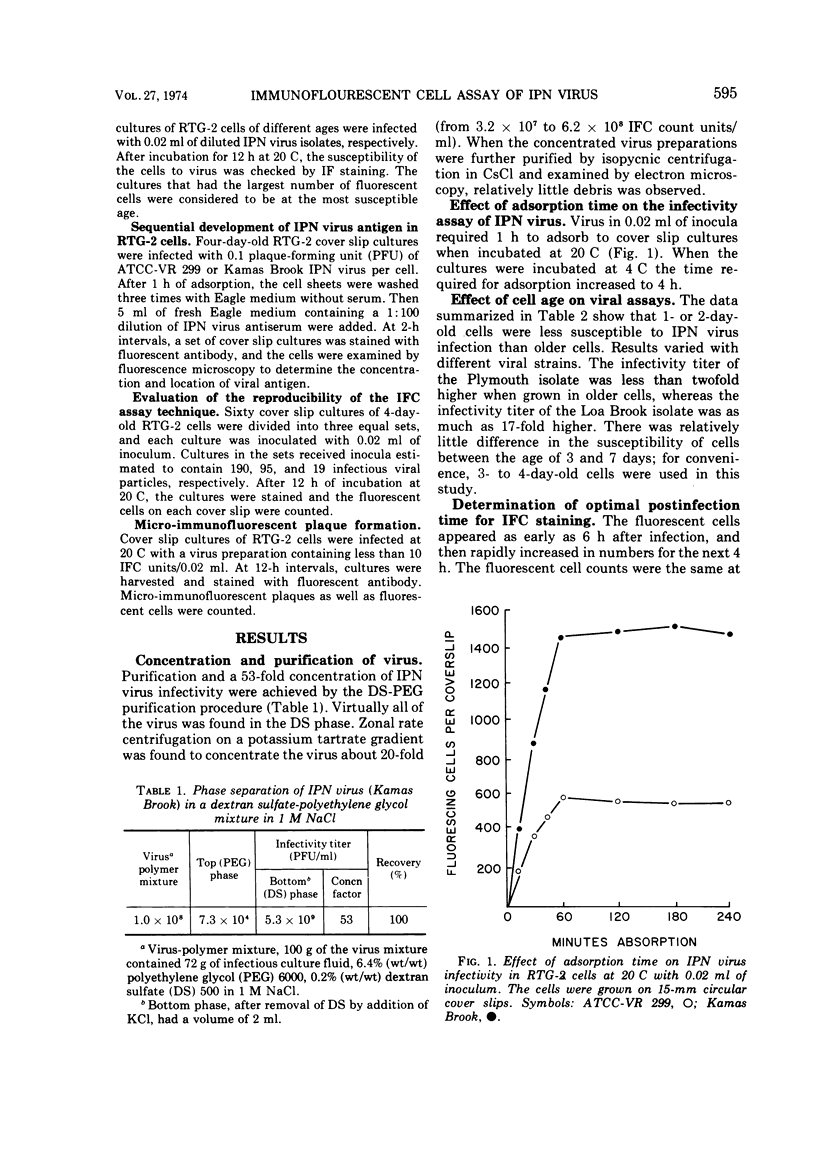
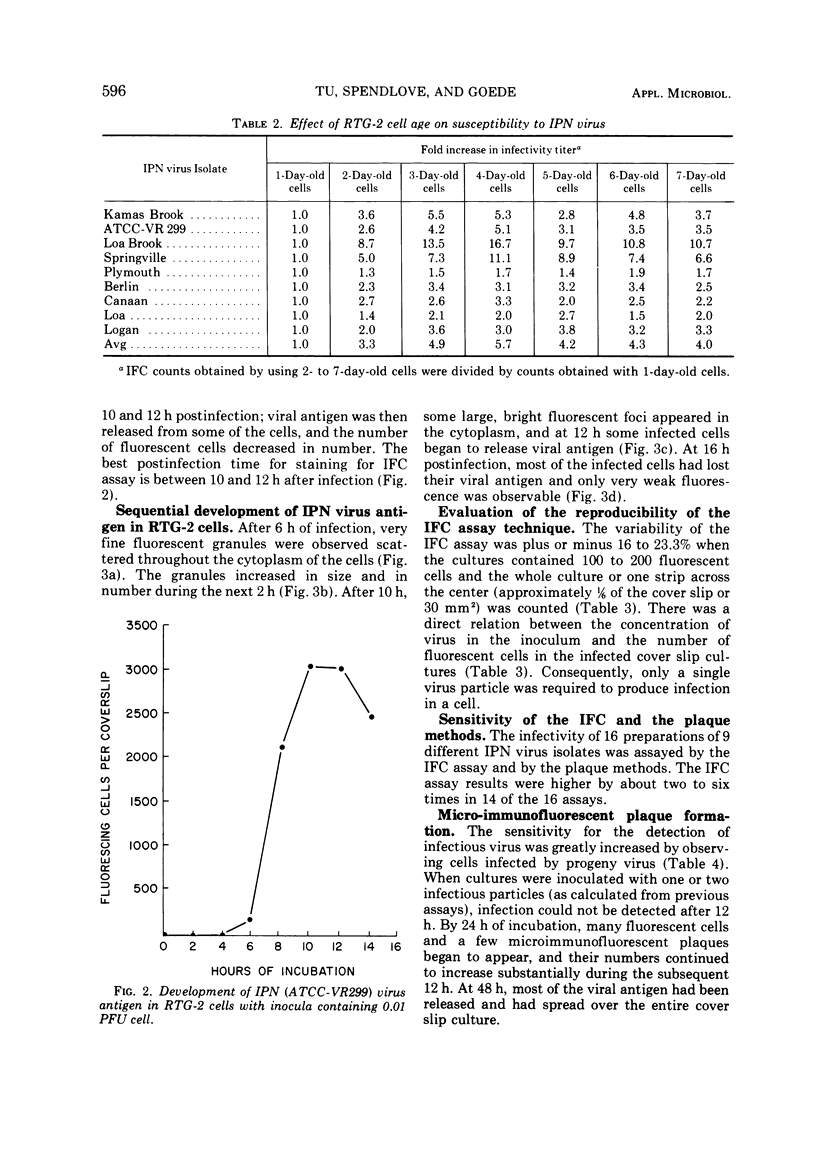
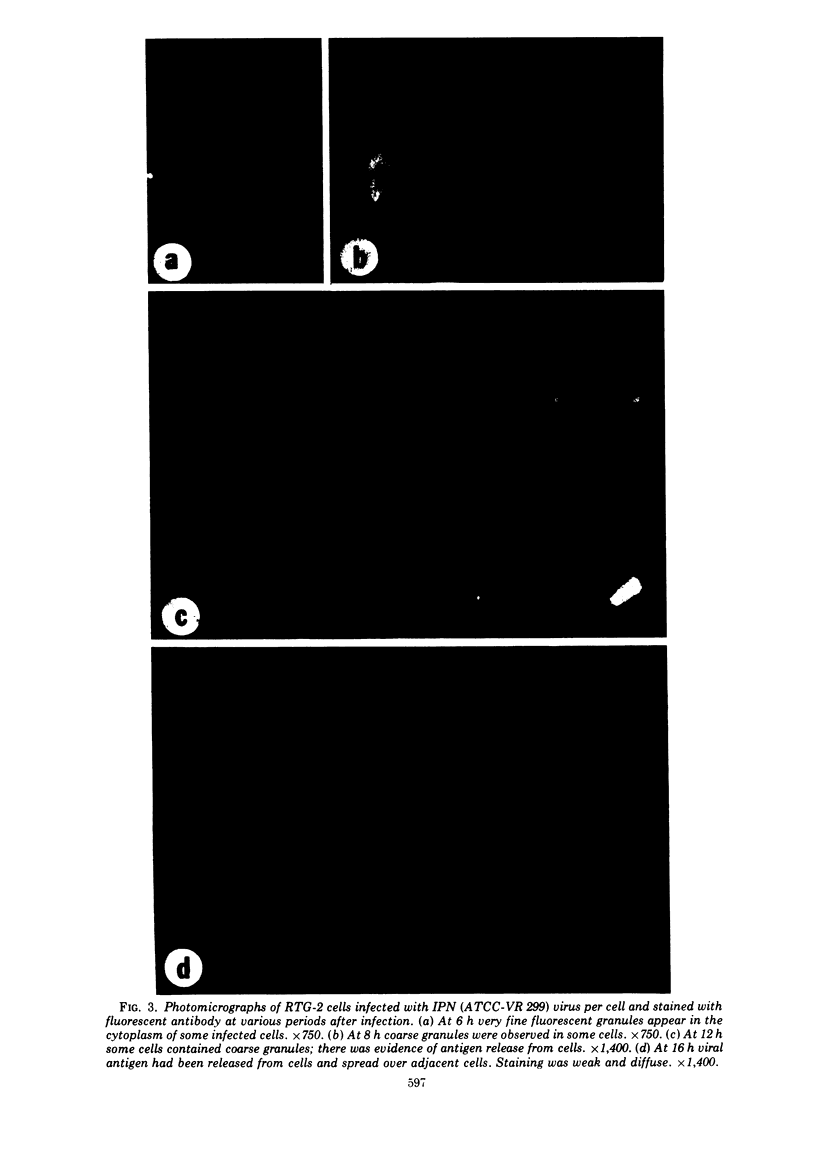
Abstract
An immunofluorescent cell (IFC) assay technique was developed for the quantification of infectious pancreatic necrosis (IPN) virus of salmonid fishes. Cover slip cultures of rainbow trout gonad (RTG-2) cells were infected with diluted virus preparations. After incubation to permit antigen development, the cells were stained with antiviral fluorescent antibody, and the number of fluorescing (infected) cells was counted. Optimal conditions for the IFC assay procedure are: (i) the use of RTG-2 cells cultured for at least 3 days at 20 C; (ii) 1-h absorption of IPN virus to RTG-2 cells at 20 C or alternatively, 4 h at 4 C; (iii) staining the infected cell cultures at 10 to 12 h postinfection. A linear relationship between the relative concentration of virus in the inoculum and the number of fluorescent cells in the first cycle of infection was observed. The IFC assay method is