Abstract
Aim
Patients hospitalized for acute heart failure (AHF) differ with respect of many clinical characteristics which may influence their prognosis and response to treatment. We have assessed possible differences in the effects of serelaxin on dyspnoea relief, 60 Day outcomes and 180 Day mortality across patient subgroups in the RELAX-AHF trial.
Methods and results
Subgroups were based on pre-specified covariates (age, sex, race, geographic region, estimated glomerular filtration rate, time from presentation to randomization, baseline systolic blood pressure, history of diabetes, atrial fibrillation, ischaemic heart disease, cardiac devices, i.v. nitrates at randomization). Other covariates which may modify the efficacy of AHF treatment were also analysed. Subgroup analyses did not show any difference in the effects of serelaxin vs. placebo on dyspnoea relief or on the incidence of cardiovascular death or rehospitalizations for heart failure or renal failure at 60 days. Nominally significant interactions between some patient subgroups and the effects of serelaxin on 180 days cardiovascular and all-cause mortality were noted but should be interpreted cautiously due to the number of comparisons and the low incidence of deaths in the subgroups at lower risk.
Conclusion
The effects of serelaxin vs. placebo appeared to be similar across subgroups of patients in RELAX-AHF.
Keywords: Acute heart failure, Serelaxin, Mortality, Subgroups
See page 3100 for the editorial comment on this article (doi:10.1093/eurheartj/eht380)
Introduction
Heart failure (HF) is the most important cause of hospitalization for subjects older than 65 years and these hospitalizations are associated with high mortality rates, up to 10–20% in the 6 months following discharge, and 5–15-fold higher than those of ambulatory patients with chronic HF.1–3 However, treatment of acute HF (AHF) has not changed in the recent decades and major trials with new therapies have failed to show clinically meaningful benefits.4–6
Serelaxin is a recombinant form of human relaxin-2, a naturally occurring peptide hormone which mediates the physiological cardiovascular (CV) and renal adaptations of pregnancy.7,8 In the RELAX-AHF trial, a 48-h i.v. infusion of serelaxin to patients with AHF was associated with an improvement in dyspnoea, measured by the visual analogue scale (VAS) to Day 5, but without a significant effect on the other primary endpoint of dyspnoea assessed by the Likert scale, and no change in the two secondary endpoints related with 60-day outcomes.9 Cardiovascular mortality at Day 180 (a protocol-specified additional efficacy analysis) and all-cause mortality at Day 180 (a pre-specified safety endpoint) were significantly reduced by serelaxin administration, which was consistent with the trend observed in the Pre-RELAX-AHF phase II trial.9–11
Patients hospitalized for HF are a heterogeneous population and differ with respect to many characteristics, including the triggers of acute decompensation, and comorbidities.1,12–14 The aim of the present study was to compare the effects of serelaxin vs. placebo on dyspnoea, 60-day outcomes and 180-day mortality, in the major subgroups of patients enrolled in the RELAX-AHF trial.
Methods
The background, design and main results of the RELAX-AHF trial (NCT00520806) have been published.9,15 Briefly, RELAX-AHF was a phase III randomized, double-blind, placebo-controlled, parallel-group, international trial comparing a 48-h i.v. infusion of serelaxin with placebo in patients hospitalized for AHF. From the start of study drug infusion, patients were followed daily to Day 5 or discharge, and then at Days 14, 60, and, only for mortality, at Day 180.
Study design
Patients eligible for enrolment had to be hospitalized for AHF within 16 h of presentation, with dyspnoea at rest or with minimum exertion, pulmonary congestion on chest radiograph, B-type natriuretic peptide (BNP) ≥350 ng/L or N-terminal prohormone of BNP (NT-proBNP) ≥1400 ng/L, mild-to-moderate renal dysfunction [simplified Modification of Diet in Renal Disease estimated glomerular filtration rate (eGFR) of 30–75 mL/min per 1.73 m2], systolic blood pressure (SBP) >125 mmHg, and treatment with at least 40 mg i.v. furosemide or its equivalent before screening. Exclusion criteria have been described previously.9,15
After randomization, patients received either serelaxin 30 µg/kg per day or placebo administered as a continuous i.v. infusion for up to 48 h. Protocol-defined dose adjustment rules were applied in case of an excessive SBP decrease or a fall in SBP to below than 100 mmHg or if a serious or intolerable adverse event or clinically significant laboratory abnormality occurred.
The trial had two primary efficacy endpoints: the difference in patient-reported dyspnoea as quantified by the area under the curve (AUC) of the change from baseline of dyspnoea severity, reported on a 0 to 100 mm VAS, assessed from baseline to Day 5; and the proportion of patients with a moderate or marked improvement in dyspnoea at 6, 12, and 24 h (all three), assessed by a 7-level Likert scale. Secondary efficacy endpoints were: (i) the rate of the combined endpoint of CV death or rehospitalization for HF or renal failure (RF) to Day 60, and (ii) the days alive and out of the hospital to Day 60 in the serelaxin group, compared with placebo. Cardiovascular deaths to Day 180 and all-cause deaths to Day 180 were assessed as a pre-specified additional efficacy endpoint and a safety endpoint, respectively.
