Significance
We discovered that SAMP1/YitFc (SAMP) mice, which develop spontaneous Crohn’s disease (CD)-like ileitis in the absence of nucleotide-binding oligomerization domain-containing 2 (NOD2) genetic mutations, fail to respond to muramyl dipeptide and display impaired bacterial clearance. These results support the concept that a dysregulated NOD2 in SAMP mice predisposes them to chronic intestinal inflammation. We believe that our study provides a paradigm shift by demonstrating that CD-like ileitis is caused by an innate immune defect, rather than an overly aggressive adaptive immune response. Therefore, preventive and curative treatments for CD should be directed to boost, rather than suppress, mucosal innate immune responses.
Abstract
Nucleotide-binding oligomerization domain-containing 2 (NOD2) is an intracellular receptor that plays an essential role in innate immunity as a sensor of a component of the bacterial cell wall, muramyl dipeptide (MDP). Crohn’s disease (CD)-associated NOD2 variants lead to defective innate immune responses, including decreased NF-κB activation and cytokine production. We report herein that SAMP1/YitFc (SAMP) mice, which develop spontaneous CD-like ileitis in the absence of NOD2 genetic mutations, fail to respond to MDP administration by displaying decreased innate cytokine production and dysregulated NOD2 signaling compared with parental AKR control mice. We show that, unlike in other mouse strains, in vivo administration of MDP does not prevent dextran sodium sulfate-induced colitis in SAMP mice and that the abnormal NOD2 response is specific to the hematopoietic cellular component. Moreover, we demonstrate that MDP fails to enhance intracellular bacterial killing in SAMP mice. These findings shed important light on the initiating molecular events underlying CD-like ileitis.
Nucleotide-binding oligomerization domain-containing 2 (NOD2) is an intracellular pattern recognition receptor (PRR) and member of the NOD-like receptor protein family that is mainly expressed in monocyte-derived cells (1). NOD2 has the essential role of initiating innate immune responses upon intracellular exposure to muramyl dipeptide (MDP), a breakdown product of peptidoglycan that is present in the cell wall of both Gram-negative and Gram-positive bacteria (2, 3). Upon MDP recognition, NOD2 binds to a downstream adaptor molecule, receptor-interacting protein-2 kinase (RIP-2), via caspase recruitment domain interactions and initiates RIP-2 polyubiquitination. Activated RIP-2 induces ubiquitination of IκB kinase-γ, which in turn allows the recruitment of TAK-1 and leads to downstream activation of both NF-κB and MAPK (4–6). In addition to activating the NF-κB and MAPK signaling pathways, NOD2 activation has recently been shown to influence MHC cross-presentation (7), autophagy induction, and resistance to intracellular bacterial infection (8, 9). Thus, although most well known for its acute signaling effects, NOD2 activation causes a variety of cell biologic changes in vivo that are also likely important for immunologic homeostasis.
The importance of NOD2 is underscored by the finding that polymorphisms within the NOD2 gene confer an increased risk for developing Crohn’s disease (CD), a chronic inflammatory disorder of the bowel (10–12). The associated risk is dose dependent, with heterozygous carriers of the NOD2 gene polymorphisms harboring a twofold to fourfold increased risk of CD, and homozygous or compound heterozygous carriers having a 20- to 40-fold increased risk. Notably, the CD-associated NOD2 gene polymorphisms cause a loss of function in the NOD2 pathway (3, 13). Although the exact mechanism by which this innate immune dysfunction leads to inflammatory bowel disease (14) is still unclear, it is generally thought that decreased NOD2 function manifests itself in a failure to respond to pathogens, causing an increased bacterial load, abnormal interactions between the gut mucosal immune system and luminal antigens, and subsequent chronic intestinal inflammation. Because NOD2 polymorphisms are associated with only 15–20% of CD patients (15), it is possible that the remaining 85% lacking the NOD2 mutations may display a combined or separate functional defect in innate immunity, possibly mediated by NOD2, which like the genetic mutation, renders them unable to mount effective innate immune responses.
The goal of our study was to determine the functional role of NOD2 during intestinal inflammation by studying the effects of MDP stimulation in the SAMP1/YitFc (SAMP) murine model of experimental CD-like ileitis. This strain was originally derived from brother–sister breeding of AKR mice. These mice do not carry genetic NOD2 variants, yet they spontaneously develop severe chronic ileitis by 20 wk of age without chemical, genetic, or immunological manipulation. Furthermore, the resulting ileitis in these mice bears remarkable phenotypic similarities to CD with regard to disease location, histological features, extraintestinal manifestations, and response to therapies that are effective in treating the human disease. Our group and others have extensively characterized this model and have provided insights into the mechanisms of experimental chronic ileitis (16).
