Significance
In the thymus, developing T cells undergo negative selection to remove T-cell specificities that react to self-antigens. This process generates a T-cell repertoire that is tolerant to one’s own tissues. It depends on antigen-presenting cells to present self-antigens to developing T cells. This paper characterizes the development and antigen-presenting function of a poorly understood population of B cells within the thymus. These cells develop intrathymically, are phenotypically distinct from all other B-cell lineages, and have properties that make them extremely efficient at presenting self-antigens to developing T cells. In particular, they accomplish this by binding to self-antigens with their specific B-cell receptors.
Abstract
The thymus contains a population of B cells that colocalize with dendritic cells and medullary thymic epithelial cells in the thymic medulla. The development and functional significance of these cells are largely unknown. Using recombination-activating gene 2 GFP reporter mice along with parabiosis experiments, we demonstrate that the vast majority of thymic B cells develop from progenitors within the thymus. Thymic B cells express unique phenotypic markers compared with peripheral B cells; particularly they express high levels of MHC class II, suggesting that they are poised to present self-antigens efficiently. Using Ig knock-in and T-cell receptor transgenic mice specific for the self-antigen glucose-6-phosphate isomerase, we show that autoreactive thymic B cells serve as efficient antigen-presenting cells for T cell negative selection even when they are present at low frequencies. Furthermore, the endogenous thymic B-cell repertoire also functions in this capacity. These results suggest that developing thymic B cells could efficiently capture a broad array of autoantigens through their B-cell receptors, presenting peptides derived from those autoantigens to developing thymocytes and eliminating cognate T cells.
Negative selection purges autoreactive T cells from the immune repertoire and is the major mechanism of central tolerance in the thymus. This process depends on presentation of self-peptides to developing thymocytes by antigen-presenting cells (APCs). The question of which APC presents self-antigen for negative selection has been investigated extensively. Initial studies using bone marrow chimeras found that bone-marrow-derived hematopoietic cells are required for negative selection (reviewed in refs. 1 and 2). Many subsequent studies have demonstrated that cortical and medullary thymic epithelial cells (mTECs) can be quite efficient for negative selection as well (1–3). The role of mTECs in deleting T cells specific for tissue-restricted antigens has been highlighted by autoimmunity in both humans and mice possessing mutations in the AIRE gene, which controls the expression of tissue-specific self-antigens in mTECs (4).
Bone-marrow-derived APCs include dendritic cells (DCs), B cells, and macrophages. In vitro assays comparing their relative antigen presentation efficiency showed that DCs were the most efficient, leading to the conclusion that DCs were largely responsible for negative selection in the thymus (5). Although B cells are poor at presenting antigens via nonspecific uptake, they capture and internalize cognate antigens that are bound by their B-cell receptors and present them very efficiently (6, 7). Therefore, antigen-specific B cells could be the most efficient APC on a per cell basis for a particular antigen.
The thymus contains a small population of B cells that make up around 0.1–0.5% of thymocytes (8–12), similar to the proportion of DCs and mTECs in the thymus (13–15). The origin of thymic B cells has been debated, and development from intrathymic progenitors and migration from the peripheral circulation have both been suggested (10, 12). Because thymic B cells preferentially reside at the junction of thymic cortex and medulla, an area where negative selection is thought to occur, they have been proposed to play a role in T cell negative selection (8, 9). Although the capacity of thymic B cells to mediate T cell negative selection has been shown in superantigen and self-antigen overexpression models (16, 17), it remains unclear what kinds of antigens thymic B cells present under normal conditions, the role of their antigen specificity, and what their overall influence on the T-cell repertoire is.
In these studies, we demonstrate that the thymic B cells develop from Rag-expressing progenitors within the thymus, and that recirculating peripheral B cells play a minor role in sustaining this population. Using Ig knock-in mice and T-cell receptor (TCR) transgenic mice that are specific for the same cognate self-antigen glucose-6-phosphate isomerase (GPI), we show that anti-GPI B cells are efficiently selected into the thymic B-cell compartment and express high levels of MHC class II and activation makers compared with those in periphery. Increasing the frequency of anti-GPI B cells results in more stringent T cell negative selection in the thymus in a B cell-autonomous manner. Furthermore, in B cell-deficient mice, negative selection toward GPI is decreased suggesting that the wild-type thymic B-cell repertoire contributes to negative selection for this physiologically relevant self-antigen. These results suggest that thymic B cells could efficiently capture a broad array of autoantigens through their B-cell receptors (BCRs) and present peptides derived from these autoantigens to developing thymocytes, linking B-cell autoreactivity with a mechanism for removing T cells with a shared specificity in the thymus.
Results
The Identification of Thymic B-Cell Progenitors in Thymus.