Statistics
An evaluation of the possible interaction between the effect of serelaxin on the two primary and key secondary efficacy endpoints (dyspnoea VAS AUC, moderately or markedly better dyspnoea by Likert, CV death or HF/RF rehospitalization through Day 60, and days alive out of hospital through Day 60) was pre-specified for the following covariates: age (<65 vs. ≥65 and <75 and ≥75 years), sex, race (white/Caucasian vs. other), geographic region (Western Europe, Eastern Europe, Israel, USA, Argentina), eGFR (<50 vs. ≥50 and >60 vs. ≥60 mL/min/1.73 m2), time from presentation to randomization (<6 vs. ≥6 h), baseline SBP (<140 vs. ≥140 mmHg), history of diabetes, history of atrial fibrillation, atrial fibrillation present at screening, left ventricular (LV) ejection fraction (LVEF, <40 vs. ≥40%), history of ischaemic heart disease, history of cardiac resynchronization therapy or implantable cardioverter defibrillator, on i.v. nitrates at randomization. Additional post hoc analyses of serelaxin effects on CV and all-cause mortality through Day 180 were also undertaken in these subgroups. Additional subgroups were defined post hoc and included hospitalization for HF in the previous year, heart rate (<80 vs. ≥80 b.p.m.), ACEi/ARB use at baseline, beta-blocker use at baseline, and lymphocyte proportion (≤12 vs. >12%). These covariates were examined as they may modify the effects of AHF therapy and interfere with the vasodilatatory and anti-inflammatory actions of serelaxin.7,8 All P-values were two-sided, and values <0.05 were considered statistically significant.
Analyses of the efficacy outcomes and CV mortality by baseline levels of biomarkers measured at a central laboratory were also pre-specified: troponin T (>99th percentile of the upper reference limit vs. not), NT-proBNP (by tertiles), and cystatin C (by tertiles). Because few patients had a troponin T value below the 99th percentile of the upper reference limit, this covariate was also grouped by tertiles. As previously described in detail,11 biomarkers were analyzed using the Roche high sensitivity cardiac troponin T assay, the Roche Elecsys proBNP assay and the Gentian Cystatin C immunoassay.
The possible differential effect of serelaxin was tested using a separate regression model for each outcome and each covariate that included the effects of serelaxin, the covariate, and the serelaxin-by-covariate interaction. Treatment effects (odds ratio, mean difference, or hazard ratio) were estimated from these regression models: logistic for moderately/markedly better dyspnoea at 6, 12, and 24 h; ANCOVA for the VAS AUC to Day 5 and days alive and out of hospital to Day 60; and Cox for the time to CV death or HF/RF rehospitalization to Day 60 and CV and all-cause mortality through Day 180.
Analyses were conducted on an intent-to-treat basis. SAS© release 9.2 (SAS Institute, Cary, NC, USA) was used for analysis.
Results
The results of RELAX-AHF have been reported in detail.9,11 Enrolment occurred from October 2009 to February 2012 and included 1161 patients (placebo, n = 580; serelaxin, n = 581), of whom 1138 (98%) received randomized study medication. Vital status at 180 days was ascertained for all but 14 patients (two lost-to-follow up; 12 withdrew consent).
Efficacy of serelaxin for subgroups
Patient characteristics, with respect to the baseline variables used to define subgroups, are shown in the Table 1.
Table 1.
Subgroups of characteristics at baseline
| Characteristic | Placebo n/N (%) | Serelaxin n/N (%) |
|---|---|---|
