongly, as do others, that investigating and manipulating multiple cell signaling pathways, likely simultaneously, will provide the best method to achieve significant human β-cell proliferation (53). Finally, the effect of Nodal on β-cell function is also of keen interest in light of the known positive effects of activin on glucose-stimulated insulin secretion (35).
In conclusion, Nodal is pro-proliferative in freshly isolated human β-cells in vitro and has no adverse effect on cellular viability. Our findings suggest that Nodal signaling may be important for β-cell replication and may be useful for the expansion of human islets for the treatment of type 1 diabetes. Nodal is one of a limited number of human β-cell mitogens identified to date, and therefore, Nodal signaling warrants additional investigation in the quest to stimulate significant, clinically applicable human β-cell proliferation.
Supplementary Material
Acknowledgments
We are grateful to members of the laboratory of N. E. Sarvetnick including Robert Harms and Dr Victoria Lao for helpful discussion pertaining to this work. We also greatly appreciate the critical review of the manuscript provided by Dr Jennifer Larsen (University of Nebraska Medical Center).
This work is supported by generous funding from the Kieckhefer Foundation (to N.E.S.) and by National Institutes of Health Grant 1UO1AI102012-01 (to N.E.S.). B.P.B. is supported by the University of Nebraska Medical Center, College of Medicine, Physician Scientist Training Award. N.M.G. is supported by an individual National Institutes of Health postdoctoral fellowship (DK091991).
Disclosure Summary: The authors have nothing to declare.
For News & Views see page 3965
- BrdU
- 5-bromo-2-deoxyuridine
- CK19
- cytokeratin 19
- DAPI
- 4′,6-diaminido-2-phenylindole
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- HPNE
- human pancreatic nestin-expressing
- IEQ
- islet equivalent
- ITS
- insulin/transferrin/selenium
- SMAD
- mothers against decapentaplegic
- SOX
- Sry-type high-mobility-group box transcription factor
- TUNEL
- terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling.
References
- 1. Gremizzi C, Vergani A, Paloschi V, Secchi A. Impact of pancreas transplantation on type 1 diabetes-related complications. Curr Opin Organ Transplant. 2010;15:119–123 [DOI] [PubMed] [Google Scholar]
- 2. Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tharavanij T, Betancourt A, Messinger S, et al. Improved long-term health-related quality of life after islet transplantation. Transplantation. 2008;86:1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weir GC, Cavelti-Weder C, Bonner-Weir S. Stem cell approaches for diabetes: towards β cell replacement. Genome Med. 2011;3:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fiaschi-Taesch NM, Salim F, Kleinberger J, et al. Induction of human β-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes. 2010;59:1926–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rutti S, Sauter NS, Bouzakri K, Prazak R, Halban PA, Donath MY. In vitro proliferation of adult human β-cells. PLoS One. 2012;7:e35801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Labriola L, Montor WR, Krogh K, et al. Beneficial effects of prolactin and laminin on human pancreatic islet-cell cultures. Mol Cell Endocrinol. 2007;263:120–133 [DOI] [PubMed] [Google Scholar]
- 8. Tian L, Gao J, Weng G, et al. Comparison of exendin-4 on β-cell replication in mouse and human islet grafts. Transpl Int. 2011;24:856–864 [DOI] [PubMed] [Google Scholar]
- 9. Vasavada RC, Gonzalez-Pertusa JA, Fujinaka Y, Fiaschi-Taesch N, Cozar-Castellano I, Garcia-Ocana A. Growth factors and β cell replication. Int J Biochem Cell Biol. 2006;38:931–950 [DOI] [PubMed] [Google Scholar]
- 10. Raya A, Izpisua Belmonte JC. Left-right asymmetry in the vertebrate embryo: from early information to higher-level integration. Nat Rev Genet. 2006;7:283–293 [DOI] [PubMed] [Google Scholar]
- 11. Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034 [DOI] [PubMed] [Google Scholar]
- 12. Mesnard D, Guzman-Ayala M, Constam DB. Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning. Development. 2006;133:2497–2505 [DOI] [PubMed] [Google Scholar]
