Significance
Ongoing losses of biodiversity underscore the need to understand how species loss affects infectious diseases. Recognizing that most communities include multiple hosts and pathogens, we tested how variation in host and parasite diversity influenced disease risk. By combining field surveys and experiments involving amphibian hosts and trematode parasites, we show that realistic changes in host and parasite richness inhibit transmission of the deadliest parasite, Ribeiroia ondatrae. Increased host richness consistently reduced infections by Ribeiroia and the total parasite community. Importantly, however, parasite richness further dampened pathogen transmission, and the most diverse assemblages reduced Ribeiroia transmission by >50%. These findings emphasize the “hidden” role of parasite communities in diversity–disease interactions and the value of a community-based approach to infectious disease.
Keywords: biodiversity, dilution effect, community assembly, amphibian decline, disease ecology
Abstract
Host–parasite interactions are embedded within complex communities composed of multiple host species and a cryptic assemblage of other parasites. To date, however, surprisingly few studies have explored the joint effects of host and parasite richness on disease risk, despite growing interest in the diversity–disease relationship. Here, we combined field surveys and mechanistic experiments to test how transmission of the virulent trematode Ribeiroia ondatrae was affected by the diversity of both amphibian hosts and coinfecting parasites. Within natural wetlands, host and parasite species richness correlated positively, consistent with theoretical predictions. Among sites that supported Ribeiroia, however, host and parasite richness interacted to negatively affect Ribeiroia transmission between its snail and amphibian hosts, particularly in species-poor assemblages. In laboratory and outdoor experiments designed to decouple the relative contributions of host and parasite diversity, increases in host richness decreased Ribeiroia infection by 11–65%. Host richness also tended to decrease total infections by other parasite species (four of six instances), such that more diverse host assemblages exhibited ∼40% fewer infections overall. Importantly, parasite richness further reduced both per capita and total Ribeiroia infection by 15–20%, possibly owing to intrahost competition among coinfecting species. These findings provide evidence that parasitic and free-living diversity jointly regulate disease risk, help to resolve apparent contradictions in the diversity–disease relationship, and emphasize the challenges of integrating research on coinfection and host heterogeneity to develop a community ecology-based approach to infectious diseases.
One of the most fundamental challenges facing contemporary disease ecology involves understanding host–parasite interactions within complex communities (1, 2). Whereas epidemiological research has historically focused on interactions between individual host and parasite species, growing evidence indicates that incorporating more realistic levels of diversity is essential for managing infectious diseases. Because most emerging parasites use or depend on multiple host species (3, 4), understanding transmission dynamics often demands detailed information on the host community (e.g., refs. 5 and 6). Concurrently, interactions among parasites are increasingly recognized in affecting disease outcomes (7, 8). Virtually all hosts in nature support a diverse assemblage of pathogenic and nonpathogenic microorganisms (9), which can interact both directly (e.g., competition for space or resources) or indirectly through host immunity (10, 11). Thus far, however, there have been few empirical efforts to unite these disparate lines of research and understand disease dynamics within multihost, multiparasite communities, in part owing to the challenges involved in understanding complex interactions operating across different scales (e.g., within and between hosts) (12, 13).
A central question, therefore, concerns how host and parasite assemblages interact to determine transmission and disease severity within ecological communities. Thus far, two divergent perspectives have emerged regarding the relationship between biodiversity and parasitism. On one hand, recent interest has focused on the dilution effect hypothesis, which posits that increases in host diversity will reduce transmission or disease risk when accompanied by declines in the overall competence of the community (e.g., refs. 14 and 15). On the other hand, increases in host diversity are also hypothesized to promote parasite diversity by enhancing colonization opportunities and supporting a wider suite of parasite life cycles (host diversity begets parasite diversity hypothesis; ref. 16), such that host diversity and parasite diversity correlate positively (17, 18). Rather than contradicting each other, these seemingly divergent perspectives on the diversity–disease relationship emphasize differences in both terminology and ecological process. Parasite diversity is not equivalent to disease risk; given that many parasitic species cause relatively little harm to the host under normal conditions, disease risk is better equated to the abundance or prevalence of the most virulent parasites, rather than the overall richness of parasites per se (5, 19). Increases in parasite diversity may even function to reduce transmission if parasites are antagonistic with one another within hosts (19–22).
Static patterns of parasite richness or species composition may also offer relatively little insight into transmission, or the capacity of a parasite to move locally between hosts (15, 23). Thus, even if more species-rich communities support higher parasite diversity owing to greater colonization opportunities, this provides limited information about how diversity per se affects parasite transmission within the community. Indeed, if parasite diversity alters transmission of the most virulent infections, it could function to amplify or mask any host-driven dilution effects, emphasizing the importance of field and experimental studies that explicitly measure transmission dynamics. These observations raise an intriguing question: If parasite diversity increases with host diversity and higher parasite richness can function to reduce disease risk, what are the individual and combined contributions of free-living and parasitic diversity to the diversity–disease relationship? Given the ubiquity of coinfection in natural host communities and the predominant tendency of diversity–disease research to focus on field-based correlations, studies that can disentangle the magnitude and relative contributions of differing components of community diversity—both free-living and parasitic—are increasingly essential.