| Gender | ||
| Male | 357/580 (61.6) | 368/581 (63.3) |
| Female | 223/580 (38.4) | 213/581 (36.7) |
| Age | ||
| <65 years | 119/580 (20.5) | 145/581 (25.0) |
| ≥65 years | 461/580 (79.5) | 436/581 (75.0) |
| <75 years | 296/580 (51.0) | 315/581 (54.2) |
| ≥75 years | 284/580 (49.0) | 266/581 (45.8) |
| Region | ||
| Eastern Europe | 282/580 (48.6) | 280/581 (48.2) |
| Western Europe | 101/580 (17.4) | 103/581 (17.7) |
| South America | 37/580 (6.4) | 34/581 (5.9) |
| North America | 55/580 (9.5) | 59/581 (10.2) |
| Israel | 105/580 (18.1) | 105/581 (18.1) |
| Race | ||
| White/Caucasian | 552/580 (95.2) | 544/581 (93.6) |
| Other | 28/580 (4.8) | 37/581 (6.4) |
| Hospitalization for heart failure in past year | ||
| Yes | 181/580 (31.2) | 216/581 (37.2) |
| No | 399/580 (68.8) | 365/581 (62.8) |
| SBP (mmHg) | ||
| <140 | 284/578 (49.1) | 298/577 (51.6) |
| ≥140 | 294/578 (50.9) | 279/577 (48.4) |
| Heart rate (b.p.m.) | ||
| <80 | 296/580 (51.0) | 314/581 (54.0) |
| ≥80 | 284/580 (49.0) | 267/581 (46.0) |
| LVEF (%) | ||
| <40 | 295/539 (54.7) | 303/552 (54.9) |
| ≥40 | 244/539 (45.3) | 249/552 (45.1) |
| History of IHD | ||
| Yes | 307/580 (52.9) | 296/581 (50.9) |
| No | 273/580 (47.1) | 285/581 (49.1) |
| History of ICD or CRT | ||
| Yes | 141/580 (24.3) | 153/581 (26.3) |
| No | 439/580 (75.7) | 428/581 (73.7) |
| History of DM | ||
| Yes | 272/580 (46.9) | 279/581 (48.0) |
| No | 308/580 (53.1) | 302/581 (52.0) |
| History of AF | ||
| Yes | 305/580 (52.6) | 297/581 (51.1) |
| No | 275/580 (47.4) | 284/581 (48.9) |
| AF at screening | ||
| Yes | 246/579 (42.5) | 233/580 (40.2) |
| No | 333/579 (57.5) | 347/580 (59.8) |
| Time from presentation to randomization | ||
| <6 h | 266/580 (45.9) | 275/581 (47.3) |
| ≥6 h | 314/580 (54.1) | 306/581 (52.7) |
| ACEI/ARB use at baseline | ||
| Yes | 398/580 (68.6) | 390/581 (67.1) |
| No | 182/580 (31.4) | 191/581 (32.9) |
| Beta-blocker use at baseline | ||
| Yes | 407/580 (70.2) | 387/581 (66.6) |
| No | 173/580 (29.8) | 194/581 (33.4) |
| MRA use at baseline | ||
| Yes | 173/580 (29.8) | 192/581 (33.0) |
| No | 407/580 (70.2) | 389/581 (67.0) |
| IV nitrates at baseline | ||
| Yes | 42/580 (7.2) | 39/581 (6.7) |
| No | 538/580 (92.8) | 542/581 (93.3) |
| Lymphocytes at baseline | ||
| ≤12% | 127/536 (23.7) | 110/535 (20.6) |
| >12% | 409/536 (76.3) | 425/535 (79.4) |
| Troponin T at baseline (µg/L) | ||
| ≤0.024 | 165/541 (30.5) | 176/533 (33.0) |
| 0.025–0.045 | 196/541 (36.2) | 182/533 (34.1) |
| >0.045 | 180/541 (33.3) | 175/533 (32.8) |
| NT-proBNP at baseline (ng/L) | ||
| <5000 | 279/551 (50.6) | 288/550 (52.4) |
| ≥5000 | 272/551 (49.4) | 262/550 (47.6) |
| ≤3346 | 190/551 (34.5) | 177/550 (32.2) |
| 3347–7281 | 174/551 (31.6) | 194/550 (35.3) |
| >7281 | 187/551 (33.9) | 179/550 (32.5) |
| Cystatin C at baseline (mg/L) | ||
| ≤1.26 | 180/551 (32.7) | 189/550 (34.4) |
| 1.27–1.65 | 191/551 (34.7) | 180/550 (32.7) |
| >1.65 | 180/551 (32.7) | 181/550 (32.9) |
| eGFR at baseline (mL/min/1.73 m2) | ||
| <60 | 408/568 (71.8) | 409/564 (72.5) |
| ≥60 | 160/568 (28.2) | 155/564 (27.5) |
| <50 | 272/568 (47.9) | 268/564 (47.5) |
| ≥50 | 296/568 (52.1) | 296/564 (52.5) |
ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; b.p.m., beats per minute; CRT, cardiac resynchronization therapy; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; h, hours; ICD, implantable cardioverter defibrillator; IHD, ischaemic heart disease; IV, intravenous; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; SBP, systolic blood pressure.
