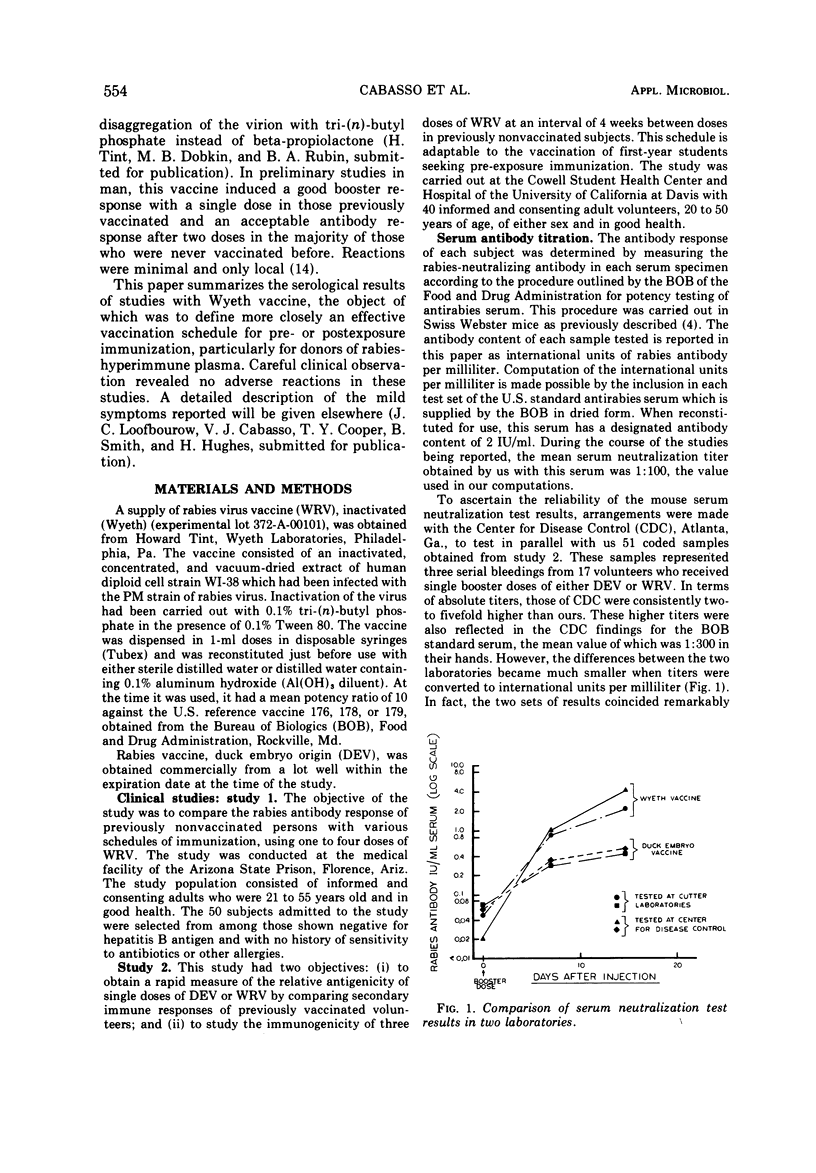
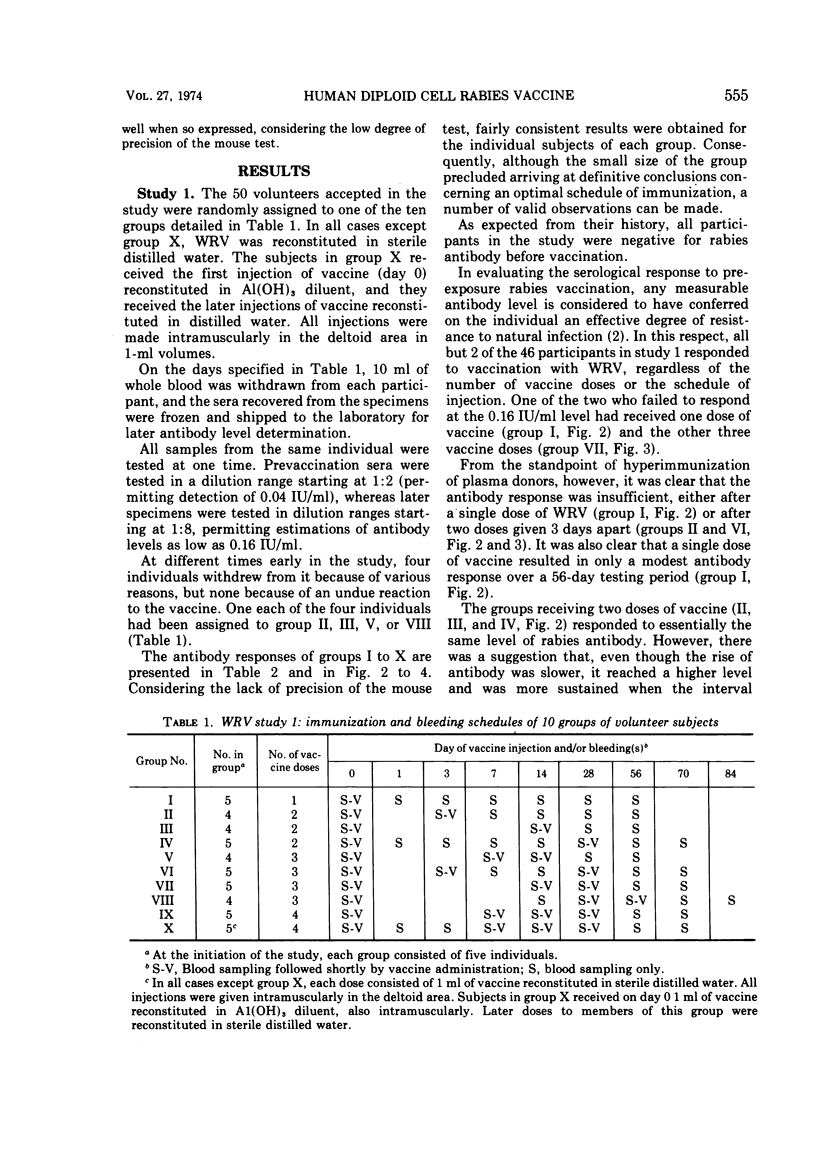
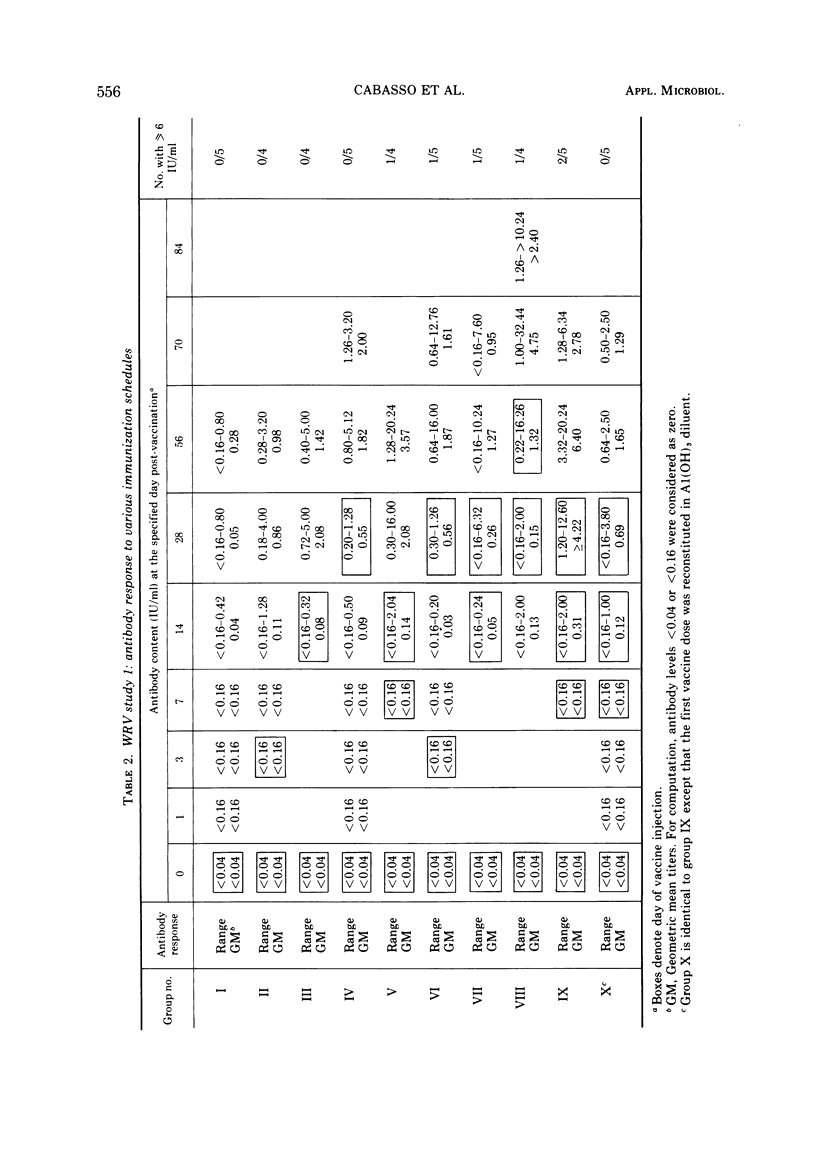
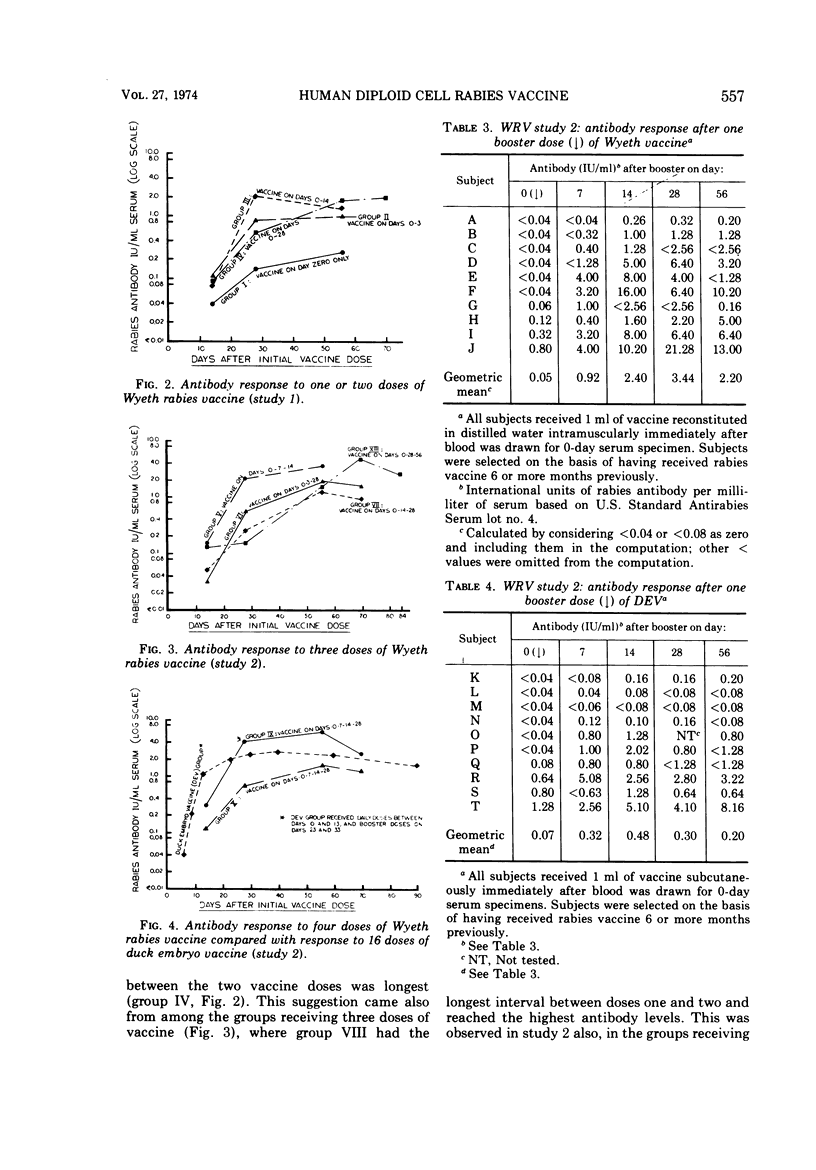
Abstract
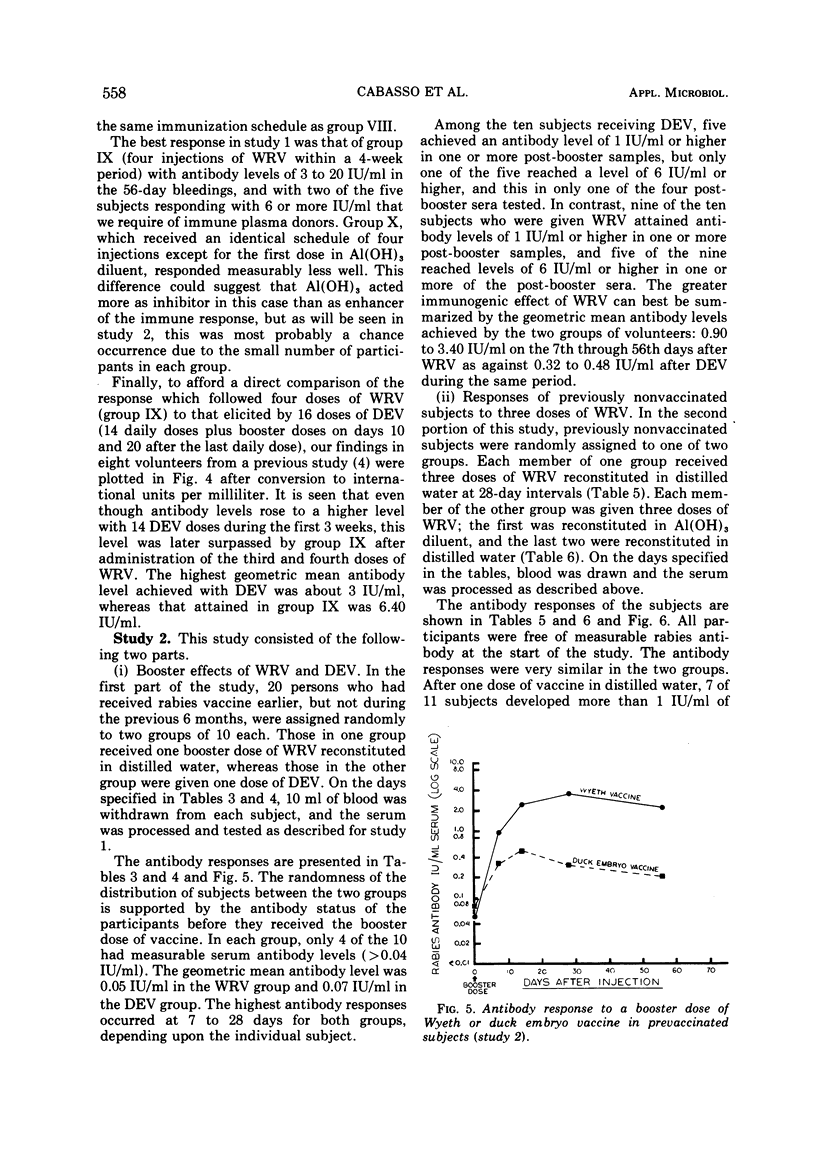
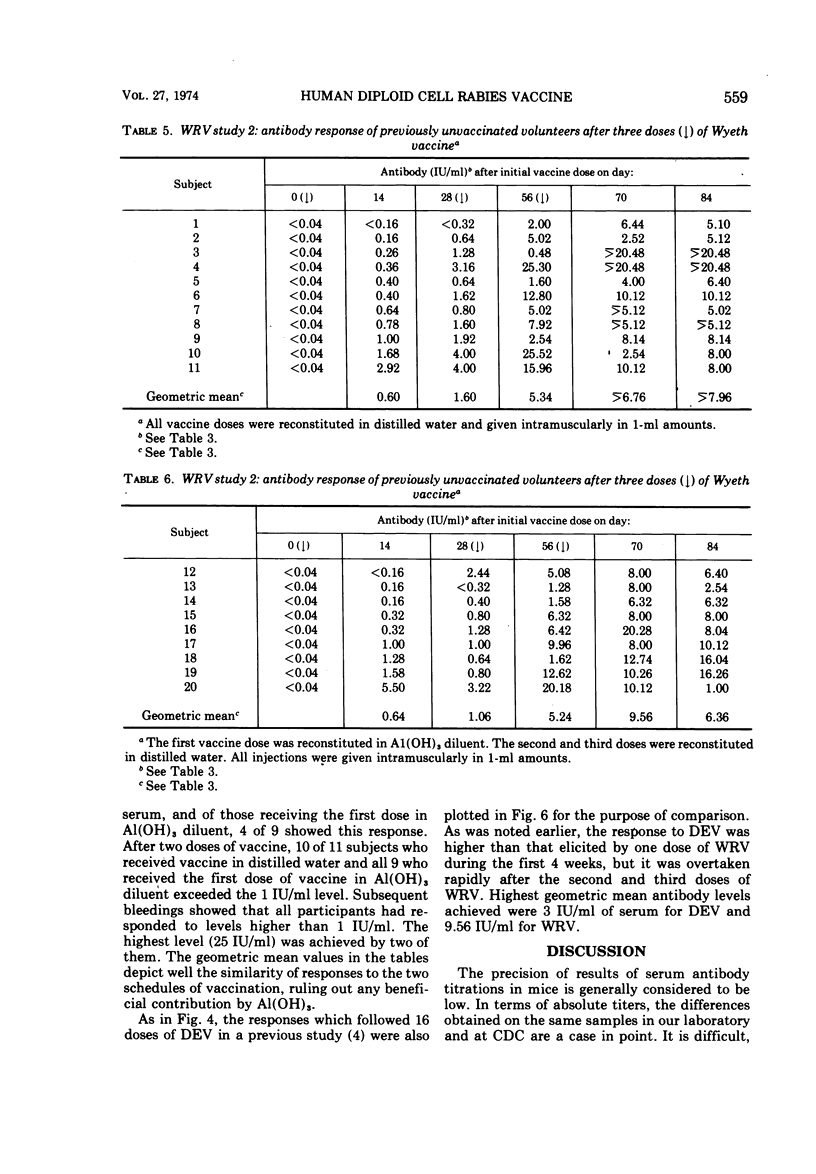
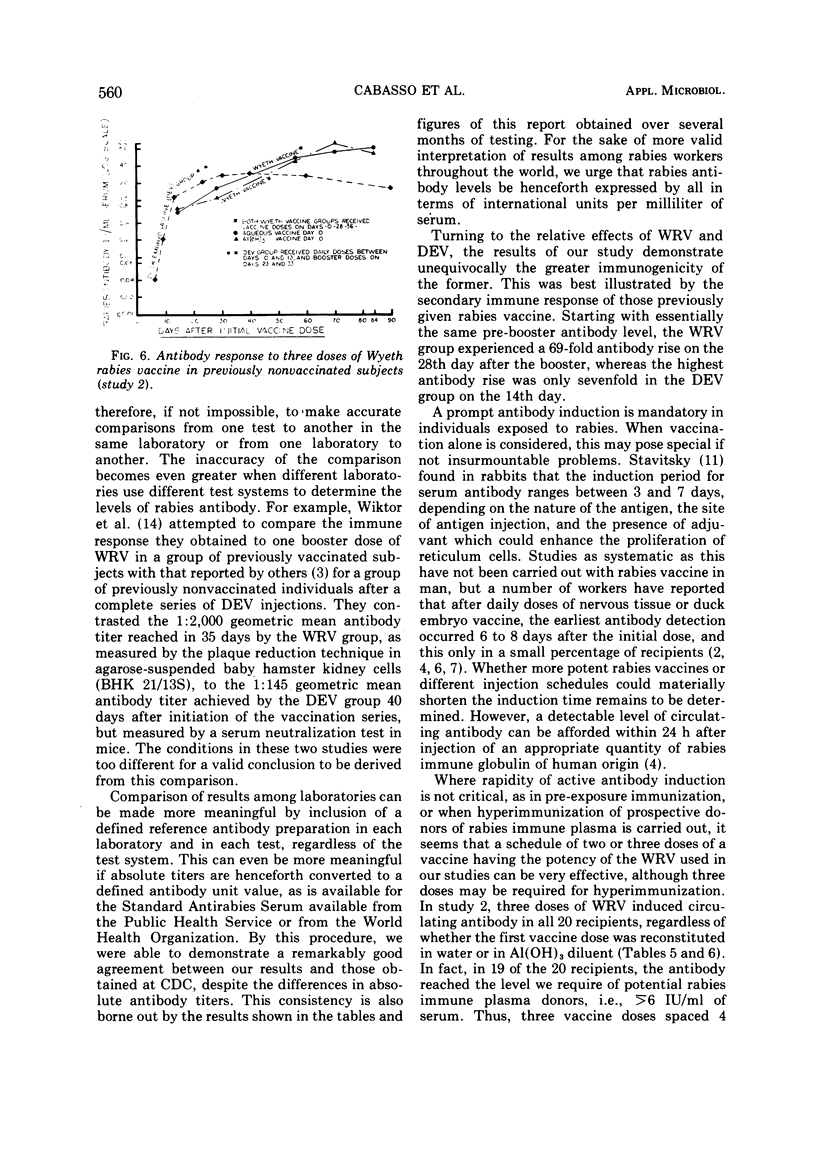
An experimentally killed rabies virus vaccine prepared in a human diploid cell strain (WI-38)—Wyeth rabies vaccine (WRV)—was used by various injection schedules in two separate studies to define more closely in human volunteer subjects an effective vaccination schedule for pre- or postexposure immunization, particularly for donors of rabies-hyperimmune plasma. To permit valid comparisons between our results and those of other workers, antibody levels achieved were expressed in terms