Here, we integrated field surveys and experimental manipulations of a multihost, multiparasite system to concurrently test the effects of host and parasite richness on transmission of a virulent parasite. By linking previously collected field data on host richness from 345 wetlands in California with new information on the full macroparasite communities of 1,686 hosts, we tested the influence of amphibian host and parasite richness on realized transmission of the pathogenic trematode Ribeiroia ondatrae, which causes mortality and malformations in amphibians (24–26). However, because we expected host diversity, parasite diversity, and parasite load to correlate in the field owing to links between colonization and diversity, we used an experimental approach first in the laboratory and then in seminatural outdoor mesocosms to decouple the unique effects of each form of diversity on infection by Ribeiroia and the total parasite community. While building upon previous efforts examining the individual effects of host and parasite richness in isolation (15, 19, 27–30), this study combines cross-sectional coinfection data, field-based measurements of transmission, and new experiments to explicitly examine the effects of host species richness on infection by an entire parasite assemblage, thereby mechanistically evaluating the joint effects of host and parasite diversity on disease risk.
Results
Field Sampling.
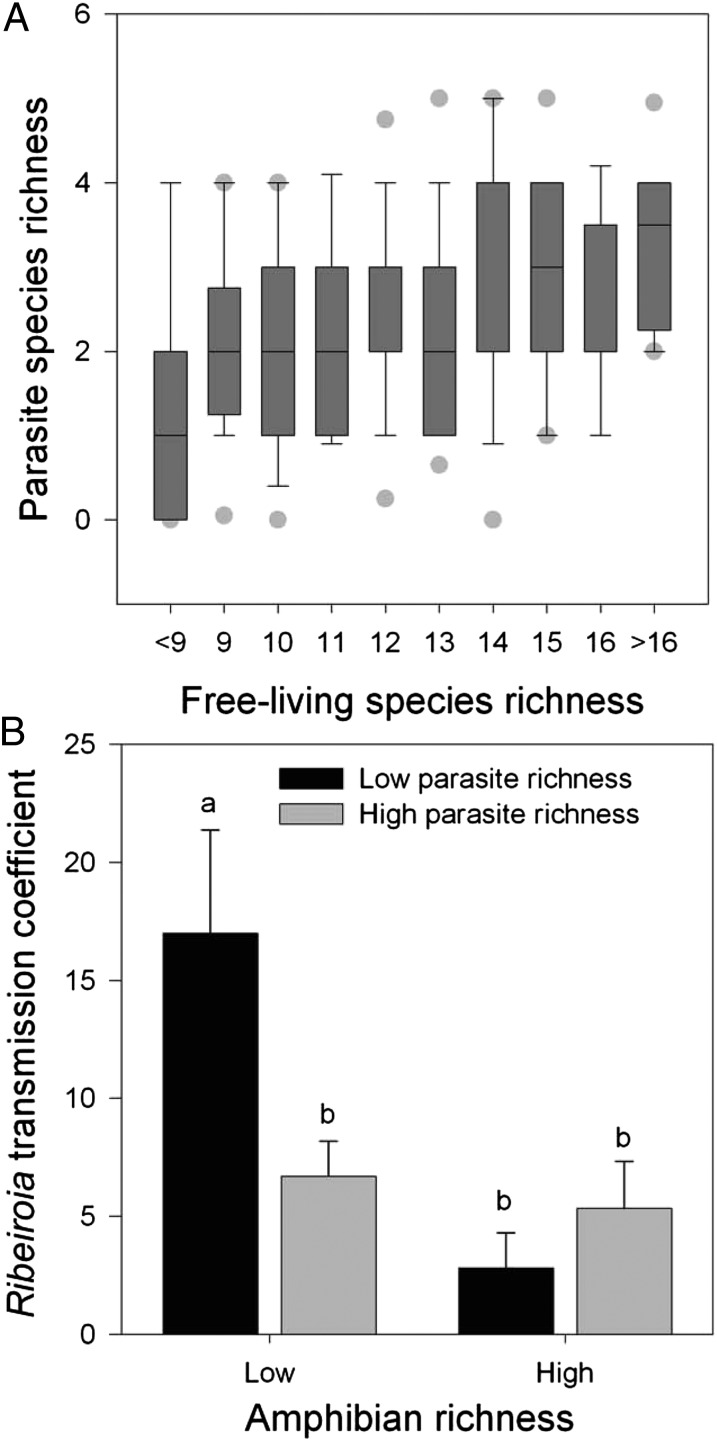
Cross-sectional field sampling of 345 wetlands provided insights into the relationship between host and parasite richness as well as how each component affected transmission of the most virulent trematode, R. ondatrae, between its snail and amphibian hosts. Trematode richness within the most common amphibian (Pseudacris regilla) correlated positively with both larval amphibian host richness (ρ = 0.196, P = 0.002) and with total free-living richness (ρ = 0.285, P < 0.0001) (Poisson regression: all χ2 > 8, P < 0.005) (Fig. 1A). Richness values, which aligned well with a Poisson distribution, ranged up to 6 for larval amphibians, 7 for larval trematodes, and 20 for all free-living taxa (i.e., larval amphibians, aquatic insects, molluscs, and other invertebrates; Table S1). Among wetlands that supported Ribeiroia (n = 136), the density of infected ramshorn snails (Helisoma trivolvis) positively predicted average Ribeiroia infection load per P. regilla, consistent with snails’ release of free-living stages (cercariae) infectious to larval amphibians. However, parasite richness interacted significantly with both the density of infected snails and amphibian richness to determine P. regilla infection (generalized linear mixed model three-way interaction, P = 0.013; n = 136 wetlands and 1686 hosts) (Fig. 1B). This model had a lower Akaike information criterion (AIC) value than models without parasite richness (ΔAIC = 8) or host richness (ΔAIC = 11). At high-parasite richness (four to seven parasite species), only the density of infected snails predicted P. regilla infection (realized transmission coefficient β′ = 5.672 ± 1.186, df = 42, P < 0.0001). At low-parasite richness (one to three parasite species), P. regilla infection depended on the interaction between amphibian richness and infected snail density, such that the influence of infected snail density was greater at low-host and low-parasite richness (β′ = 16.986 ± 4.387, df = 58, P = 0.0003) than at low-parasite but high-host richness (β′ = 2.79 ± 1.508, df = 29, P = 0.074). This indicates that infected snail density positively predicts amphibian infection by Ribeiroia, but the magnitude of this relationship (β′) is moderated by both the free-living and parasitic communities.