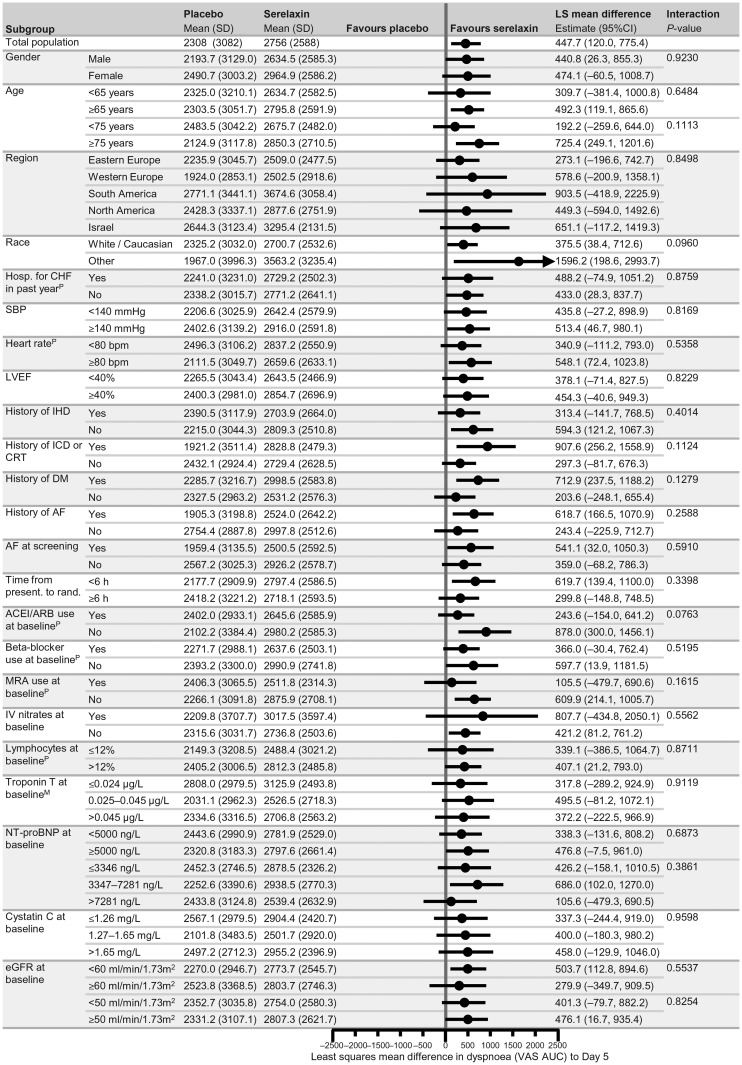
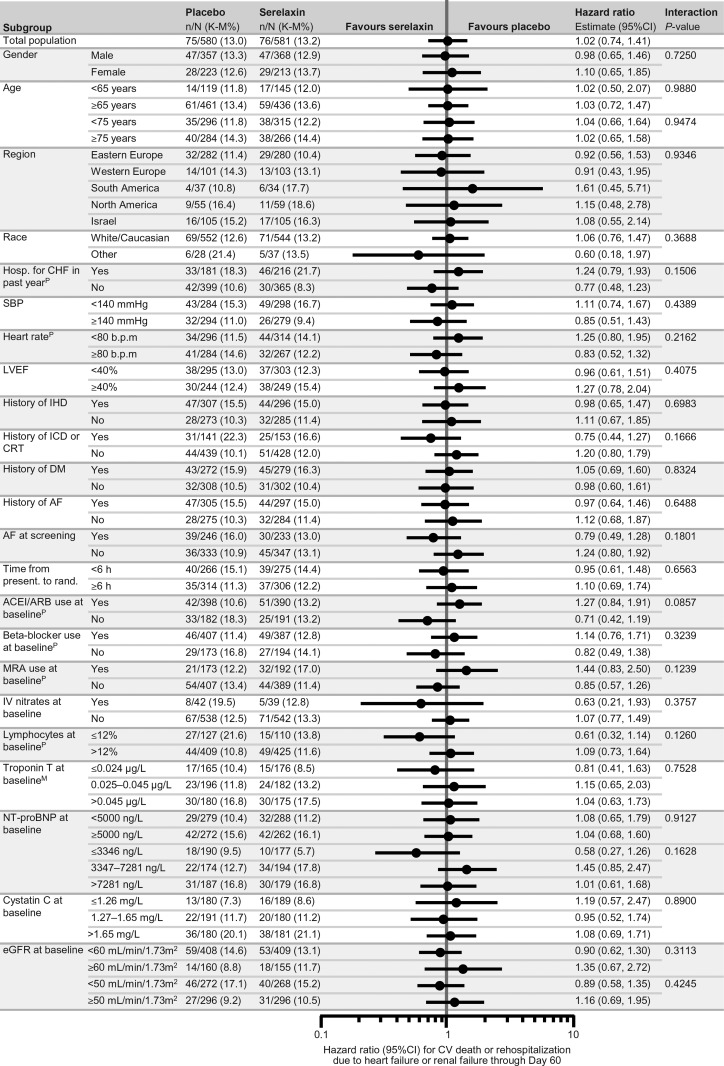
Subgroup analyses regarding the primary and secondary endpoints of RELAX-AHF and CV and all-cause mortality at Day 180 are shown in Figures 1–3 and in Supplementary material online, Figures 1 and 2. The magnitude of the effect of serelaxin relative to placebo on VAS AUC was similar in all the subgroups (Figure 1). No differences were also observed by subgroups analysis with respect of the other primary efficacy endpoint of dyspnoea by the Likert scale (Supplementary material online, Figure 1) and of the hazard ratio for the secondary endpoint of CV death or HF/RF rehospitalization through Day 60 (Figure 2, P > 0.05 in all cases).
Figure 1.
Forest plots of subgroup analysis for dyspnoea Visual Analogue Scale area under the curve change from baseline to Day 5. PPost hoc subgroup analysis, MModification of categories for pre-specified subgrouping variable. ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; b.p.m., beats per minute; CI, confidence interval; CRT, cardiac resynchronization therapy; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; h, hours; hosp., hospitalization; ICD, implantable cardioverter defibrillator; IHD, ischaemic heart disease; IV, intravenous; LS, least squares; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; MRA, mineralocorticoid receptor antagonist; present., presentation; rand., randomization; SBP, systolic blood pressure; SD, standard deviation.