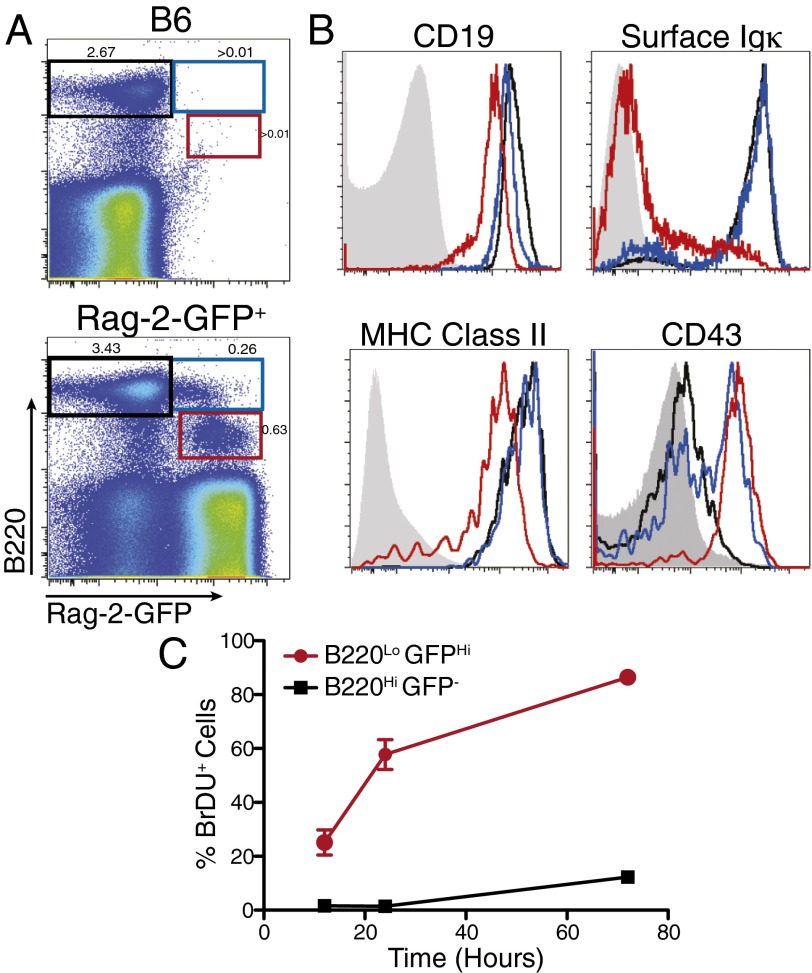
The origin of thymic cells is still not well understood. Both intrathymic development and contribution from recirculating B cells have been reported (10, 12). To visualize developing thymic B cells, we used the Rag2-GFP transgenic mice (18). GFP expression marks cells that are actively undergoing either TCR or BCR rearrangement, and due to the relatively long half-life of GFP protein, low levels persist in cells after its expression has been turned off, marking cells that have recently expressed Rag (19). To enrich for developing B cells in thymus, we depleted bulk thymocytes with anti-CD4 and anti-CD8 antibodies. As shown in Fig. 1A, three populations of B220+ cells were identified: B220hiGFP−, B220hiGFPlo, and B220loGFP+. Staining with additional B-cell markers showed that the largest population B220hiGFP− cells express high levels of CD19, MHC class II, and surface Igκ, suggesting they are more mature thymic B cells with rearranged BCRs (Fig. 1B). B220loGFP+ cells expressed lower levels of CD19 and MHC class II, and are CD43hi and Igκ negative, suggesting that they are most likely the equivalent of pre- and pro-B cells rearranging the BCR. B220hiGFPlo cells have very similar expression patterns as B220hiGFP− cells suggesting that they are more mature thymic B cells that have recently completed successful BCR rearrangement (Fig. 1B).
Fig. 1.
B-cell development in the thymus. (A) Costaining of B220 and GFP in CD4/CD8 depleted thymi of Rag2-GFP− (B6) and Rag2-GFP+ mice. (B) Characterization of three B-cell populations defined in A: B220hiGFP− (black line), B220hiGFPlo (blue line), B220loGFP+ cells (red line), and B220− thymocytes (gray-filled) for all panels except CD43 where B220+ splenocytes (gray-filled) are shown. (C) BrdU incorporation in thymic B cells with continuous labeling for the indicated duration. n = 3–5 mice for each time point.
These data suggest a developmental relationship between these three populations as they transition from progenitors to more mature stages, which we confirmed by continuous BrdU labeling experiments. The B220loGFP+ population is rapidly dividing with 22% of the cells incorporating BrdU after 12 h and 85% becoming labeled after 3 d, suggesting they have a high turnover rate (Fig. 1C). In contrast, only 10% of B220hiGFP− B cells incorporated BrdU over a 3-d period, suggesting a much slower turnover for the mature B-cell compartment in the thymus.
Recirculation Has a Minimal Contribution to Thymic B-Cell Compartment.
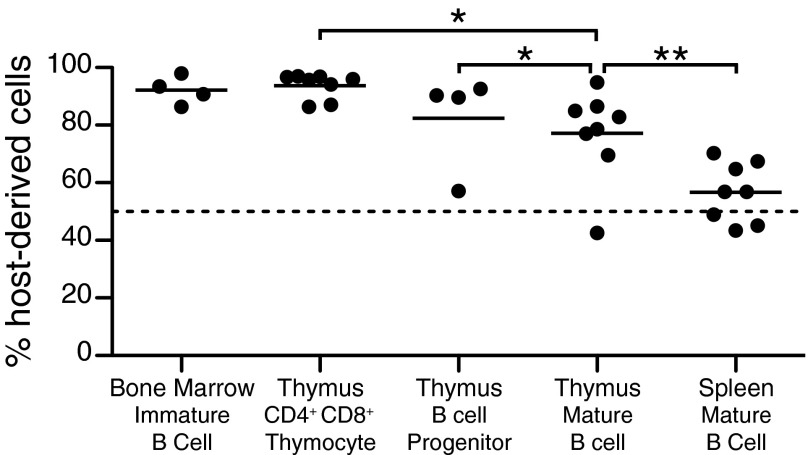
Although these data support the hypothesis that thymic B cells develop in the thymus, they do not directly address the contribution of recirculating peripheral B cells to the maintenance of this population. We studied parabiotic mice to determine the extent to which circulating B cells migrate to the thymus under steady state. Congenic CD45.1 and CD45.2 mice were parabiotically joined to allow for exchange of cells through the blood. Although splenic B cells exchange readily and reach equilibrium in a short period, the thymic B-cell compartment showed significantly less exchange, with an average of only 23% of cells being derived from the parabiotic partner after 6–8 wk of joining (Fig. 2). Furthermore, within that 23%, some proportion can be attributed to exchange of thymic B-cell progenitors, as an 18% exchange was observed for CD19loB220lo cells with a similar phenotype to the thymic B-cell progenitors described in Fig. 1. For comparison, we also analyzed CD4+CD8+ T cells in the thymus and immature B cells in the bone marrow, and detected little exchange (6% and 8%, respectively), consistent with what has been reported (20). Altogether, these data suggest a minor contribution of recirculation to the maintenance of the thymic B-cell compartment.
Fig. 2.
Analysis of thymic B-cell recirculation by parabiosis. A CD45.1+ mouse was joined to a CD45.2+ partner for 6–8 wk. The percent of host-derived cells in each population, BM immature B cell (B220loIgM−), thymus B-cell progenitor (CD19loB220lo), thymus mature B cell (CD19hiB220hi), and spleen mature B cell (CD19+B220+) was determined by CD45.1 and CD45.2 expression. Each symbol represents an individual mouse.