of international units (IU) per milliliter of serum. Antibody response of previously nonvaccinated persons were only modest after a single dose of WRV, never reaching a level higher than 0.80 IU/ml over a 56-day testing period. Moreover, antibody was not detected at 0.16 IU/ml before the 14th day, either after a single dose or after two doses given 3 days apart. The best response followed four doses of WRV given within 4 weeks. This schedule resulted in the highest rate of seroconversion to the ≥6 IU/ml antibody level required of potential rabies-immune plasma donors. Giving the first vaccine dose in aluminum hydroxide diluent did not enhance the antibody response. There was a definite suggestion in the various injection schedules that higher and more sustained antibody levels were reached when the interval between the first and second vaccine doses was longest. The greater immunogenicity of WRV as compared with duck embryo vaccine was best demonstrated by the fact that a single booster dose of duck embryo vaccine to previously vaccinated individuals resulted in only a sevenfold antibody rise during the following 56 days, whereas a booster dose of WRV elicited a 69-fold rise. Al(OH)3 in the first dose of WRV had no effect, but the enhancing effect of a longer interval between vaccine doses was noted once again; 20 of 20 subjects who received three doses of WRV with 4 weeks between doses developed good levels of rabies antibody, and 19 exceeded 6 IU/ml.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATANASIU P., BAHMANYAR M., BALTAZARD M., FOX J. P., HABEL K., KAPLAN M. M., KISSLING R. E., KOMAROV A., KOPROWSKI H., LEPINE P. Rabies neutralizing antibody response to different schedules of serum and vaccine inoculations in non-exposed persons. Bull World Health Organ. 1956;14(4):593–611. [PMC free article] [PubMed] [Google Scholar]
- CULBERTSON C. G., PECK F. B., Jr, POWELL H. M. Duck-embryo rabies vaccine; study of fixed virus vaccine grown in embryonated duck eggs and killed with beta-propiolactone (BPL). J Am Med Assoc. 1956 Dec 8;162(15):1373–1376. [PubMed] [Google Scholar]
- Cabasso V. J., Loofbourow J. C., Roby R. E., Anuskiewicz W. Rabies immune globulin of human origin: preparation and dosage determination in non-exposed volunteer subjects. Bull World Health Organ. 1971;45(3):303–315. [PMC free article] [PubMed] [Google Scholar]