In the present study, we provide evidence that SAMP mice have dysregulated NOD2 responses. This manifests itself in vivo as an inability of MDP to ameliorate both the spontaneous CD-like ileitis and the dextran sodium sulfate (DSS)-induced colitis in SAMP mice. This dysfunctional response is specifically present in the hematopoietic cellular component of SAMP mice. SAMP macrophages produce less cytokines in response to MDP administration and demonstrate delayed acute signaling responses to MDP stimulation. In addition, MDP fails to enhance intracellular Salmonella killing in SAMP macrophages, a feature common with NOD2 dysfunction (9, 17). Finally, SAMP mice display increase susceptibility to Salmonella infection in vivo. The end result is an ineffective maintenance of immunologic mucosal homeostasis due to dysregulation of NOD2-induced bacterial clearance with concomitant inflammatory disease susceptibility in the presence of a WT NOD2 genotype.
Results
MDP Administration Does Not Protect Against SAMP CD-Like Ileitis.
Increasing evidence suggests that one of the physiological functions of NOD2 activation via MDP is to provide a temporal down-regulation of the inflammatory responses through inhibition of multiple TLR pathways. This evidence is based on in vitro studies showing that NOD2 deficiency causes impaired tolerance to infection with pathogenic and commensal bacteria in macrophages that are rendered tolerant to LPS and MDP (18). Furthermore, in vivo studies in normal mice show that administration of MDP leads to the amelioration of both DSS and 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis, and that this effect is abrogated in NOD2-deficient mice (19).
These findings led us to study the ability of MDP to protect SAMP mice from the development of spontaneous CD-like ileitis. Preinflamed SAMP mice were administered MDP (100 μg or PBS, i.p.) twice weekly for a total of 6 wk. Histological assessment of ileal inflammation, based on active inflammation, chronic inflammation, and villous distortion, showed no significant differences in total inflammatory scores between MDP- and PBS-treated mice (Fig. S1). These data suggest that, unlike in previous studies of DSS- and TNBS-induced colitis in normal mice, MDP does not confer protection against spontaneous ileitis in SAMP mice.
MDP Administration Does Not Protect SAMP Mice from DSS-Induced Colitis.
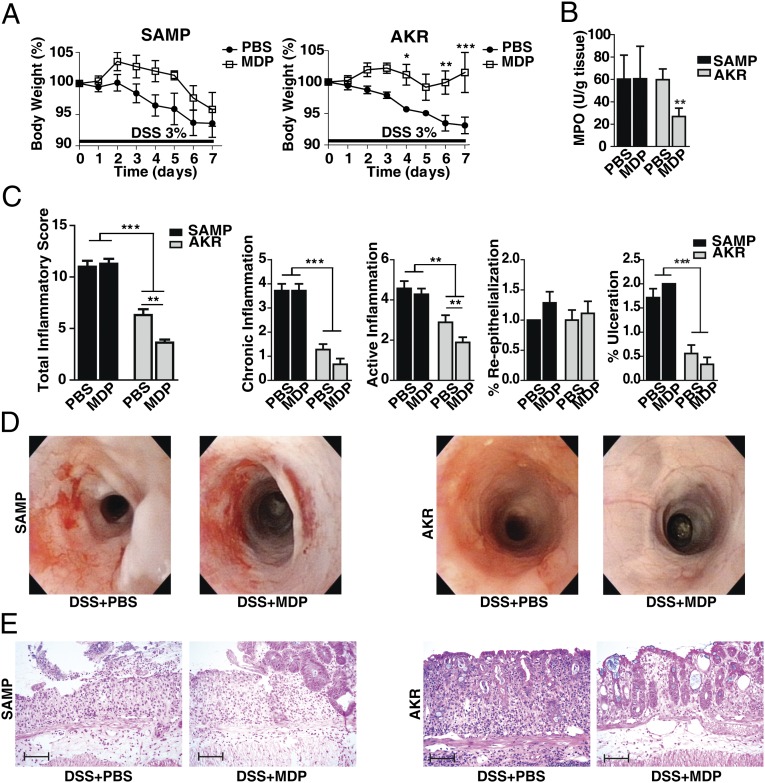
To test whether the in vivo protective effects of MDP are specific for colitis, we treated SAMP mice with 3% (wt/vol) DSS in drinking water for 7 d. By causing exposure of the lamina propria of the colon to resident bacteria, this model tests the acute inflammatory response and its repair in the colon. MDP (through NOD2) activation is known to be protective in this acute colitis model (19). DSS-treated SAMP and AKR control mice were administered MDP (100 μg or PBS, i.p.) for 3 consecutive days (days 0, 1, and 2 of colitis induction) to assess the protective effects of MDP in this model of colitis. As shown in Fig. 1A, AKR