Thymic B Cells Are Distinct from the B1 Cell Lineage.
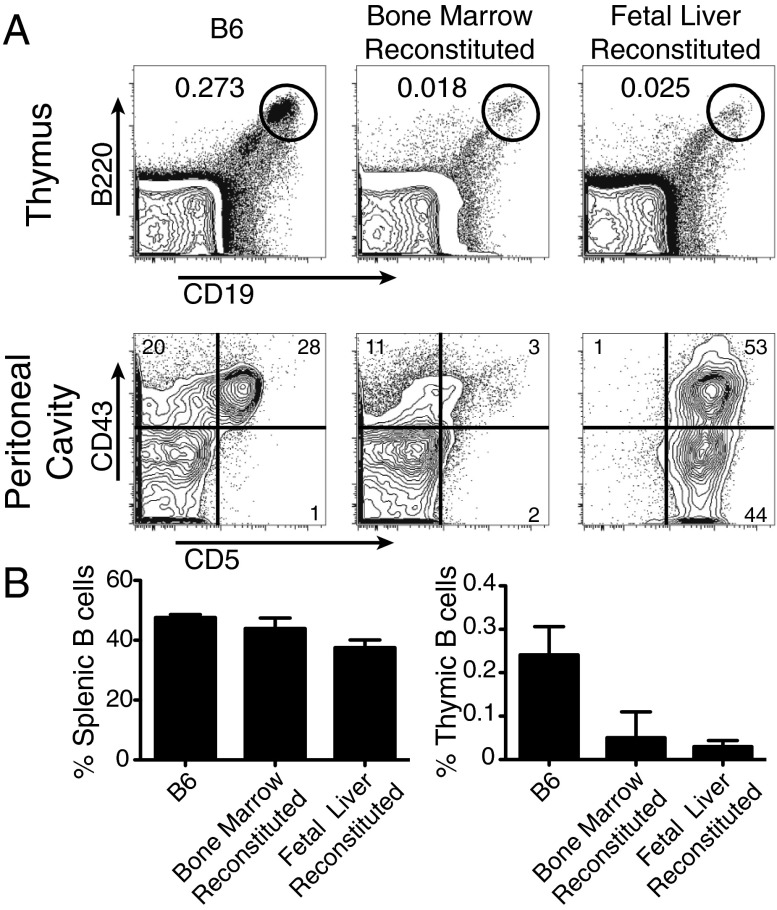
The initial characterization of thymic B cells showed that they express CD5, a marker of the B1a B-cell lineage in mice. By performing a more rigorous analysis of B1 lineage markers, we found that, although thymic B cells do express CD5, they possess a distinct and far more diffuse staining pattern of CD43 and no staining of CD11b (Fig. S1). B-cell development initially occurs in the fetal liver and then transitions to the bone marrow following birth. B1 B cells are preferentially generated from cells from the fetal liver, but inefficiently generated by bone marrow progenitors. We compared the capacity of fetal liver and bone marrow cells to reconstitute the thymic B-cell compartment of Rag-1−/− mice that had been lethally irradiated. As expected, both fetal liver and bone marrow cells efficiently restored normal B-cell development in the spleen, whereas only fetal liver cells were capable of reconstituting B1 B cells in the peritoneal cavity (Fig. 3A). Fetal liver and bone marrow cells restored the thymic B-cell compartment equally well, although neither of these sources managed to fully restore thymic B cells to their normal numbers (Fig. 3B). These data suggest that thymic B cells and B1 cells are of different lineages and that both fetal liver and bone marrow cells are likely to contribute to thymic B-cell development.
Fig. 3.
Reconstitution of thymic B cells by fetal liver and bone marrow cells in Rag−/− host. (A) Representative FACS plots in the thymus (gated on lymphocytes) and peritoneal cavity (gated on CD19+B220+ cells). (B) Quantitation of B cells as a percentage of total cells in the thymus and spleen. n = 4 mice per group.
Thymic B Cells Show a Unique Phenotype of Differentiation and Activation Markers.
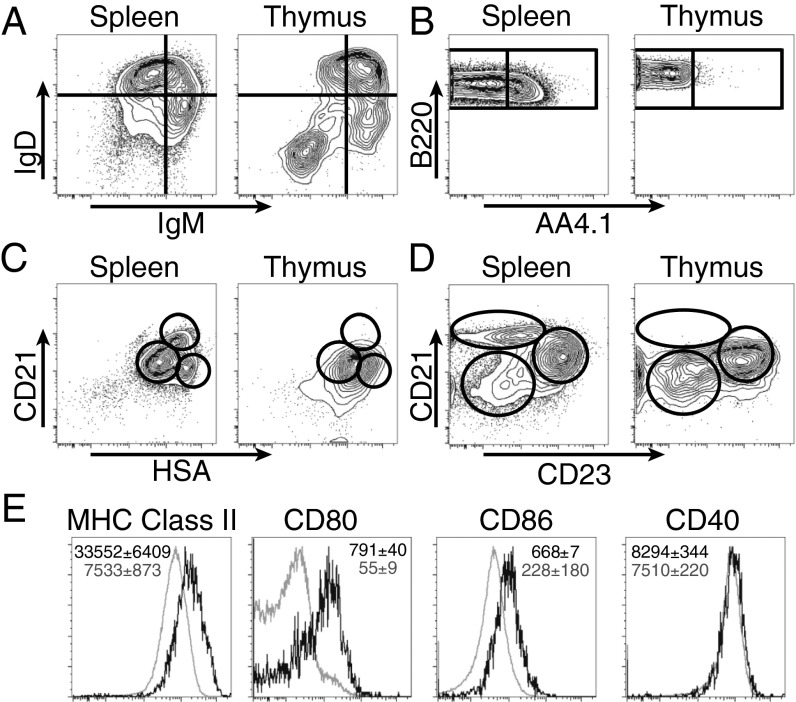
We further characterize the phenotypes of thymic B cells by staining with common markers expressed on other well-characterized B-cell subsets in periphery. Thymic B cells have a distinct profile of IgM/IgD compared with splenic B cells (Fig. 4A). Whereas mature follicular B cells possess an IgMloIgDhi phenotype, the majority of thymic B cells express high levels of both IgM and IgD. Interestingly, there is also a substantial population of IgM−IgD− cells. CD93 (AA4.1) is a marker for B-cell precursors up to transitional B-cell stage. Surprisingly, thymic B cells are almost entirely negative for CD93 (Fig. 4B). This absence of CD93 expression is unique to the thymic B-cell lineage rather than to a specific stage of thymic B-cell development as even the Rag2-GFP+B220lo progenitors do not express CD93. The combination of CD21 and HSA is able to distinguish transitional, marginal zone, and follicular B cells in the spleen (21); however, thymic B cells showed a rather homogeneous profile that does not overlap cleanly with any of the splenic populations (Fig. 4C). The combination of CD21 and CD23 is useful for separating marginal zone and follicular B cells, and thymic B cells do not contain cells with the marginal zone B-cell phenotype (Fig. 4D).