Figure 2.
Forest plots of subgroup analysis for cardiovascular death or heart failure or renal failure rehospitalization through Day 60. PPost hoc subgroup analysis, MModification of categories for pre-specified subgrouping variable. ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; b.p.m., beats per minute; CI, confidence interval; CRT, cardiac resynchronization therapy; CV, cardiovascular; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; h, hours; hosp., hospitalization; ICD, implantable cardioverter defibrillator; IHD, ischaemic heart disease; IV, intravenous; K–M, Kaplan–Meier; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; MRA, mineralocorticoid receptor antagonist; present., presentation; rand., randomization; SBP, systolic blood pressure.
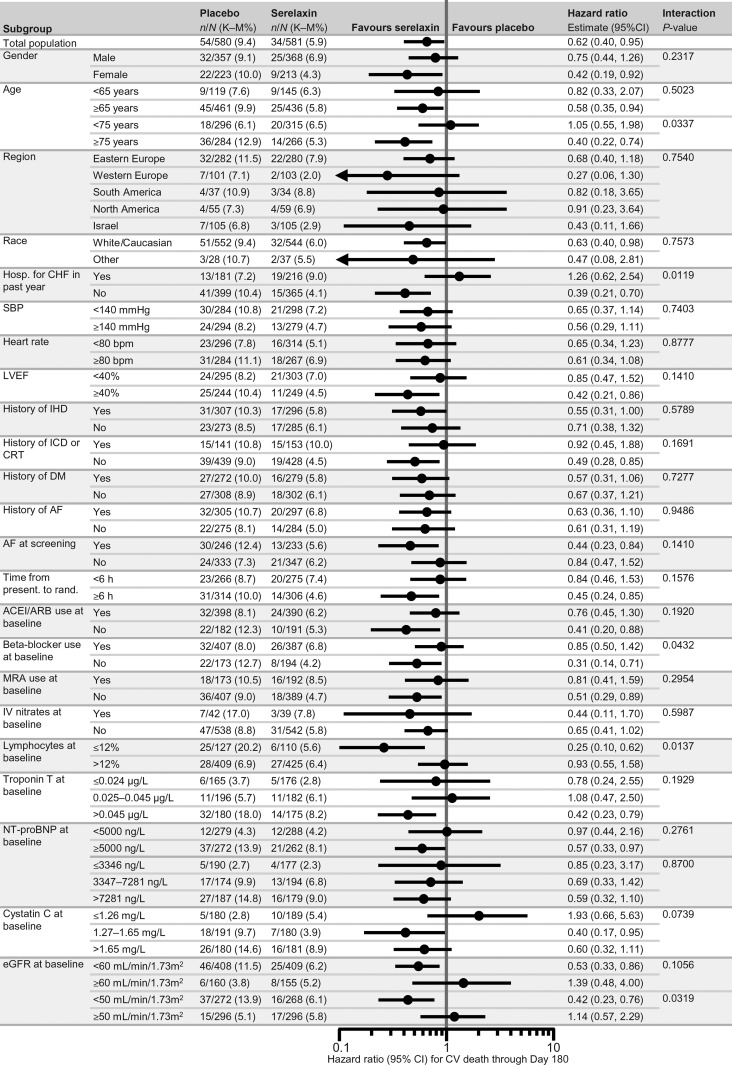
Figure 3.
Forest plots of subgroup analysis for cardiovascular death through Day 180. All these analyses were post hoc. ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; b.p.m., beats per minute; CI, confidence interval; CRT, cardiac resynchronization therapy; CV, cardiovascular; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; h, hours; hosp., hospitalization; ICD, implantable cardioverter defibrillator; IHD, ischaemic heart disease; IV, intravenous; K-M, Kaplan-Meier; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; MRA, mineralocorticoid receptor antagonist; present., presentation; rand., randomization; SBP, systolic blood pressure.
Among all the analyses performed on the five clinical endpoints there were five and four nominally significant (P < 0.05) treatment-by-subgroup interactions which were found with the CV and all-cause mortality endpoints, respectively. A larger reduction in CV mortality, with serelaxin vs. placebo, was noted in the patients aged ≥75 years (P = 0.0337), those with no HF hospitalization in the previous year (P = 0.0119), no beta-blocker use at baseline (P = 0.0432), with blood lymphocytes ≤12% (P = 0.0137), and with an eGFR <50 mL/min/m2 (P = 0.0319) (Figure 3). Similar data were found when 180-day all-cause mortality was considered. The reduction in all-cause mortality, with serelaxin vs. placebo, was larger in the patients aged ≥75 years (P = 0.0473), with no HF hospitalization in the previous year (P = 0.0222), with blood lymphocytes ≤12% (P = 0.0298) and with an eGFR <50 mL/min/m2 (P = 0.0286). No significant interaction was found with any other covariate (Supplementary material online, Figure 2).
Discussion
Our study shows consistency of the results of the RELAX-AHF trial across different patient subgroups based on baseline clinical characteristics. These subgroups included all the pre-specified subgroups as well as additional ones generally considered as clinically important in AHF studies.