Fig. 1.
Host diversity, parasite diversity, and Ribeiroia infection in California wetlands. (A) Positive correlation between larval trematode species richness and free-living species richness, including larval amphibians, molluscs, aquatic insects, and oligochaete among 246 wetlands. (B) Effects of host and parasite diversity on realized transmission, that is, the regression coefficient (+ 1 SE) between the density of infected snails (log10-transformed) and average Ribeiroia infection within Pseudacris regilla. Although analyses were performed using richness values as a continuous variable, they are broken into categories here for ease of visualization. Low and high host richness corresponds to one to three species and four to six species, respectively, whereas low and high parasite richness are one to three species and four to seven species among sites that supported Ribeiroia (n = 136). Letters (a vs. b) indicate significant differences (P ≤ 0.05).
Laboratory Experiment.
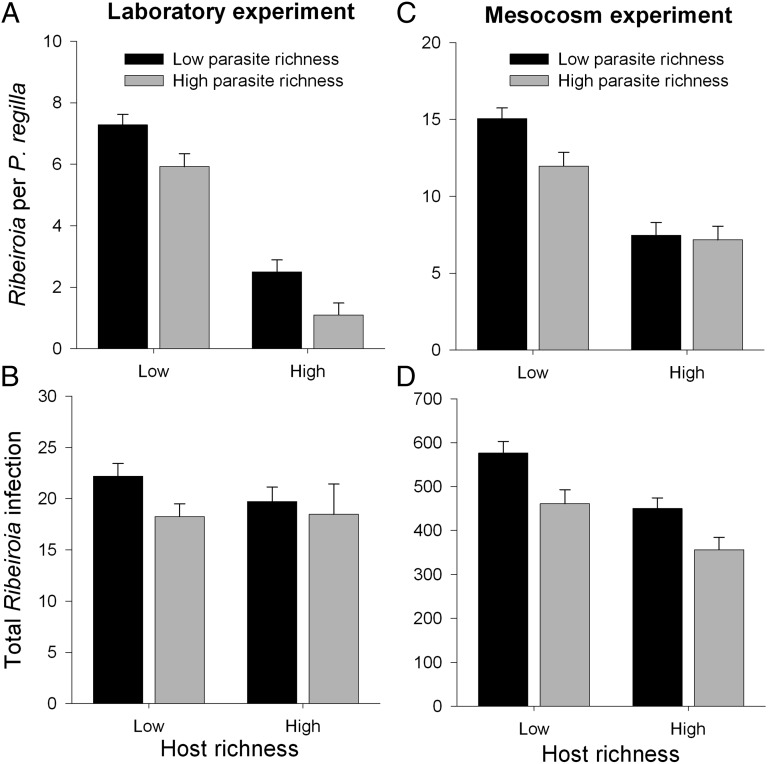
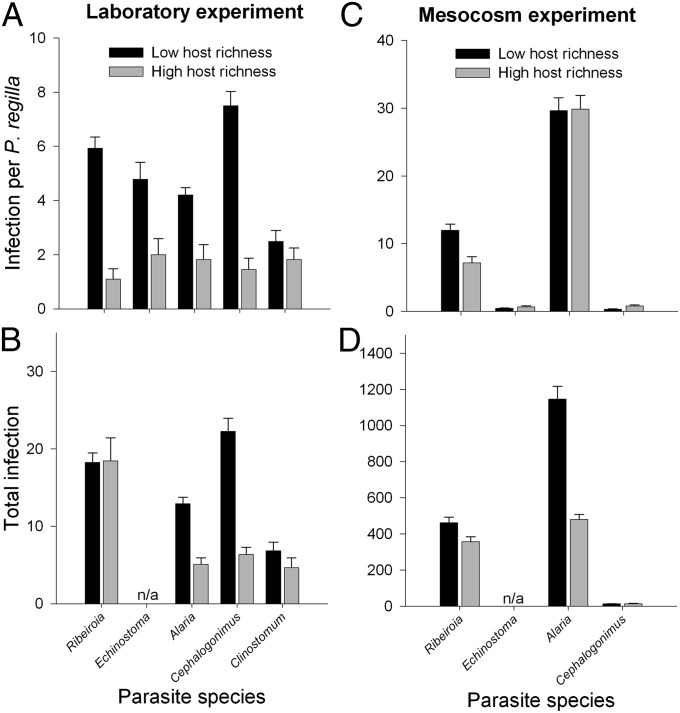
Because host richness, parasite richness, and parasite load correlated with one another in the field data, we used a complementary experimental approach to better understand the underlying mechanisms. In laboratory microcosms, both amphibian richness (one vs. three species) and parasite richness (one vs. five species) reduced infection by Ribeiroia within individual amphibian hosts (host diversity coefficient = −1.305 ± 0.141, df = 52, P < 0.00001; parasite diversity coefficient = −0.195 ± 0.089, df = 52, P = 0.0339; interaction term not significant). The effect of amphibian richness on Ribeiroia in P. regilla was particularly strong (71.6% decrease in infection) compared with the additional effect of parasite richness (24.1% decrease) (Fig. 2A). Total Ribeiroia (summed among all hosts) decreased with parasite richness but not host richness (P = 0.04) (Fig. 2B). Increases in host richness also caused substantial yet variable reductions in average infections by other parasite species both within P. regilla (Echinostoma trivolvis: 61.8% reduction, P = 0.0033; Alaria sp. 2: 60.5% reduction, P = 0.0012; Cephalogonimus americanus: 82.3% reduction, P < 0.00001; Clinostomum: 33.2% reduction, P = 0.24; Fig. 3A) and in their totals summed among hosts (Alaria: coefficient = −1.752 ± 0.352, P < 0.00001; Cephalogonimus: coefficient = −2.07 ± 0.399, P < 0.00001; Clinostomum: coefficient = −1.752 ± 0.352, P = 0.009; Fig. 3B) (Supporting Information gives host-specific results). At the community level, total infection of all parasites summed among all hosts decreased by 42.6% between low and high host richness.