Fig. 4.
Comparison of B-cell markers and costimulatory molecules between thymic and splenic B cells. Representative flow plots of IgM and IgD (A), B220 and AA4.1 (B), CD21 and HSA (C), CD21 and CD23 (D), and MHC class II and costimulatory markers (E) on thymic B cells (black) relative to bulk splenic B cells (gray). Average median fluorescence intensities and SDs are listed for each marker, n = 3–5 mice per group, representative of three independent experiments. Gated on CD19+B220+ cells.
We next assessed the activation phenotype of the most mature thymic B cells by gating on CD19hiB220hi cells. Thymic B cells expressed MHC class II at levels that were 4.5 times higher than splenic B cells (Fig. 4E). Thymic B cells also expressed significantly higher levels of CD80 and CD86 compared with splenic B cells, and expressed similar levels of CD40, which is already up-regulated on splenic B cells (22). We also confirmed that thymic B cells colocalized with dendritic cells in the thymic medulla by immunofluorescence (Fig. S2). All together, these data show that thymic B cells highly express the appropriate accessory molecules to participate in T cell negative selection and are properly localized in the thymic medulla where negative selection is thought to occur.
Development of Autoreactive B Cells in Thymus.
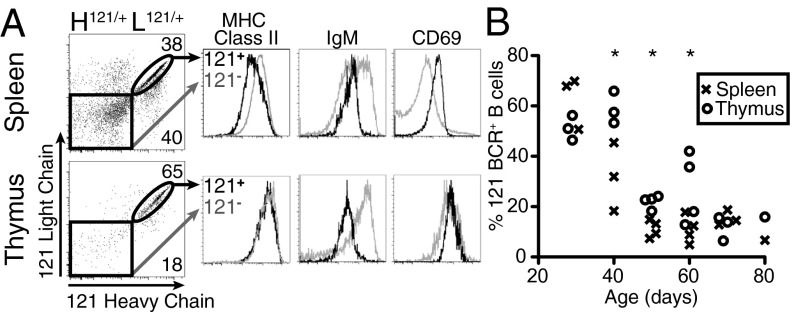
We next wanted to test whether autoreactive thymic B cells could be efficient APCs for the cognate antigens of their BCRs. We used the 121 BCR knock-in mice specific for self-antigen GPI, derived from the K/BxN mouse model of rheumatoid arthritis (23). We first examined the development of autoreactive anti-GPI B cells in the thymus and compared them to their counterparts in the spleen. To identify 121 B cells independent of their binding to GPI, we developed clonotypic antibodies (8H2 and 6B9) against the 121 heavy and light chain respectively (Fig. S3). As shown in Fig. 5A, 121 heavy and light chains are efficiently expressed in thymic B cells (B cells expressing both the heavy and light chain are referred to here as 121+). 121+ B cells were previously shown to be arrested in their maturation in the spleen and were anergic to BCR stimulation (23). Consistent with their inability to fully mature, 121+ B cells in the spleen expressed lower levels of MHC class II than 121− B cells. However, 121+ thymic B cells expressed the characteristic high levels of MHC class II and showed no reduction relative to 121− thymic B cells. This lack of reduction in MHC class II expression is not due to ignorance of the antigen, as 121+ populations in both the spleen and thymus express lower levels of IgM and higher levels of CD69, indicating antigen experience. Similar to MHC II expression, both 121+ and 121− B cells in the thymus showed much higher levels of CD69 than on splenic B cells.
Fig. 5.
Development of autoreactive B cells in the thymus. (A) Expression of MHC class II, IgM, and CD69 in 121+ (black) and 121− (gray) B cells. Gated on CD19+ B cells. Representative of five mice. (B) Quantification of the percentage of 121+ as detected in A in the spleen versus the thymus over time. Each symbol represents an individual mouse. Asterisks represent significant difference between thymus and spleen at the time point assayed.
The number of 121+ B cells in periphery decreases over time as they are outcompeted by allelically-included B cells and B cells that have undergone receptor editing (23). 121+ B cells in the thymus showed a similar trend of decreased survival with slightly slower kinetics (Fig. 5B). These results suggest that autoreactive B cells still undergo some form of tolerance in the thymus; however, autoreactivity does not seem to impair their capacity to present antigens.
Autoreactive Thymic B Cells Present Cognate Antigen for T Cell Negative Selection.
KRN T cells recognize peptide 283–292 of GPI presented by the MHC class II molecule I-Ag7; however, negative selection of these autoreactive transgenic T cells is incomplete (24). To study whether 121+ thymic B cells were capable of presenting GPI and mediating KRN T cell negative selection in vivo, we crossed the 121 BCR knock-in mice with the KRN TCR transgenic mice and B6.H-2g7 congenic mice. This strategy allows us to selectively enhance GPI presentation by thymic B cells without affecting other thymic APC populations or altering the overall expression level of GPI in the thymus.