- 13. Topczewska JM, Postovit LM, Margaryan NV, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932 [DOI] [PubMed] [Google Scholar]
- 14. Burren OS, Adlem EC, Achuthan P, Christensen M, Coulson RM, Todd JA. T1DBase: update 2011, organization and presentation of large-scale data sets for type 1 diabetes research. Nucleic Acids Res. 2011;39:D997–D1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kutlu B, Kayali AG, Jung S, et al. Meta-analysis of gene expression in human pancreatic islets after in vitro expansion. Physiol Genomics. 2009;39:72–81 [DOI] [PubMed] [Google Scholar]
- 16. Zhang YQ, Sterling L, Stotland A, Hua H, Kritzik M, Sarvetnick N. Nodal and lefty signaling regulates the growth of pancreatic cells. Dev Dyn. 2008;237:1255–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vo BT, Khan SA. Expression of nodal and nodal receptors in prostate stem cells and prostate cancer cells: autocrine effects on cell proliferation and migration. Prostate. 2011;71:1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee CC, Jan HJ, Lai JH, et al. Nodal promotes growth and invasion in human gliomas. Oncogene. 2010;29:3110–3123 [DOI] [PubMed] [Google Scholar]
- 19. Zhao F, Huang F, Tang M, et al. Nodal induces apoptosis through activation of the ALK7 signaling pathway in pancreatic INS-1 β-cells. Am J Physiol Endocrinol Metab. 2012;303:E132–E143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Santis ML, Kannan S, Smith GH, et al. Cripto-1 inhibits β-casein expression in mammary epithelial cells through a p21ras-and phosphatidylinositol 3′-kinase-dependent pathway. Cell Growth Differ. 1997;8:1257–1266 [PubMed] [Google Scholar]
- 21. Ebert AD, Wechselberger C, Frank S, et al. Cripto-1 induces phosphatidylinositol 3′-kinase-dependent phosphorylation of AKT and glycogen synthase kinase 3β in human cervical carcinoma cells. Cancer Res. 1999;59:4502–4505 [PubMed] [Google Scholar]
- 22. Zhang YQ, Cleary MM, Si Y, et al. Inhibition of activin signaling induces pancreatic epithelial cell expansion and diminishes terminal differentiation of pancreatic β-cells. Diabetes. 2004;53:2024–2033 [DOI] [PubMed] [Google Scholar]
- 23. Gray PC, Vale W. Cripto/GRP78 modulation of the TGF-β pathway in development and oncogenesis. FEBS Lett. 2012;586:1836–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lonardo E, Hermann PC, Mueller MT, et al. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–446 [DOI] [PubMed] [Google Scholar]
- 25. Xing PX, Hu XF, Pietersz GA, Hosick HL, McKenzie IF. Cripto: a novel target for antibody-based cancer immunotherapy. Cancer Res. 2004;64:4018–4023 [DOI] [PubMed] [Google Scholar]
- 26. Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor β signaling and enhance cell growth. Mol Cell Biol. 2008;28:666–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Negi S, Jetha A, Aikin R, Hasilo C, Sladek R, Paraskevas S. Analysis of β-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PLoS One. 2012;7:e30415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao R, Ustinov J, Korsgren O, Otonkoski T. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. Diabetologia. 2005;48:2296–2304 [DOI] [PubMed] [Google Scholar]
- 29. Hanley SC, Pilotte A, Massie B, Rosenberg L. Cellular origins of adult human islet in vitro dedifferentiation. Lab Invest. 2008;88:761–772 [DOI] [PubMed] [Google Scholar]
- 30. Peakman M, McNab GL, Heaton ND, Tan KC, Vergani D. Development of techniques for obtaining monodispersed human islet cells. Transplantation. 1994;57:384–393 [DOI] [PubMed] [Google Scholar]
- 31. Brown ML, Schneyer AL. Emerging roles for the TGFβ family in pancreatic β-cell homeostasis. Trends Endocrinol Metab. 2010;21:441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wiater E, Vale W. Roles of activin family in pancreatic development and homeostasis. Mol Cell Endocrinol. 2012;359:23–29 [DOI] [PubMed] [Google Scholar]
- 33. Yamaoka T, Idehara C, Yano M, et al. Hypoplasia of pancreatic islets in transgenic mice expressing activin receptor mutants. J Clin Invest. 1998;102:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goto Y, Nomura M, Tanaka K, et al. Genetic interactions between activin type IIB receptor and Smad2 genes in asymmetrical patterning of the thoracic organs and the development of pancreas islets. Dev Dyn. 2007;236:2865–2874 [DOI] [PubMed] [Google Scholar]