Fig. 2.
Effects of host and parasite richness on Ribeiroia infection within experiments. Influence of host and parasite richness on average Ribeiroia infection intensity per Pseudacris regilla and total Ribeiroia infection (summed among co-occurring host species) in the laboratory (A and B) and mesocosm (C and D) experiments. All values are mean + 1 SE.
Fig. 3.
Effects of host richness on infection by all parasite species. (A) and (B) represent the influence of host richness on average infection Ribeiroia load per Pseudacris regilla and total Ribeiroia (summed among co-occurring host species) in the laboratory experiment; (C) and (D) depict the same response variables in the mesocosm experiment. Values are mean + 1 SE. Note: equal numbers of each of five parasite species were added within the laboratory experiment, whereas variable numbers of four parasite species were added in the mesocosm study. The total number of Echinostoma infections could not be calculated owing to preexisting infections within Lithobates catesbeianus hosts.
Mesocosm Experiment.
In outdoor mesocosms, host and parasite diversity both negatively influenced Ribeiroia infection (host diversity coefficient = −0.62 ± 0.084, P < 0.0001; parasite diversity coefficient = −0.225 ± 0.0722, P = 0.0035; interaction term not significant; n = 40 mesocosms and 854 hosts). Higher host and parasite richness lowered the average number of Ribeiroia per P. regilla and the total number of Ribeiroia (P. regilla: F3,36 = 20.69, P < 0.00001; total Ribeiroia: F3,36 = 10.492, P < 0.00001; Fig. 2 C and D). Thus, in the high-host, high-parasite richness treatments, we detected 52.4% fewer Ribeiroia per P. regilla and 38.2% fewer Ribeiroia overall relative to the low-host, low-parasite richness condition—despite the fact that more diverse host treatments in our mesocosms supported approximately fivefold greater total biomass. Among experimental assemblages exposed to all parasite species, higher host richness was associated with a 48.6% decrease in total infection (summed among all parasite species) and a 58.9% decrease in total Alaria (coefficient = −666.34 ± 76.38, P < 0.0001), with no effect on total Cephalogonimus, average Alaria or echinostomes per P. regilla, and a slight positive effect on Cephalogonimus per P. regilla (coefficient = 0.48 ± 0.15, P = 0.005) (Fig. 3 C and D). Importantly, however, host species identity exhibited substantial yet variable differences in infection by the four parasite species (all χ2 > 35, df = 3, P < 0.0001; Fig. S1).
Discussion
Divergent lines of inquiry into the influence of both host diversity (2, 15, 30) and parasite coinfections (10, 27, 31) have gained considerable momentum in disease ecology research over the past decade, but thus far few studies have simultaneously integrated these topics. Here, we combined results from field surveys, a simplified laboratory experiment, and a more realistic outdoor mesocosm experiment to evaluate the relationships among host diversity, parasite diversity, and transmission. Within natural wetlands, host and parasite diversity correlated positively, as predicted based on the “host diversity begets parasite diversity” hypothesis and the greater colonization opportunities afforded by richer host communities (16, 32). Importantly, however, among sites that supported the virulent parasite Ribeiroia, increases in either host or parasite richness functioned to weaken transmission success from infected snails to the most common amphibian host by ∼50%, highlighting the concurrent effects of both the free-living and parasitic communities in regulating infection. Models that included both host and parasite richness as explanatory terms provided a superior fit relative to those including host diversity or infected snail density alone. These findings indicate that apparently separate lines of research into the diversity–disease relationship are not contradictory; even if host diversity tends to enhance parasite diversity via greater colonization, as shown here, increases in richness can still impair transmission.
Results from experiments modeled after naturally occurring host–parasite assemblages further supported the joint roles of free-living and parasitic communities in regulating Ribeiroia infection success and helped to quantify their relative contributions. In laboratory and outdoor mesocosm manipulations, decreases in host richness caused a 50–65% decrease in per capita Ribeiroia infections within the most common hosts (P. regilla). Although variable, total infection success by each of the parasite species also tended to decrease across the host richness gradient (five of seven cases; Fig. 3 B and D), leading to a 42–48% decline in the total community-level infection (all parasite species within all host species). Concurrently, increases in parasite richness in both experiments led to further decreases in Ribeiroia transmission, such that focal hosts in the high-host, high-parasite richness conditions supported 52–85% fewer Ribeiroia while the community load of Ribeiroia decreased by 16–39% relative to the simplest assemblages. The fact that, in general, both per capita infection and total infection decreased suggests that increases in community richness diminished parasite infection success or persistence (although in some cases decreases in per capita infection occurred without a change in total infection, suggesting a redistribution of parasites among host species; Fig. 3 and Fig. S1). These results hint that some of the effects ascribed to host diversity based on correlational studies may, in fact, be due to “hidden” changes in parasite diversity, emphasizing the importance of linking experimental and survey approaches to decompose the diversity–disease relationship. Given the clear link between Ribeiroia infection and amphibian mortality and malformations (15, 24, 25), observed changes in infection also have immediate relevance for host disease risk.