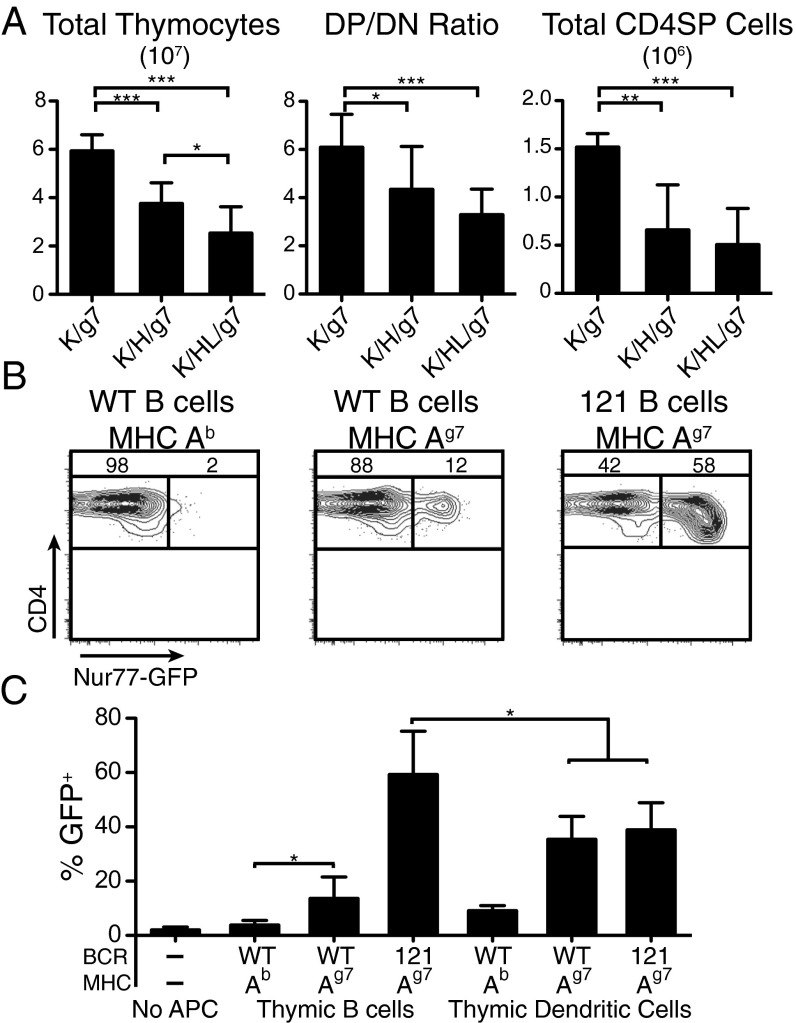
We compared three types of mice that differed in the composition of their B-cell repertoire: wild-type mice with an unmanipulated repertoire, H121/+ mice that carry the 121 heavy chain paired with an endogenous light chain, and H121/+L121/+ mice that carry both the 121 heavy and light chain. As shown previously, in H121/+ mice, only 1–4% of B cells binds GPI, and do so with a much lower affinity than B cells in H121/+L121/+ mice (23). All experimental mice also carry KRN TCR transgene and I-Ag7/b alleles, and are therefore designated as K/g7, K/H/g7, and K/HL/g7, respectively. Relative to K/g7 controls, K/HL/g7 mice that bear both the heavy and light chain of the 121 BCR had a 2.3-fold reduction in total thymic cellularity and a 46% decrease in the CD4 CD8 double positive (DP)/double negative (DN) ratio, demonstrating increased death of developing thymocytes and enhanced negative selection along with a threefold reduction in the number of CD4 single positive (SP) thymocytes (Fig. 6A). Analysis of K/H/g7 mice, which express only the 121 heavy chain and must rely on pairing with an endogenous light chain for GPI reactivity, showed an intermediate phenotype, with a 1.5-fold reduction in cellularity, a 29% decrease in DP/DN ratio, and a 2.5-fold reduction in the number of CD4 SP thymocytes, suggesting that, even when the B-cell repertoire is skewed toward GPI at a more physiological affinity and precursor frequency, thymic B cells are still capable of shaping T-cell selection.
Fig. 6.
Increased negative selection with enhanced thymic B-cell presentation of GPI in KRN TCR transgenic mice. (A) Thymic cellularity, DP/DN ratio, and number of CD4 single positive cells of KRN TCR+ mice expressing the Ag7 MHC, with either a WT B-cell repertoire (K/g7), the heavy chain of the 121 BCR (K/H/g7) or the heavy and light chain of the 121 BCR (K/HL/g7). (B) Representative FACS plot of Nur77-GFP induction in KRN-TCR DP thymocytes (maintained on B6 background) by thymic B cells of B6, K/g7, or K/HL/g7 mice. Gated on Thy1+ DP cells. (C) Quantification of GFP+ DP thymocytes after culturing with various APCs. n = 3–6 mice per group from five independent experiments.
To confirm that the observed phenotype was due to enhanced presentation of GPI by thymic B cells rather than indirect contribution to other APCs (25, 26), thymic B cells and DCs were tested for their capacity to present antigen to thymocytes in a classical in vitro assay in which DP thymocytes were cultured with APCs (27–29). We used nuclear steroid orphan receptor Nur77 induction in DP thymocytes as readout because it was more robust than CD4/CD8 coreceptor dulling or apoptosis. B cells and DCs were enriched from B6, K/BxN, or K/HL/g7 mice, and cultured with thymocytes from KRN TCR transgenic mice crossed to a Nur77-GFP reporter mice (30), which were maintained on a B6 background and had not previously encountered GPI. As expected, thymic APCs from B6 mice induced very little Nur77 expression in KRN DP thymocytes as they do not bear the Ag7 allele that is required for GPI presentation. Thymic B cells from K/HL/g7 mice were capable of inducing substantial Nur77 up-regulation, and interestingly, even the normal repertoire of B cells from K/BxN mice was capable of inducing significant up-regulation over B6 controls (Fig. 6 B and C).