control mice administered MDP lost significantly less body weight than AKR mice receiving PBS. In contrast, SAMP mice treated with MDP exhibited comparable body weight loss to SAMP mice treated with PBS. Body weight correlated with myeloperoxidase activity evaluated in colons of treated mice (Fig. 1B), and with the histological assessment of colitis (Fig. 1C). Colonoscopy revealed that, in AKR mice, more severe inflammation was associated with PBS treatment, demonstrated by increased inflammatory cellular infiltrates in the lamina propria, whereas MDP-treated mice showed only mild inflammation with slight vascular changes and granularity. In SAMP mice, severe inflammation, including marked wall thickening, irregular vascular patterns, fibrin, granularity, and bleeding, was observed in mice treated with both PBS and MDP (Fig. 1D). Representative histological sections are shown in Fig. 1E. These data suggest that the previously reported in vivo protective effects of MDP against DSS-induced murine colitis are also observed in AKR control mice, but not in SAMP mice, suggesting that SAMP mice have an abnormal innate immune response to MDP administration.
Fig. 1.
MDP administration in vivo reduces DSS colitis in AKR mice, but not in SAMP mice. SAMP and AKR mice were treated with 3% DSS in their drinking water for 7 d (n = 8–11 per group). At the early phase of colitis induction (days 0, 1, 2), mice were administered either MDP (100 μg, i.p.) or PBS daily. (A) Changes in body weight in SAMP and AKR mice administered MDP or PBS (two-way ANOVA repeated measures, MDP protective effect for AKR was significant at P = 0.023, but not for SAMP, P = 0.125). (B) Myeloperoxidase (MPO) activity calculated from the colons of treated mice (Kruskal–Wallis, P < 0.01, Dunn’s). (C) Colonic total inflammatory scores, as determined by the sum of chronic inflammation, active inflammation, percentage reepithelialization, and percentage of ulceration (one-way ANOVA, P < 0.001; pairwise Bonferroni). (D) High-resolution endoscopic images of the proximal colon after 7 d of DSS treatment show severe inflammation in both groups of SAMP mice (PBS and MDP) and mild inflammation (including slight vascular changes and mild granularity) in AKR control mice treated with MDP compared with PBS. (E) Representative histopathological sections show active, severe ulcers, adjacent regenerative crypts, active cryptitis, and increased inflammatory cells in the lamina propria of SAMP mice treated with PBS and MDP. Sections from AKR mice treated with MDP show regenerative colonic mucosa with focal mild, active cryptitis, and more minimal increased inflammatory cells compared with PBS-treated AKR mice. (Scale bars, 100 µm.) Data are represented as mean ± SEM. The single asterisk (*), double asterisk (**), and triple asterisk (***) denote significant differences at P < 0.05, P < 0.01, and P < 0.001, respectively. Results are representative of three independent experiments.
Defective Function of NOD2 Signaling in SAMP Mice Is Derived from Hematopoietic Sources.