Mechanistically, the changes in parasite infection success with increases in host richness likely owe to differences in host species’ attractiveness to colonizing parasites as well as their capacity to support infections. Larger hosts, such as bullfrog larvae, probably functioned as more prominent targets for free-swimming parasites (Fig. S1), even if their long-term suitability to support infections was lower. Host competence, or the tendency of each host species to become infected and maintain infection, also varies among species (6, 15, 30). Previous work involving Ribeiroia has shown that total host community competence decreases in more diverse assemblages with the progressive addition of “poor-quality” hosts (15), consistent with results of our field and experimental studies. Antagonistic effects among parasites could result from competition for a shared resource (e.g., space or host cells) or interactions mediated through host immunity (7). Based on the differences in infection sites among the parasites included here and their low metabolic demands within second intermediate hosts, cross-reactive immunity is perhaps the most probable mechanism, consistent with a growing emphasis on the role of host immunity in determining coinfection outcomes (31, 33–36). That host diversity generally had stronger inhibitory effects on parasite loads than did parasite diversity may stem from the greater potential for low-competence hosts to function as population sinks for invading parasites, whereas immune-mediated competition associated with coinfection acts primarily to weaken persistence of parasites that have already colonized (2, 37).
Unlike in experiments, however, parasite and host richness in the field also interacted with one another, such that the dampening effects of one manifested only at low richness values of the other. Although the cause of this interaction is uncertain, it could indicate nonlinearities and threshold effects in the influence of diversity on transmission. For instance, diversity-mediated inhibition of transmission may be strongest when infection pressure is low, which would suggest that high levels of infection pressure could “swamp out” any dilution effect and lead to a nonlinear relationship between diversity and transmission. Alternatively, this interaction may have been artifactual, stemming from the high overall correlations among host richness, parasite richness, and Ribeiroia load in the field. Our experiments allowed us to decouple the independent effects of host and parasite diversity and thereby overcome some of the challenges associated with identifying the influence of variables that are often highly correlated in nature. However, future efforts that vary parasite dosage—alongside parasite richness—are needed to understand the range of conditions under which such effects will be most important. Similarly, extensions to consider a broader range of parasite taxa (e.g., micro- and macroparasites) and life histories (e.g., host generalists and specialists) will be essential for theory development and testing.
Although our results highlight the simultaneous effects of both host and parasite communities in controlling pathogen transmission, we emphasize that these findings do not necessitate that more diverse assemblages will have less disease. This is due to at least two factors. First, our emphasis here is on transmission, or the ability of Ribeiroia to move effectively between its snail and amphibian hosts. While we found that community diversity significantly moderated transmission, the primary determinant of disease risk was infection pressure (i.e., the density of infected snails), which will be affected by numerous factors including definitive host activity, snail population growth, and climate. Thus, if more diverse wetlands support a high density of infected snails, overall infection and disease risk in amphibians could nonetheless be high in these systems. Second, the net effects of increasing free-living or parasite diversity will depend strongly on community assembly patterns. Increases in diversity will tend to reduce disease when high-competence host species and high-virulence parasites dominate in low-richness assemblages, with progressive decreases in disease as less-competent hosts and lower-virulence parasites are added. Although our experiments used realistic patterns of host and parasite assembly (15, 19), the predictability of patterns involving host–parasite coassembly will be a key factor in determining the outcome of the diversity–disease relationship in other systems. If host communities assemble randomly with respect to competence (e.g., owing to stochastic or legacy-driven effects), for instance, but parasites assemble in order of increasing virulence, variation in parasite diversity will more sharply regulate disease risk than will host diversity.
These findings underscore both the challenges and necessity of approaches that better integrate free-living and parasitic assemblages to understand disease dynamics within natural communities. Although an increasing number of studies have reported negative correlations between host diversity and disease risk (29, 38, 39), the hidden effects of concurrent changes in parasite communities have rarely been explored (19, 31, 40). Future studies examining the biodiversity–disease relationship should focus more heavily on the relative importance of multiple components of biodiversity (and how they covary) in driving observed patterns in disease risk (41–43), including the direct and indirect effects of hosts, nonhosts (e.g., predators and competitors), parasites, as well as other microorganisms. An important frontier in continued efforts to understand disease within complex communities will therefore involve cross-system comparisons of host–parasite combinations to determine (i) the predictability of community assembly/disassembly (ii), the relationship between species assembly order and either host competence or parasite virulence, and (iii) the degree to which parasitic and free-living communities assemble additively or substitutively (14, 15, 19, 44–46).
Materials and Methods
Field Surveys.