In confirmation of previous reports that thymic DCs are capable of presenting GPI (31), CD11c+ DCs from the thymus could induce Nur77 when they expressed the appropriate MHC allele. However, no enhancement of Nur77 induction was observed when DCs from K/HL/g7 mice and K/BxN mice were compared (Fig. 6C), suggesting that changing the B-cell receptor repertoire had little effect on the antigen presentation by DCs in this system, and that the enhanced negative selection (Fig. 6A) was due to the increased antigen presentation by B cells. It is also interesting to note that thymic B cells from K/HL/g7 mice induced significantly higher levels of Nur77 than thymic DC’s as measured by median fluorescence intensity of GFP. Although thymic B cells from K/BxN mice induced relatively fewer GFP+ cells, they induced equivalent levels of GFP compared with thymic DCs (Fig. S4).
Endogenous B-Cell Repertoire Contributes to Negative Selection of KRN T Cells.
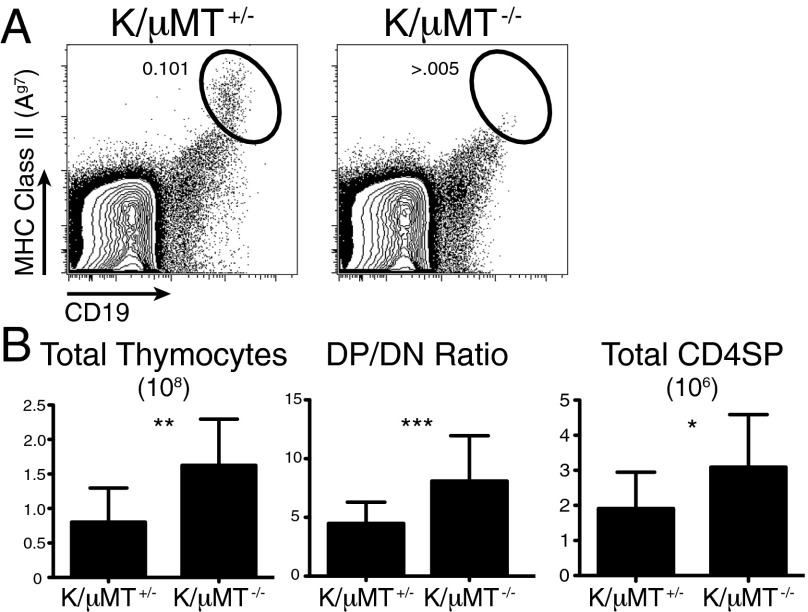
Given the ability of low affinity B cells to induce deletion in the K/H/g7 mice and the capacity of the wild-type Ag7 thymic B cells to induce Nur77 in the in vitro assay, it seemed plausible that the endogenous thymic B-cell compartment could contribute to negative selection of GPI reactive T cells and that negative selection should be less efficient in B cell-deficient mice. To test this hypothesis, we crossed the K/BxN mouse model on to a μMT−/− background (K/BxN/μMT−/−). K/BxN/μMT−/− mice had no mature B cells in the thymus (Fig. 7A). They had double the thymic cellularity of μMT+/− littermates along with a 55% increase of DP/DN ratio and a 61% increase in the number of CD4 SP thymocytes. These results demonstrate that endogenous thymic B cells contribute to negative selection of KRN T cells.
Fig. 7.
Decreased negative selection of KRN TCR in mice lacking B cells. (A) Absence of mature thymic B cells in K/BxN mice on μMT+/− or μMT−/− background. (B) Thymic cellularity, DP/DN ratio, and number of CD4 SP thymocytes in μMT+/− and μMT−/− littermates analyzed at 4 wk of age (n = 11 and 14, respectively).
Discussion
Our studies demonstrate that thymic B cells develop de novo within the thymus with minimal contribution from recirculating peripheral B cells. These thymic B cells express high levels of MHC class II molecules and possess unique activation characteristics and are capable of presenting self-antigens efficiently to mediate deletion of autoreactive CD4+ T cells. Previous studies on the role of thymic B cells in negative selection have used superantigen or B cell-specific expression of a model antigen (16, 17). Whereas those studies demonstrated the capacity of thymic B cells in negative selection, our current study examined the importance of antigen specificity for thymic B cells. We propose that thymic B cells are most efficient for antigens they recognize and promote the deletion of “cognate” autoreactive T cells. Therefore, recognition of self-antigens by thymic B cells and T cells are linked. These data also suggest that thymic B cells could specialize in presenting a subset of self-antigens.
B-cell development is classically thought to be restricted to the bone marrow and, in early life, the fetal liver, but early thymic precursors retain some residual B-cell potential (reviewed in ref. 32). Still, it remained unclear whether thymic B cells developed intrathymically as a result of this latent B-cell potential or arrived as mature B cells from the peripheral recirculation. Supporting the idea of intrathymic development, earlier studies reported the presence of a B220loCD43+IgM− population within the murine thymus that when purified and injected intrathymically could give rise to mature B220hiCD43−IgM+ B cells (10, 12). The Rag2-GFP+B220lo population that we describe falls within this B220loCD43hi population. However, GFP+ cells only constitute 20–30% of the B220loCD43hi population, suggesting that B220loCD43+ cells are not simply the equivalents of pro-B cells in the bone marrow and that it may contain earlier progenitors. Thus, GFP+ cells represent a better-defined B-cell precursors progressing through the B-cell development, as evidenced by the up-regulation of surface Ig on a subset of B220loGFP+ cells. The detection of more mature B220hi thymic B cells with residual GFP expression also strongly argues that B-cell development is an ongoing process in the thymus. Earlier attempts to quantify the recirculation of peripheral B cells to the thymus used transfers of very large numbers of splenic B cells and found very little thymic chimerism; however, the conclusions were limited by an inability to account for the contribution of nonsplenic B-cell populations and poor survival of transferred cells (10). Our studies show that from the pool of all recirculating B cells, there is very little thymus homing capacity, and that the thymic B-cell compartment more closely mimics other cell populations that develop within the thymus.
The combination of markers used to distinguish immature, transitional, marginal zone, or mature follicular B cells were not very informative for differentiating thymic B-cell subsets. Although thymic B cells do express CD5 and CD43, markers with distinct expression patterns on B1 cells in the peritoneum, thymic B cells appear to have a more continuous range of expressions. Even though CD5 is commonly associated with the B1 lineage, it is more broadly recognized as a marker of ongoing BCR and TCR stimulation (33, 34). Increased autoreactivity along with the activated phenotype of thymic B cells could also account for the up-regulation of CD5. Two additional lines of evidence suggest that thymic B cells are not related to the B1 lineage. First, it has been shown that B cells bearing the 121 BCR are not selected into the B1 B-cell compartment (23); however, a high proportion of thymic B cells express this specificity in the knock-in mice. Second, both bone marrow and fetal liver cells can reconstitute thymic B-cell compartment equally well (Fig. 3 and ref. 35).