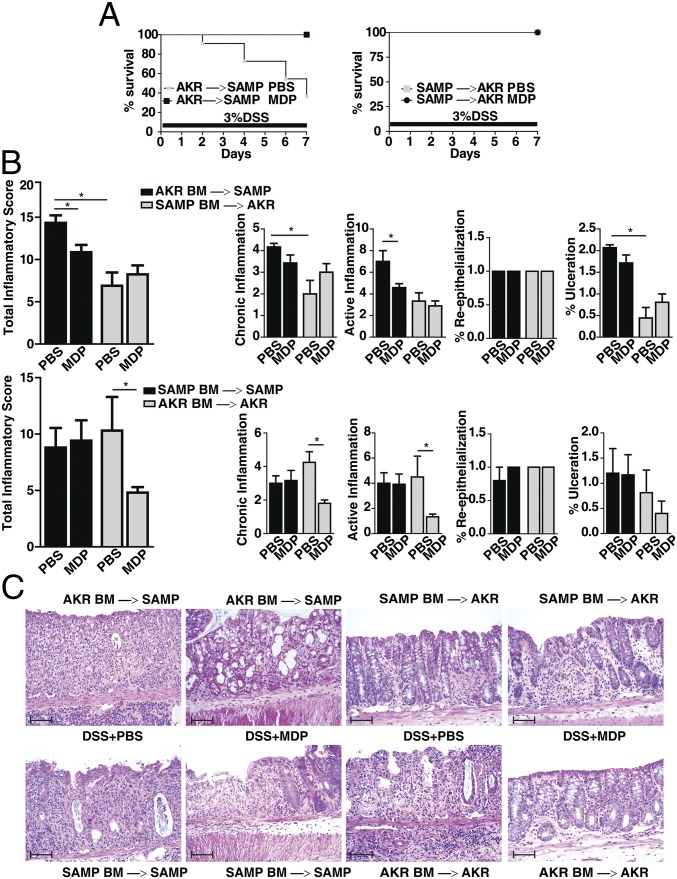
Because NOD2 is an intracellular PRR expressed in a limited number of cell types (1), we next used bone marrow (BM) chimera experiments to identify the specific cellular compartment that is responsible for the abnormal immune response to MDP in SAMP mice. We generated BM chimera mice by adoptively transplanting BM from AKR donor mice into irradiated SAMP mice (AKR BM→SAMP) and BM from SAMP donor mice into irradiated AKR mice (SAMP BM→AKR); irradiated AKR mice transplanted with AKR BM (AKR BM→AKR) and irradiated SAMP mice transplanted with SAMP BM (SAMP BM→SAMP) were used as controls. After 6 wk of hematopoietic reconstitution to achieve chimerism, all groups were treated with 3% DSS for 7 d in their drinking water to induce colitis, as well as 3 d of MDP or PBS stimulation.
Markedly less mortality was observed in AKR BM→SAMP mice administered MDP vs. PBS. Because no mortality was observed in the other chimeric groups (Fig. 2A), it is likely that the increased mortality in the AKR BM→SAMP treated with PBS is due to the primary epithelial dysfunction and increased permeability characteristic of SAMP mice (20). Notably, as shown by histological assessment of colitis, AKR BM→SAMP mice treated with MDP had lower total inflammatory scores compared with those treated with PBS; similar results were seen in AKR BM→AKR mice treated with MDP vs. PBS (Fig. 2B). However, MDP treatment did not lower inflammatory scores in SAMP BM→AKR mice or SAMP BM→SAMP mice, consistent with data shown previously. The fact that irradiated AKR mice reconstituted with SAMP BM do not display protective effects strongly suggests that the abnormal NOD2 response to MDP stimulation is specifically associated with the hematopoietic compartment in SAMP mice. This result is further strengthened by our finding that the protective effect associated with MDP stimulation was restored in irradiated SAMP mice reconstituted with AKR BM.
Fig. 2.
The abnormal response to MDP in SAMP mice is contained within the hematopoietic compartment. AKR and SAMP mice (n = 9 per group) were transplanted with SAMP and AKR BM, respectively (n = 5 per group), and administered MDP or PBS during the first 3 d of 3% DSS treatment. (A) Percentage survival of chimeric mice during 3% DSS treatment. (Log-rank test, hazard ratio for AKR→SAMP with DSS/PBS was 4.85 times higher than for DSS/MDP, 95% confidence interval (CI) of hazard ratio = 0.8, 26.7, P = 0.090; no effect on hazard ratio for SAMP→AKR, P = 1.0.) (B) Colonic total inflammatory scores, as determined by the sum of chronic inflammation, active inflammation, percentage reepithelialization, and percentage of ulceration. (C) Representative histopathological sections for colons in each chimeric group. AKR BM→SAMP mice treated with MDP showed more attenuated intensity of colitis and active inflammation compared with control (PBS treatment); no difference were seen in SAMP BM→AKR mice treated with MDP or PBS, as well as SAMP BM→SAMP mice treated with MDP or PBS, all of which showed severe ulceration with severe active and chronic inflammation. AKR BM→AKR mice showed no ulceration and mild active and chronic inflammation with some regenerative changes in the group treated with MDP compared with control (PBS). (Scale bars, 100 µm.) Data are represented as mean ± SEM. The asterisks (*) denote significant differences at P < 0.05. Results are representative of three independent experiments.
SAMP Mice Display Abnormal Cytokine Production and Dysregulated NOD2 Signaling in Response to MDP Stimulation.