During the summers of 2009–2011 we surveyed 345 wetlands in the San Francisco Bay Area of California (see ref. 15). Each wetland was visited once between May and June to determine whether it supported the requisite hosts for Ribeiroia. Ramshorn snails in the genus Helisoma function as obligate first intermediate hosts for Ribeiroia, releasing free-swimming infectious stages (cercariae) that infect larval amphibians. Although most larval amphibians are susceptible to infection, species vary widely in their ability to support infection (i.e., competence) (25). Wetlands that supported H. trivolvis and at least one amphibian species were visited a second time between July and August. On both visits, we measured the richness and density of larval amphibians, aquatic insects, molluscs, and other invertebrates (Table S1) using standardized dipnet sweeps every 3–5 m around a pond’s perimeter and three to five habitat-stratified seine hauls. We quantified Ribeiroia infection prevalence in H. trivolvis by dissecting 50–100 mature individuals (≥5 mm shell width). During the second visit, we collected ∼10 metamorphosing P. regilla and quantified infection by all larval trematode species using standard necropsy techniques (see ref. 15 for additional details). We ensured that our sampling methods adequately captured host and parasite richness using rarefaction curves and richness estimators (see refs. 15 and 19 and Table S2 in Supporting Information).
Laboratory Experiment.
We conducted a 2 × 2 laboratory experiment that manipulated host richness (one vs. three species) and parasite richness (one vs. five species) with 14 replicates per treatment. Egg masses (P. regilla and Rana luteiventris) or early-stage larvae (Lithobates catesbeianus) were field-collected and reared to Gosner (47) stage 28–31, at which point they were randomly assigned to a treatment. We included five trematodes that use H. trivolvis and amphibians as first and second intermediate hosts, respectively: R. ondatrae, E. trivolvis, C.s americanus, Alaria sp. 2 and Clinostomum sp., of which Ribeiroia is the most deadly (see, e.g., ref. 19). Three amphibian larvae representing either one species (P. regilla only) or all three species were placed into 2-L experimental microcosms and exposed to either 50 cercariae of R. ondatrae or 50 cercariae of each of the five parasite species (n = 250 parasites total). We used an additive design both because host pathology is often driven by the abundance of the most virulent parasite and because empirical data on trematode communities support an additive assembly structure (i.e., richness correlates positively with total infection load; ref. 19). Parasites were isolated from field-collected snails, counted under a dissecting microscope, and administered directly to the containers within 4 h of their release from snails. Thus, unlike in the field survey, here we quantified the number of infectious parasites directly rather than using infected snail density as a proxy. Parasites were administered over two infection events separated by 5 d. Five days after the second exposure, we ended the experiment and recorded the numbers and identities of each parasite.
Mesocosm Experiment.
The mesocosm experiment manipulated larval amphibian host richness (one vs. four species) and parasite richness (one vs. four species) (10 replicates per treatment). Mesocosms consisted of 378-L tanks filled with well water and initiated using standardized techniques (Supporting Information). We maintained constant host densities by stocking 40 P. regilla in the low-host-diversity treatment and 10 P. regilla, 10 Anaxyrus boreas, 10 Taricha torosa, and 10 L. catesbeianus in the high diversity treatment (see Supporting Information for initial sizes). All of these host species are susceptible to the selected parasites, albeit to differing degrees, which is a key principle underlying the dilution effect hypothesis (15, 25). This design was chosen to avoid concurrent changes in richness and host density. In accordance with natural variation in parasite availability, we added ∼2,338 Ribeiroia cercariae to every mesocosm and 694 Echinostoma, 793 Cephalogonimus, and 11,141 Alaria cercariae to mesocosms in the high-parasite-richness treatments (see Supporting Information for variance estimates). Collectively, these amphibian and trematode assemblages mirror those species commonly encountered in northern California ponds. Parasite additions began 1 wk after establishment of amphibian communities and continued for 12 d (Supporting Information).
As with any manipulative experiment involving ecological communities, the results will be sensitive to the particular species assemblages included. Here, we focused on manipulations of host richness (rather than density) so as not to mix different epidemiological mechanisms affecting transmission. Because increases in host density strongly influence Ribeiroia transmission (15, 48), using an additive design that increased the total density of all hosts would confound density and diversity (although previous work has shown that host richness reduces transmission in additive experimental designs after accounting for density; see ref. 15). We included the most common amphibian species (P. regilla) in all treatments to mimic natural assemblages. For parasites, we focused on Ribeiroia as it is the most virulent parasite and used an additive design to allow us to evaluate whether other infections reduced the success of Ribeiroia. We openly acknowledge that a completely randomized order of species additions would likely lead to different outcomes than what are presented here, but we opted to focus on realistic assemblage patterns given their ecological relevance as well as the complexity of working with multihost, multipathogen communities.
Analysis.
Among sampled wetlands, we modeled “realized” transmission (β′) as the magnitude of the regression coefficient between infected snail density (log10-transformed + 1 values) and Ribeiroia infection load within P. regilla (i.e., the average number of metacercariae per host). We focused on P. regilla both because it is the most common amphibian species and because the relationship between Ribeiroia infection and malformations is well established, offering a robust link to disease risk. We expected that the density of infected snails—as a metric of infection pressure—would positively predict amphibian infection. Including amphibian density did not improve this relationship in previous analyses (see ref. 15). To evaluate how host and parasite diversity affected Ribeiroia transmission, we tested for significant interactions between the density of infected snails (i.e., infection pressure) and either larval amphibian richness or parasite richness (both calculated at the wetland level and treated as continuous variables) using a generalized linear mixed model in which hosts were nested within wetlands and infection data were modeled using a negative binomial distribution in the R package glmmADMB (49). Because host richness has already been shown to affect realized transmission (15), our primary interest here was in testing for additional influences from the richness of parasites other than Ribeiroia (as an interaction with infected snail density or larval amphibian richness). We compared among full and reduced models using ΔAIC values.