- HOSTY T. S., KISSLING R. E., SCHAEFFER M., WALLACE G. A., DIBBLE E. H. Human antirabies gamma globulin. Bull World Health Organ. 1959;20:1111–1119. [PMC free article] [PubMed] [Google Scholar]
- Hattwick M. A., Rubin R. H., Music S., Sikes R. K., Smith J. S., Gregg M. B. Postexposure rabies prophylaxis with human rabies immune globulin. JAMA. 1974 Jan 28;227(4):407–410. [PubMed] [Google Scholar]
- Loofbourow J. C., Cabasso V. J., Roby R. E., Anuskiewicz W. Rabies immune globulin (human). Clinical trials and dose determination. JAMA. 1971 Sep 27;217(13):1825–1831. [PubMed] [Google Scholar]
- Sikes R. K., Cleary W. F., Koprowski H., Wiktor T. J., Kaplan M. M. Effective protection of monkeys against death from street virus by post-exposure administration of tissue-culture rabies vaccine. Bull World Health Organ. 1971;45(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- WIKTOR T. J., KOPROWSKI H. SUCCESSFUL IMMUNIZATION OF PRIMATES WITH RABIES VACCINE PREPARED IN HUMAN DIPLOID CELL STRAIN WI-38. Proc Soc Exp Biol Med. 1965 Apr;118:1069–1073. doi: 10.3181/00379727-118-30048. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Plotkin S. A., Grella D. W. Human cell culture rabies vaccine. Antibody response in man. JAMA. 1973 May 21;224(8):1170–1171. [PubMed] [Google Scholar]
- Wiktor T. J., Sokol F., Kuwert E., Koprowski H. Immunogenicity of concentrated and purified rabies vaccine of tissue culture origin. Proc Soc Exp Biol Med. 1969 Jul;131(3):799–805. doi: 10.3181/00379727-131-33981. [DOI] [PubMed] [Google Scholar]