The de novo development of B cells in thymus raises the question of how the thymic B-cell repertoire is selected compared with that of the bone marrow or the periphery. In 121 knock-in mice, most of the tolerance checkpoints that act on the 121+ B cells take place in the spleen, where they are arrested in maturation, have a fast turnover rate, and are outcompeted by nonautoreactive B cells with age (23). The high proportion of 121+ B cells in thymus particularly in young animals more closely resembles the development in the bone marrow. However, the fact that 121+ B cells are gradually outcompeted in the thymic B-cell compartment suggests that autoreactive thymic B cells are counterselected similar to peripheral B cells.
As APCs, B cells are unique in that they are most efficient for antigens recognized by their BCRs, but the frequency of a given specificity is rare within the repertoire. Are these low-frequency B cells sufficient to purge autoreactive T cells? A similar scenario has been encountered for mTECs as only a small fraction (∼1%) of mTECs will express any particular tissue-specific protein (15). Using the KRN TCR transgenic system, we were able to demonstrate that even when GPI-specific B cells are present at low frequencies (less than 5% in H/g7/K mice) that they are still capable of exerting a significant effect on T-cell selection. We further confirmed that GPI-presenting B cells are detectable in the unmanipulated thymic B-cell repertoire, and the data from the K/BxN/μMT−/− mice suggests that even this endogenous frequency of GPI-reactive B cells contributes to negative selection of KRN T cells. It has been proposed that thymocytes have time and the occasion to be scrutinized (36). Thymocytes reside in medulla for at least 4–5 d and are extremely motile. This behavior, along with the low turnover rate and perfect location of thymic B cells, may facilitate the interaction of cognate T and B cells in the thymus.
What kind of autoantigens do thymic B cells preferentially recognize and present? The Nussensweig laboratory and others have demonstrated that even after central tolerance in the bone marrow, between 55% and 75% of B cells in the immature B-cell compartment show some degree of autoreactivity or polyreactivity, and that these specificities are not removed until they reach peripheral tolerance checkpoints in the spleen (37). In addition, thymic B cell may also present peptides from the highly diverse regions of the B-cell receptor itself, as T cells that are specific for BCR peptides can become pathogenic if they escape into the periphery (38, 39). Recent studies tracking autoreactive B-cell populations in the wild-type repertoire have argued that for some classes of self-antigens, B-cell tolerance is achieved not by deletion or anergy but largely through a lack of cognate T-cell help (40). Overall, these data support a model where thymic B cells couple a broad recognition of self-antigens with an enhanced antigen-presentation capacity to purge cognate T cells from the developing repertoire.
Materials and Methods
Mice.
Rag2-GFP were maintained on B6 background. The 121 BCR knockin mice, KRN TCR, μMT−/−/B6, μMT−/−/NOD, B6.H2g7, and Nur77-GFP mice were described (23, 24, 30). Unless otherwise stated, 6- to 12-wk-old mice were used in experiments. See SI Materials and Methods for generation of parabiotic mice and bone marrow chimeras. All mice were housed in a specific pathogen-free facility, and experiments were approved by the University of Chicago Institutional Animal Care and Use Committee.
Analysis of Thymic B Cells.
Thymi were dissected carefully to avoid the thymic lymph nodes. Thymic B cells were enriched by either depleting bulk thymocytes with anti-CD4 (GK1.5) and anti-CD8 (2.43.1), or by positive selection with anti-B220 (RA3-6B2) using MACS microbeads (Miltenyi Biotec). For BrdU incorporation studies, mice were injected intraperitoneally with 1 mg of BrdU (Sigma) every 12 h for the indicated period. BrdU was detected by a BrdU Flow kit following the manufacturer’s protocol (BD Biosciences). See SI Materials and Methods for antibodies used in flow cytometry.
In Vitro Negative Selection Assay.
Thymi from individual mice were collagenase digested, and enriched for either B220+ or CD11c+ cells by double enrichment using AutoMACS separator (to greater than 80% purity as determined by CD19 or CD11c costaining with MHC Class II). A total of 1.0 × 105 KRN TCR transgenic thymocytes were mixed with 0.5 × 105 sorted thymic APCs in a round bottom 96-well plate. After 12 h, cultures were stained with anti-CD4, anti-CD8, and anti-Thy1.2. Expression of Nur77 in DP cells was assessed in Thy1.2+ cells within the lymphocyte gate.
Statistical Analysis.
Statistical analysis was performed by Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Material
Acknowledgments
We thank Drs. D. Mathis and C. Benoist for various mice, Drs. M. Nussenweig and P. Fink for Rag2-GFP mice, Dr. A. Bendelac for help with parabiosis experiments and critical reading of the manuscript, X. Liu and C. Rayon for help with mice, and the Fitch Monoclonal Antibody Core Facility (partially supported by National Cancer Institute Cancer Center Support Grant 5P30CA014599-38) for the generation of anti-121 clonotypic antibodies. This work was supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) Grant R01 AI087645 (to H.H.). J.P. was partially supported by NIH/NIAID Grant 2T32AI007090.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313001110/-/DCSupplemental.