In the experiments, we added known quantities of free-swimming cercariae to each microcosm or mesocosm such that transmission success was simply observed infection load within amphibian hosts. We therefore analyzed how host richness, parasite richness, and their interaction affected the average load of each parasite within the focal host (P. regilla) as well as total numbers of parasites (summed among hosts). Our analyses often involved generalized linear mixed models with a negative binomial distribution, host species identity as a fixed effect, and container or mesocosm as a random effect. For analyses involving average infection, we used log10-transformations as needed to help normalize values. We also assessed treatment effects on P. regilla size and survival (Supporting Information). Because field-collected L. catesbeianus inadvertently supported preexisting echinostome infections, we could not analyze effects on total E. trivolvis infection and excluded echinostomes in calculating total parasite infection.
Supplementary Material
Acknowledgments
We thank M. Baragona, I. Buller, K. Gietzen, B. Goodman, J. Jenkins, E. Kellermanns, T. McDevitt-Galles, J. McFarland, and S. Paull for assistance in collecting and organizing data, two anonymous reviewers and the editor for helping improve the manuscript, and East Bay Regional Parks, East Bay Municipal Utility District, Santa Clara County Parks, Hopland Research and Extension Center, Blue Oak Ranch Reserve, California State Parks, The Nature Conservancy, Open Space Authority and Midpeninsula Regional Open Space District for access to properties and logistical support. This work was supported through US National Science Foundation Grants DEB-0841758 and DEB-1149308 and the David and Lucile Packard Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310557110/-/DCSupplemental.
References
- 1.Pongsiri MJ, et al. Biodiversity loss affects global disease ecology. Bioscience. 2009;59:945–954. [Google Scholar]
- 2.Keesing F, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468(7324):647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356(1411):983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holt RD, Dobson AP. Extending the principles of community ecology to address the epidemiology of host-pathogen systems. In: Collinge SK, Ray C, editors. Disease Ecology: Community Structure and Pathogen Dynamics. Oxford: Oxford Univ Press; 2006. pp. 28–40. [Google Scholar]
- 6.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proc R Soc Biol Sci B. 2006;273(1599):):2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen AB, Fenton A. Emphasizing the ecology in parasite community ecology. Trends Ecol Evol. 2007;22(3):133–139. doi: 10.1016/j.tree.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Lello J, Norman RA, Boag B, Hudson PJ, Fenton A. Pathogen interactions, population cycles, and phase shifts. Am Nat. 2008;171(2):176–182. doi: 10.1086/525257. [DOI] [PubMed] [Google Scholar]
- 9.Belden LK, Harris RN. Infectious diseases in wildlife: The community ecology context. Front Ecol Environ. 2007;5:533–539. [Google Scholar]
- 10.Graham AL. Ecological rules governing helminth-microparasite coinfection. Proc Natl Acad Sci USA. 2008;105(2):566–570. doi: 10.1073/pnas.0707221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenton A, Perkins SE. Applying predator-prey theory to modelling immune-mediated, within-host interspecific parasite interactions. Parasitology. 2010;137(6):1027–1038. doi: 10.1017/S0031182009991788. [DOI] [PubMed] [Google Scholar]
- 12.Seabloom EW, Borer ET, Mitchell CE, Power AG. Viral diversity and prevalence gradients in North American Pacific Coast grasslands. Ecology. 2010;91(3):721–732. doi: 10.1890/08-2170.1. [DOI] [PubMed] [Google Scholar]
- 13.Thieltges DW, et al. Host diversity and latitude drive trematode diversity patterns in the European freshwater fauna. Glob Ecol Biogeogr. 2011;20:675–682. [Google Scholar]
- 14.Ostfeld R, Keesing F. Effects of host diversity on infectious disease. Annu Rev Ecol Evol Syst. 2012;43:157–182. [Google Scholar]
- 15.Johnson PTJ, Preston DL, Hoverman JT, Richgels KLD. Biodiversity decreases disease through predictable changes in host community competence. Nature. 2013;494(7436):230–233. doi: 10.1038/nature11883. [DOI] [PubMed] [Google Scholar]
- 16.Hechinger RF, Lafferty KD. ) Host diversity begets parasite diversity: Bird final hosts and trematodes in snail intermediate hosts. Proc R Soc Biol Sci B. 2005;272(1567):1059–1066. doi: 10.1098/rspb.2005.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson PTJ, Thieltges DW. Diversity, decoys and the dilution effect: how ecological communities affect disease risk. J Exp Biol. 2010;213(6):961–970. doi: 10.1242/jeb.037721. [DOI] [PubMed] [Google Scholar]
- 18. Wood CL, Lafferty KD (2012) Biodiversity and disease: A synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol Evol 28(4):239–247. [DOI] [PubMed]
- 19.Johnson PTJ, Hoverman JT. Parasite diversity and coinfection determine pathogen infection success and host fitness. Proc Natl Acad Sci USA. 2012;109(23):9006–9011. doi: 10.1073/pnas.1201790109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris JB, et al. Immunologic responses to Vibrio cholerae in patients co-infected with intestinal parasites in Bangladesh. PLoS Negl Trop Dis. 2009;3(3):e403. doi: 10.1371/journal.pntd.0000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Elsas JD, et al. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci USA. 2012;109(4):1159–1164. doi: 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgdorfer W, Hayes SF, Mavros AJ. In: Non-Pathogenic Rickettsiae in Dermacentor andersoni: A Limiting Factor for the Distribution of Rickettsia rickettsii. Rickettsiae and Rickettsial Diseases. Burgdorfer W, Anacker RL, editors. New York: Academic; 1981. pp. 585–594. [Google Scholar]