- 35. Florio P, Luisi S, Marchetti P, et al. Activin A stimulates insulin secretion in cultured human pancreatic islets. J Endocrinol Invest. 2000;23:231–234 [DOI] [PubMed] [Google Scholar]
- 36. Szabat M, Johnson JD, Piret JM. Reciprocal modulation of adult β cell maturity by activin A and follistatin. Diabetologia. 2010;53:1680–1689 [DOI] [PubMed] [Google Scholar]
- 37. Brun T, Franklin I, St-Onge L, et al. The diabetes-linked transcription factor PAX4 promotes β-cell proliferation and survival in rat and human islets. J Cell Biol. 2004;167:1123–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanley S, Rosenberg L. Transforming growth factor β is a critical regulator of adult human islet plasticity. Mol Endocrinol. 2007;21:1467–1477 [DOI] [PubMed] [Google Scholar]
- 39. Rankin SA, Kormish J, Kofron M, Jegga A, Zorn AM. A gene regulatory network controlling hhex transcription in the anterior endoderm of the organizer. Dev Biol. 2011;351(2):297–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reissmann E, Jornvall H, Blokzijl A, et al. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Munir S, Xu G, Wu Y, Yang B, Lala PK, Peng C. Nodal and ALK7 inhibit proliferation and induce apoptosis in human trophoblast cells. J Biol Chem. 2004;279:31277–31286 [DOI] [PubMed] [Google Scholar]
- 42. He Z, Jiang J, Kokkinaki M, Dym M. Nodal signaling via an autocrine pathway promotes proliferation of mouse spermatogonial stem/progenitor cells through Smad2/3 and Oct-4 activation. Stem Cells. 2009;27:2580–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerstrom C, Andersson A. Major species differences between humans and rodents in the susceptibility to pancreatic β-cell injury. Proc Natl Acad Sci USA. 1994;91:9253–9256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lai E, Bikopoulos G, Wheeler MB, Rozakis-Adcock M, Volchuk A. Differential activation of ER stress and apoptosis in response to chronically elevated free fatty acids in pancreatic β-cells. Am J Physiol Endocrinol Metab. 2008;294:E540–E550 [DOI] [PubMed] [Google Scholar]
- 45. Mamin A, Philippe J. Activin A decreases glucagon and arx gene expression in α-cell lines. Mol Endocrinol. 2007;21:259–273 [DOI] [PubMed] [Google Scholar]
- 46. Hanson MS, Park EE, Sears ML, et al. A simplified approach to human islet quality assessment. Transplantation. 2010;89:1178–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jayaraman S. A novel method for the detection of viable human pancreatic β cells by flow cytometry using fluorophores that selectively detect labile zinc, mitochondrial membrane potential and protein thiols. Cytometry A. 2008;73:615–625 [DOI] [PubMed] [Google Scholar]
- 48. Pechhold K, Zhu X, Harrison VS, et al. Dynamic changes in pancreatic endocrine cell abundance, distribution, and function in antigen-induced and spontaneous autoimmune diabetes. Diabetes. 2009;58:1175–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pechhold K, Koczwara K, Zhu X, et al. Blood glucose levels regulate pancreatic β-cell proliferation during experimentally-induced and spontaneous autoimmune diabetes in mice. PLoS One. 2009;4:e4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aikin R, Hanley S, Maysinger D, et al. Autocrine insulin action activates Akt and increases survival of isolated human islets. Diabetologia. 2006;49:2900–2909 [DOI] [PubMed] [Google Scholar]
- 51. Johnson JD, Bernal-Mizrachi E, Alejandro EU, et al. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci USA. 2006;103:19575–19580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fiaschi-Taesch NM, Kleinberger JW, Salim F, et al. Cytoplasmic-nuclear trafficking of G1/S cell cycle molecules and adult human β cell replication: a revised model of human β cell G1/S control. Diabetes. 2013;62(7):2460–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kulkarni RN, Mizrachi E, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61(9):2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.