Patients hospitalized for HF are known to be a heterogeneous group of patients. The main pathophysiological mechanisms leading to HF decompensation may range from severe LV systolic dysfunction with low cardiac output, low blood pressure, peripheral hypoperfusion, fluid retention and oedema, to increased vascular stiffness, increased pre- and afterload, impairment of LV diastolic function, pulmonary congestion, and oedema.1,13,14 RELAX-AHF was among the first trials to take these differences into account and specifically target those patients most likely to benefit from the study drug.15
Our present analysis shows that the effects of serelaxin on study outcomes were generally consistent across subgroups. Specifically, there was no interaction between the effects of serelaxin and critical variables (such as SBP, time from presentation to randomization, and baseline NT-proBNP values). However, since the study was designed to include patients with SBP >125 mmHg, within 16 h from presentation and with relatively high baseline NT-proBNP or BNP levels, it cannot be excluded that the patients selected were sufficiently homogenous so that subgroup analysis could not yield any further selection of the patients with a better response. Thus, while generally representative of most of the AHF patients, inclusion/exclusion criteria of RELAX-AHF may have resulted in the selection of a relatively homogeneous population, more likely to benefit from a treatment with a predominant vascular mode of action, and with no evident subgroup differences. Secondly, the drug may act on some mechanisms which contribute to the symptoms and the poor survival of the patients having characteristics similar to those of the patients enrolled in RELAX-AHF.7,8
Systolic blood pressure is a major prognostic factor in patients hospitalized for AHF.1,12 It is also a major determinant of the effects of therapy, with the untoward effects of new therapies associated with an excessive blood pressure drop.6,16,17 Based on these data, a SBP >125 mmHg was selected as entry criterion for the RELAX-AHF trial.9,15 This criterion still allows the inclusion of > 70% of the patients admitted for AHF.1,12 Despite its vasodilatatory effects, also shown by the larger proportion of patients who had had a protocol-defined blood-pressure-related study drug dose adjustment or study drug discontinuation,9 no interaction was found between the effects of serelaxin, compared with placebo, and the baseline SBP.
Early treatment is considered essential to obtain better effects in patients admitted for AHF. In analyses of patients included in the Acute Decompensated Heart Failure (ADHERE) Registry, Peacock et al.18 showed that early administration of vasodilators was associated with a better outcome, compared with late treatment. In the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial, patients could be enrolled 24 h from the start of furosemide treatment and the median time from the start of treatment to randomization was 16 h.19 However, these considerations were already taken into account in the design of RELAX-AHF, where patients were enrolled within 16 h from presentation with the median time from presentation to randomization being 7.9 ± 4.7 and 7.8 ± 4.6 h, in the placebo and the serelaxin groups, respectively.9 It is possible that the relatively short time from presentation may have reduced the interpatient variability and, therefore, the interaction between time from presentation to randomization and serelaxin's effect.
N-terminal prohormone of BNP is a major prognostic variable in most studies of patients hospitalized for HF.4,20,21 Increased NT-proBNP or BNP levels at baseline were required as inclusion criteria in RELAX-AHF, with the aim to avoid the enrolment of patients without congestion. This criterion has allowed the enrolment of patients at relatively high risk of events. However, also in this case, baseline NT-proBNP levels did not influence the effects of serelaxin vs. placebo and this may be related to the pre-selection of patients with high BNP or NT-proBNP levels at baseline, since this was an inclusion criterion.11
Patients with a preserved LVEF represent approximately half of the patients admitted for AHF and this proportion is growing due to ageing of the general population.22 No treatment has been shown as effective in the patients with HF and preserved LVEF, to date.4 These patients have unique clinical characteristics and were excluded or under-represented in previous large AHF trials.16,23,24 Less than 20% of all the patients included in ASCEND-HF had a LVEF <40%,19 and LVEF was available in only approximately half of all the patients in two other AHF trials.25,26
In RELAX-AHF, LVEF was available in most of the patients (Table 1) and 45% of the patients had a LVEF above the pre-specified cut-off for subgroup analysis of 40%.9 No differences in the response to serelaxin administration were found in the patients with a LVEF < or ≥40% with respect to any endpoint. These results are consistent with the presence of common pathogenetic mechanisms in AHF, such as increased LV afterload, pulmonary congestion, and end-organ damage, which are independent of LVEF, and on which serelaxin may exert beneficial effects.11,27 These results are also consistent with serelaxin's mechanism of action, which is active more on the peripheral vasculature and arterial elastance, rather than on the myocardium.7,8
Another subgroup of potential interest is that of patients on i.v. nitrates at the time of randomization. Nitrates are a treatment option for patients with AHF and high blood pressure4 and were allowed in RELAX-AHF for the patients with a SBP >150 mmHg at screening.9,15 Although, theoretically, their concomitant administration might have blunted some of the favourable effects of serelaxin, estimated treatment effects on mortality and dyspnoea were in the same direction as for the patients not on nitrates. However, the proportion of patients on i.v. nitrates at randomization was small and the confidence intervals for the estimated treatment effects were wide.