more sensitive than the plaque method for the assay of IPN virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ibrahim A. L., Loh P. C. Comparison of the immunofluorescent-cell counting and plaque methods for the assay of vaccinia virus. Appl Microbiol. 1972 Feb;23(2):214–217. doi: 10.1128/am.23.2.214-217.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain M. E., Spendlove R. S., Lennette E. H. Infectivity assay of Reoviruses: comparison of immunofluorescent cell count and plaque methods. J Immunol. 1967 Jun;98(6):1301–1308. [PubMed] [Google Scholar]
- Piper D., Nicholson B. L., Dunn J. Immunofluorescent study of the replication of infectious pancreatic necrosis virus in trout and Atlantic salmon cell cultures. Infect Immun. 1973 Aug;8(2):249–254. doi: 10.1128/iai.8.2.249-254.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENDLOVE R. S., LENNETTE E. H. A simplified immunofluorescent plaque method. J Immunol. 1962 Jul;89:106–112. [PubMed] [Google Scholar]
- Spendlove R. S., Crosbie R. B., Hayes S. F., Keeler R. F. TRICINE-buffered tissue culture media for control of mycoplasma contaminants. Proc Soc Exp Biol Med. 1971 May;137(1):258–263. doi: 10.3181/00379727-137-35556. [DOI] [PubMed] [Google Scholar]
- Spendlove R. S. Optimal labeling of antibody with fluorescein isothiocyanate. Proc Soc Exp Biol Med. 1966 Jun;122(2):580–583. doi: 10.3181/00379727-122-31196. [DOI] [PubMed] [Google Scholar]
- WOLF K., QUIMBY M. C. Established eurythermic line of fish cells in vitro. Science. 1962 Mar 23;135(3508):1065–1066. doi: 10.1126/science.135.3508.1065. [DOI] [PubMed] [Google Scholar]
- WOLF K., SNIESZKO S. F., DUNBAR C. E., PYLE E. Virus nature of infectious pancreatic necrosis in trout. Proc Soc Exp Biol Med. 1960 May;104:105–108. doi: 10.3181/00379727-104-25743. [DOI] [PubMed] [Google Scholar]
- Yamane I., Matsuya Y., Jimbo K. An autoclavable powdered culture medium for mammalian cells. Proc Soc Exp Biol Med. 1968 Jan;127(1):335–336. doi: 10.3181/00379727-127-32685. [DOI] [PubMed] [Google Scholar]