References
- 1.Stockinger B. T lymphocyte tolerance: From thymic deletion to peripheral control mechanisms. Adv Immunol. 1999;71:229–265. doi: 10.1016/s0065-2776(08)60404-6. [DOI] [PubMed] [Google Scholar]
- 2.McCaughtry TM, Hogquist KA. Central tolerance: What have we learned from mice? Semin Immunopathol. 2008;30(4):399–409. doi: 10.1007/s00281-008-0137-0. [DOI] [PubMed] [Google Scholar]
- 3.Hinterberger M, et al. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010;11(6):512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 4.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 5.Stockinger B, Hausmann B. Functional recognition of in vivo processed self antigen. Int Immunol. 1994;6(2):247–254. doi: 10.1093/intimm/6.2.247. [DOI] [PubMed] [Google Scholar]
- 6.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 7.Pierce SK, et al. Antigen-presenting function of B lymphocytes. Immunol Rev. 1988;106:149–180. doi: 10.1111/j.1600-065x.1988.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 8.Isaacson PG, Norton AJ, Addis BJ. The human thymus contains a novel population of B lymphocytes. Lancet. 1987;2(8574):1488–1491. doi: 10.1016/s0140-6736(87)92622-5. [DOI] [PubMed] [Google Scholar]
- 9.Miyama-Inaba M, et al. Unusual phenotype of B cells in the thymus of normal mice. J Exp Med. 1988;168(2):811–816. doi: 10.1084/jem.168.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akashi K, Richie LI, Miyamoto T, Carr WH, Weissman IL. B lymphopoiesis in the thymus. J Immunol. 2000;164(10):5221–5226. doi: 10.4049/jimmunol.164.10.5221. [DOI] [PubMed] [Google Scholar]
- 11.Ceredig R. The ontogeny of B cells in the thymus of normal, CD3 epsilon knockout (KO), RAG-2 KO and IL-7 transgenic mice. Int Immunol. 2002;14(1):87–99. doi: 10.1093/intimm/14.1.87. [DOI] [PubMed] [Google Scholar]
- 12.Mori S, et al. Presence of B cell progenitors in the thymus. J Immunol. 1997;158(9):4193–4199. [PubMed] [Google Scholar]
- 13.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206(3):607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerri L, et al. Analysis of APC types involved in CD4 tolerance and regulatory T cell generation using reaggregated thymic organ cultures. J Immunol. 2013;190(5):2102–2110. doi: 10.4049/jimmunol.1202883. [DOI] [PubMed] [Google Scholar]
- 15.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2(11):1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 16.Kleindienst P, Chretien I, Winkler T, Brocker T. Functional comparison of thymic B cells and dendritic cells in vivo. Blood. 2000;95(8):2610–2616. [PubMed] [Google Scholar]
- 17.Frommer F, Waisman A. B cells participate in thymic negative selection of murine auto-reactive CD4+ T cells. PLoS ONE. 2010;5(10):e15372. doi: 10.1371/journal.pone.0015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu W, et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400(6745):682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 19.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5(4):418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 20.Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148(6):1604–1612. [PubMed] [Google Scholar]
- 21.Loder F, et al. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190(1):75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keir ME, Sharpe AH. The B7/CD28 costimulatory family in autoimmunity. Immunol Rev. 2005;204:128–143. doi: 10.1111/j.0105-2896.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Kearney JF, Grusby MJ, Benoist C, Mathis D. Induction of tolerance in arthritogenic B cells with receptors of differing affinity for self-antigen. Proc Natl Acad Sci USA. 2006;103(10):3734–3739. doi: 10.1073/pnas.0600214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kouskoff V, et al. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87(5):811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 25.Townsend SE, Goodnow CC. Abortive proliferation of rare T cells induced by direct or indirect antigen presentation by rare B cells in vivo. J Exp Med. 1998;187(10):1611–1621. doi: 10.1084/jem.187.10.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey BP, et al. Editing antigen presentation: Antigen transfer between human B lymphocytes and macrophages mediated by class A scavenger receptors. J Immunol. 2008;181(6):4043–4051. doi: 10.4049/jimmunol.181.6.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swat W, Ignatowicz L, von Boehmer H, Kisielow P. Clonal deletion of immature CD4+8+ thymocytes in suspension culture by extrathymic antigen-presenting cells. Nature. 1991;351(6322):150–153. doi: 10.1038/351150a0. [DOI] [PubMed] [Google Scholar]
- 28.Vasquez NJ, Kaye J, Hedrick SM. In vivo and in vitro clonal deletion of double-positive thymocytes. J Exp Med. 1992;175(5):1307–1316. doi: 10.1084/jem.175.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogquist KA, et al. Identification of a naturally occurring ligand for thymic positive selection. Immunity. 1997;6(4):389–399. doi: 10.1016/s1074-7613(00)80282-4. [DOI] [PubMed] [Google Scholar]
- 30.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208(6):1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shih FF, Racz J, Allen PM. Differential MHC class II presentation of a pathogenic autoantigen during health and disease. J Immunol. 2006;176(6):3438–3448. doi: 10.4049/jimmunol.176.6.3438. [DOI] [PubMed] [Google Scholar]
- 32.Zediak VP, Maillard I, Bhandoola A. Closer to the source: Notch and the nature of thymus-settling cells. Immunity. 2005;23(3):245–248. doi: 10.1016/j.immuni.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Wortis HH, Teutsch M, Higer M, Zheng J, Parker DC. B-cell activation by crosslinking of surface IgM or ligation of CD40 involves alternative signal pathways and results in different B-cell phenotypes. Proc Natl Acad Sci USA. 1995;92(8):3348–3352. doi: 10.1073/pnas.92.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179(2):709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Than S, et al. Origin of thymic and peritoneal Ly-1 B cells. Eur J Immunol. 1992;22(5):1299–1303. doi: 10.1002/eji.1830220527. [DOI] [PubMed] [Google Scholar]
- 36.Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20(5):509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- 37.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 38.Detanico T, Heiser RA, Aviszus K, Bonorino C, Wysocki LJ. Self-tolerance checkpoints in CD4 T cells specific for a peptide derived from the B cell antigen receptor. J Immunol. 2011;187(1):82–91. doi: 10.4049/jimmunol.1002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munthe LA, Corthay A, Os A, Zangani M, Bogen B. Systemic autoimmune disease caused by autoreactive B cells that receive chronic help from Ig V region-specific T cells. J Immunol. 2005;175(4):2391–2400. doi: 10.4049/jimmunol.175.4.2391. [DOI] [PubMed] [Google Scholar]
- 40.Taylor JJ, et al. Deletion and anergy of polyclonal B cells specific for ubiquitous membrane-bound self-antigen. J Exp Med. 2012;209(11):2065–2077. doi: 10.1084/jem.20112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.