Some interactions were nominally statistically significant when the 180-day CV mortality and all-cause mortality data were considered (Figure 3). However, these data have to be interpreted with caution as these subgroup analyses were not pre-specified, the number of subgroups examined was large and the number of events was small. The interaction between some variables and the effects of serelaxin on mortality, compared with placebo, may be explained by the low incidence of deaths in the subgroups at lower risk, such as that of the patients ≤75 years old or with an eGFR ≥50 mL/min/m2. Some data seem, however, to suggest that serelaxin was more effective in patients with no history of HF, as shown by the subgroups with no HF hospitalization in the previous year and no concomitant treatment with neurohormonal antagonists, such as angiotensin-converting enzyme inhibitors, mineralocorticoid receptor antagonists and beta-blockers (Figures 1–3). One potential explanation is that patients not treated with neurohormonal antagonists are more likely to be more sensitive to the untoward effects of diuretic therapy, and serelaxin administration was associated with lower diuretic doses in the RELAX-AHF trial.9 The same might also apply to the subgroup with reduced eGFR as kidney dysfunction is also associated with the need to use higher doses of loop diuretics. In addition, serelaxin may be effective on neurohormonal and inflammatory mechanisms which are favourably affected also by neurohormonal antagonists.7,8
The limitations of subgroup analyses are well known.28,29 Although the results suggest a homogeneous response to serelaxin among the subgroups examined, the study was not powered to detect differential treatment effects. On the other hand, any nominally statistically significant interactions should be interpreted cautiously given the number of both pre-specified and post hoc analyses performed. The results of the present study must be limited to the patients with characteristics similar to those of the patients enrolled in RELAX-AHF with, namely, a SBP >125 mmHg and <16 h from presentation to hospital. Any extension of the present findings to patients with different characteristics from those of the patients in RELAX-AHF is not possible.
In conclusion, subgroup analyses of the RELAX-AHF trial has shown similar effects of serelaxin, when compared with placebo, across various subgroups, suggesting a consistency of the effect of serelaxin in the patients with AHF with the characteristics used in this study.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The RELAX-AHF trial was supported by Corthera, Inc., a member of the Novartis group of companies. Graham Allcock of CircleScience provided editorial assistance, which was funded by Novartis Pharma AG, Basel, Switzerland.
Conflict of interest: M.M. received consulting income from Novartis, Amgen, Bayer, Daiichi-Sankyo, Servier and Trevena and received speaker honoraria from Abbott Vascular and Novartis. P.P. received consulting income from Abbott Vascular, Amgen, Bayer, J&J, Novartis, Servier, Cardiorentis, Vifor, Cibiem, Momentum Research and Respicardia; received speaker honoraria from Abbott Vascular, Novartis, Servier, Vifor, Pfizer, Merck-Serono and Boehringer Ingelheim and received research grant support from Vifor. G.C. and B.D. are employees of Momentum Research, which has received research grants from Novartis, Corthera, Inc. (a Novartis Company), Sorbent Therapeutics, ChanRx, Amgen, Cardio3 Biosciences, Trevena and Anexon and have received travel support from Momentum Research. G.M.F. received consulting income from Novartis, Amgen, Otsuka and Trevena; received research grant support from Amgen and Otsuka; received payment for participation in review activities (e.g. data monitoring boards, endpoint committee, etc) from Novartis and received payment for development of educational presentations from Novartis. G.F. received consulting income from Corthera, Inc. (a Novartis company), Bayer and Cardiorentis; received speaker honoraria from Novartis and received research grant support from Amgen, Nanosphere and the European Union. B.H.G. received consulting income from Novartis, Celladon and Zensun; received payment for participation in review activities (e.g. data monitoring boards, endpoint committee, etc) from St. Jude's Medical, Actelion, Amgen, Bayer and Gambro; and received payment for development of educational presentations from Novartis. T.A.H. and T.S. are employees of Novartis, and receive salary and stock options from Novartis Pharmaceuticals Corporation and Novartis Pharma AG, respectively. E.U. was former employee of the sponsor, Corthera, Inc. (a Novartis Company). A.A.V. received consulting income from Novartis; received speaker honoraria from Novartis and received research grant support from Novartis. J.R.T. received consulting income from Novartis, Trevena and Amgen/Cytokinetics and received research grant support from Novartis and Amgen/Cytokinetics.