- 23.Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9(4):485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 24.Johnson PTJ, Lunde KB, Ritchie EG, Launer AE. The effect of trematode infection on amphibian limb development and survivorship. Science. 1999;284(5415):802–804. doi: 10.1126/science.284.5415.802. [DOI] [PubMed] [Google Scholar]
- 25.Johnson PTJ, et al. Living fast and dying of infection: Host life history drives interspecific variation in infection and disease risk. Ecol Lett. 2012;15(3):235–242. doi: 10.1111/j.1461-0248.2011.01730.x. [DOI] [PubMed] [Google Scholar]
- 26.Schotthoefer AM, Koehler AV, Meteyer CU, Cole RA. Influence of Ribeiroia ondatrae (Trematoda: Digenea) infection on limb development and survival of northern leopard frogs (Rana pipiens): Effects of host stage and parasite-exposure level. Can J Zool. 2003;81:1144–1153. [Google Scholar]
- 27.Telfer S, et al. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330(6001):243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzán G, et al. Experimental evidence for reduced rodent diversity causing increased hantavirus prevalence. PLoS ONE. 2009;4(5):e5461. doi: 10.1371/journal.pone.0005461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas SE, Hooten MB, Rizzo DM, Meentemeyer RK. Forest species diversity reduces disease risk in a generalist plant pathogen invasion. Ecol Lett. 2011;14(11):1108–1116. doi: 10.1111/j.1461-0248.2011.01679.x. [DOI] [PubMed] [Google Scholar]
- 30.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100(2):567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ezenwa VO, Etienne RS, Luikart G, Beja-Pereira A, Jolles AE. Hidden consequences of living in a wormy world: Nematode-induced immune suppression facilitates tuberculosis invasion in African buffalo. Am Nat. 2010;176(5):613–624. doi: 10.1086/656496. [DOI] [PubMed] [Google Scholar]
- 32.Hudson PJ, Dobson AP, Lafferty KD. Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol. 2006;21(7):381–385. doi: 10.1016/j.tree.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Maas M, Keet DF, Rutten VPMG, Heesterbeek JAP, Nielen M. Assessing the impact of feline immunodeficiency virus and bovine tuberculosis co-infection in African lions. Proc R Soc Biol Sci B. 2012;279(1745):4206–4214. doi: 10.1098/rspb.2012.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ezenwa VO, Jolles AE. From host immunity to pathogen invasion: The effects of helminth coinfection on the dynamics of microparasites. Integr Comp Biol. 2011;51(4):540–551. doi: 10.1093/icb/icr058. [DOI] [PubMed] [Google Scholar]
- 35.Cobey S, Lipsitch M. Pathogen diversity and hidden regimes of apparent competition. Am Nat. 2013;181(1):12–24. doi: 10.1086/668598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knowles SCL. The effect of helminth co-infection on malaria in mice: a meta-analysis. Int J Parasitol. 2011;41(10):1041–1051. doi: 10.1016/j.ijpara.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Madden JR, Clutton-Brock TH. Manipulating grooming by decreasing ectoparasite load causes unpredicted changes in antagonism. Proc R Soc Biol Sci B. 2009;276(1660):1263–1268. doi: 10.1098/rspb.2008.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allan BF, et al. Ecological correlates of risk and incidence of West Nile virus in the United States. Oecologia. 2009;158(4):699–708. doi: 10.1007/s00442-008-1169-9. [DOI] [PubMed] [Google Scholar]
- 39.Pagán I, et al. Effect of biodiversity changes in disease risk: Exploring disease emergence in a plant-virus system. PLoS Pathog. 2012;8(7):e1002796. doi: 10.1371/journal.ppat.1002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jolles AE, Ezenwa VO, Etienne RS, Turner WC, Olff H. Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo. Ecology. 2008;89(8):2239–2250. doi: 10.1890/07-0995.1. [DOI] [PubMed] [Google Scholar]
- 41. Vourc’h G, Plantard O, Morand S (2012) How does biodiversity influence the ecology of infectious disease? New Frontiers of Molecular Epidemiology of Infectious Diseases, eds Morand S, Beaudeau F, Cabaret J (Springer, Berlin), pp 291–309.
- 42.Holt RD, Dobson AP, Begon M, Bowers RG, Schauber EM. Parasite establishment in host communities. Ecol Lett. 2003;6:837–842. [Google Scholar]
- 43.Woolhouse MEJ, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science. 2001;292(5519):1109–1112. doi: 10.1126/science.1059026. [DOI] [PubMed] [Google Scholar]
- 44.Ostfeld RS, LoGiudice K. Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology. 2003;84:1421–1427. [Google Scholar]
- 45.Moore SM, Borer ET. The influence of host diversity and composition on epidemiological patterns at multiple spatial scales. Ecology. 2012;93(5):1095–1105. doi: 10.1890/11-0086.1. [DOI] [PubMed] [Google Scholar]
- 46.Brooks CP, Zhang HM. A null model of community disassembly effects on vector-borne disease risk. J Theor Biol. 2010;264(3):866–873. doi: 10.1016/j.jtbi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 47.Gosner KL. A simplified table for staging anuran embryos and larvae with notes and identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- 48.Johnson PTJ, Hartson RB, Larson DJ, Sutherland DR. Diversity and disease: community structure drives parasite transmission and host fitness. Ecol Lett. 2008;11(10):1017–1026. doi: 10.1111/j.1461-0248.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- 49.R Development Core Team 2008. R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna). Available at www.R-project.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.