Supplementary Material
References
- 1.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L International Working Group on Acute Heart Failure Syndromes. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 2.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA. Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Investigators. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamsson P, Swedberg K, Borer JS, Böhm M, Kober L, Komajda M, Lloyd SM, Metra M, Tavazzi L, Ford I. Risk following hospitalization in stable chronic systolic heart failure. Eur J Heart Fail. 2013;15:885–891. doi: 10.1093/eurjhf/hft032. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P Task Force for the D, Treatment of A, Chronic Heart Failure of the European Society of C. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. ESC Committee for Practice Guidelines. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. doi:10.1161/?CIR.0b013e31829e8776. Published online ahead of print 5 June 2013. [Google Scholar]
- 6.Felker GM, Pang PS, Adams KF, Cleland JG, Cotter G, Dickstein K, Filippatos GS, Fonarow GC, Greenberg BH, Hernandez AF, Khan S, Komajda M, Konstam MA, Liu PP, Maggioni AP, Massie BM, McMurray JJ, Mehra M, Metra M, O'Connell J, O'Connor CM, Pina IL, Ponikowski P, Sabbah HN, Teerlink JR, Udelson JE, Yancy CW, Zannad F, Gheorghiade M, International AHFS Working Group. Clinical trials of pharmacological therapies in acute heart failure syndromes: lessons learned and directions forward. Circ Heart Fail. 2010;3:314–325. doi: 10.1161/CIRCHEARTFAILURE.109.893222. [DOI] [PubMed] [Google Scholar]
- 7.Teichman SL, Unemori E, Dschietzig T, Conrad K, Voors AA, Teerlink JR, Felker GM, Metra M, Cotter G. Relaxin, a pleiotropic vasodilator for the treatment of heart failure. Heart Fail Rev. 2009;14:321–329. doi: 10.1007/s10741-008-9129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du XJ, Bathgate RA, Samuel CS, Dart AM, Summers RJ. Cardiovascular effects of relaxin: from basic science to clinical therapy. Nat Rev Cardiol. 2010;7:48–58. doi: 10.1038/nrcardio.2009.198. [DOI] [PubMed] [Google Scholar]
- 9.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M RELAXin in Acute Heart Failure (RELAX-AHF) Investigators. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 10.Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, Marmor A, Katz A, Grzybowski J, Unemori E, Teichman SL, Cotter G. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIB study. Lancet. 2009;373:1429–1439. doi: 10.1016/S0140-6736(09)60622-X. [DOI] [PubMed] [Google Scholar]
- 11.Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR. RELAX-AHF Investigators. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the relaxin in acute heart failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391–403. doi: 10.1016/j.jacc.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure–re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10:165–169. doi: 10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Cotter G, Moshkovitz Y, Milovanov O, Salah A, Blatt A, Krakover R, Vered Z, Kaluski E. Acute heart failure: a novel approach to its pathogenesis and treatment. Eur J Heart Fail. 2002;4:227–234. doi: 10.1016/s1388-9842(02)00017-x. [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P, Metra M, Teerlink JR, Unemori E, Felker GM, Voors AA, Filippatos G, Greenberg B, Teichman SL, Severin T, Mueller-Velten G, Cotter G, Davison BA. Design of the relaxin in acute heart failure study. Am Heart J. 2012;163:149–155. doi: 10.1016/j.ahj.2011.10.009. e141. [DOI] [PubMed] [Google Scholar]
- 16.Packer MCW, Fisher L, Massie BM, Teerlink JT, Young J, Thakkar R, Delgado-Herrera L, Salon J, Garratt C, Huang B, Sarapohja T. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. J Am Coll Cardiol Heart Fail. 2013;1:8. [Google Scholar]
- 17.Erdmann E, Semigran MJ, Nieminen MS, Gheorghiade M, Agrawal R, Mitrovic V, Mebazaa A. Cinaciguat, a soluble guanylate cyclase activator, unloads the heart but also causes hypotension in acute decompensated heart failure. Eur Heart J. 2013;34:57–67. doi: 10.1093/eurheartj/ehs196. [DOI] [PubMed] [Google Scholar]
- 18.Peacock WF, 4th, Fonarow GC, Emerman CL, Mills RM, Wynne J, Committee ASA Investigators, Adhere Study G. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in adhere. Cardiology. 2007;107:44–51. doi: 10.1159/000093612. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. New Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 20.Kociol RD, Horton JR, Fonarow GC, Reyes EM, Shaw LK, O'Connor CM, Felker GM, Hernandez AF. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF) linked to medicare claims. Circ Heart Fail. 2011;4:628–636. doi: 10.1161/CIRCHEARTFAILURE.111.962290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maisel A, Mueller C, Adams K, Jr, Anker SD, Aspromonte N, Cleland JG, Cohen-Solal A, Dahlstrom U, DeMaria A, Di Somma S, Filippatos GS, Fonarow GC, Jourdain P, Komajda M, Liu PP, McDonagh T, McDonald K, Mebazaa A, Nieminen MS, Peacock WF, Tubaro M, Valle R, Vanderhyden M, Yancy CW, Zannad F, Braunwald E. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 23.Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Poder P, Kivikko M. SURVIVE Investigators. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 24.Gheorghiade M, Konstam MA, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan I. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST clinical status trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 25.McMurray JJ, Teerlink JR, Cotter G, Bourge RC, Cleland JG, Jondeau G, Krum H, Metra M, O'Connor CM, Parker JD, Torre-Amione G, van Veldhuisen DJ, Lewsey J, Frey A, Rainisio M, Kobrin I. VERITAS Investigators. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA. 2007;298:2009–2019. doi: 10.1001/jama.298.17.2009. [DOI] [PubMed] [Google Scholar]
- 26.Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC PROTECT Investigators and Committees. Rolofylline, an adenosine a1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 27.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 28.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355:1064–1069. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]
- 29.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21:2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.