To assess the function of NOD2 signaling in the hematopoietic compartment of SAMP mice at the cellular level, we determined the effects of MDP stimulation on innate cytokine production from bone marrow-derived macrophages (BMDMs) isolated from preinflamed SAMP mice and age-matched AKR control mice. Cells were incubated with MDP for 24 h and supernatants were tested for production of innate cytokines, such as IL-1α, IL-6, IL-10, IL-12, and TNF-α. Cytokine production by BMDMs isolated from SAMP mice was significantly reduced compared with AKR control mice (Table S1).
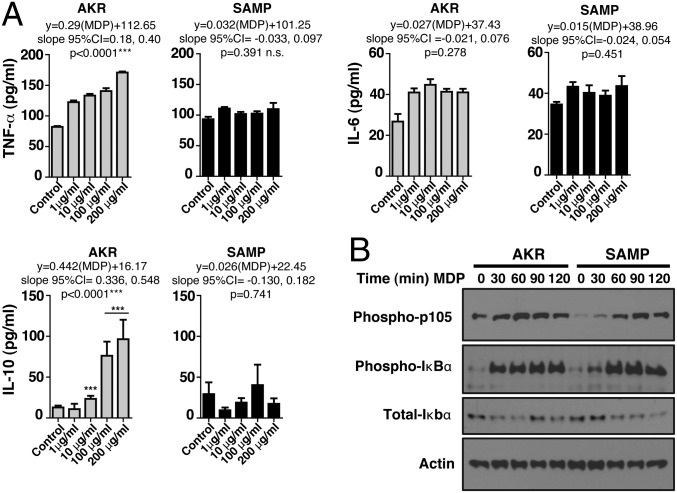
We also examined whether the decrease in MDP-stimulated cytokine production was due to a decreased sensitivity of SAMP BMDMs to MDP. BMDMs isolated from preinflamed SAMP mice and age-matched AKR control mice were stimulated using increasing concentrations of MDP for 24 h and supernatants tested for cytokine production. MDP induced a significant dose-dependent stimulation of TNF-α, IL-6, and IL-10 production in AKR but not SAMP mice (Fig. 3A). The lack of an MDP dose–response in SAMP mice demonstrates that their defective MDP response is not explained by a different threshold for activation compared with AKR control mice.
Fig. 3.
Impaired in vitro production of innate cytokines and NOD2 signaling in response to MDP in SAMP mice. (A) BMDMs isolated from preinflamed SAMP (4 wk old) and age-matched AKR control mice were incubated with different concentrations of MDP (1, 10, 100, 200 μg/mL) or control medium for 24 h. Cell-free supernatants were analyzed by ELISA for production of TNF-α, IL-6, and IL-10. AKR-derived cells responded producing significantly increased amounts of TNF-α [linear regression, F(2,48) = 22.06, AKR vs. SAMP, P < 0.00001] and IL-10 [linear regression, F(2,69) = 6.09, AKR vs. SAMP, P = 0.0037] as the MDP doses increased, a response that did not occur in SAMP-derived cells [linear regression, TNF-α, F(2,34) = 0.11, P = 0.743; IL-10, F(2,34) = 0.11, P = 0.39]. IL-6 produced by AKR and SAMP cells had a different pattern. IL-6 production significantly increased with the lowest MDP dose [1 µg/mL, generalized linear model (GLM), df = 22, P < 0.0001] but remained unchanged as the MDP concentration increased (slope not different from zero; GLM, df = 48, P > 0.59; pairwise comparisons, adjusted P > 0.23). MDP-stimulated SAMP cells produced one-half of the amount of IL-6 produced by AKR in response to all MDP doses tested (paired adjusted linear GLM coefficients, 6.91 vs. 15.28 pg/mL; mean difference, −8.37; 95% CI of difference, −13.94, −2.80; paired t test, P = 0.017). (B) BMDMs isolated from AKR and SAMP mice were left untreated or stimulated with MDP for 30, 60, 90, and 120 min. Lysates were standardized for equal protein concentration before immunoblotting with antibodies against phosphorylated p105, total and phosphorylated IκBα, and actin. Results are representative of three independent experiments. Data are represented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Because MDP induces the secretion of proinflammatory cytokines via both NF-κB and MAPK activation (4–6, 21), we next sought to determine whether this MDP-induced functional defect in SAMP mice is related to the inability of NOD2 to signal acutely through the NF-κB pathway. BMDMs isolated from both sex-matched, littermate preinflamed SAMP mice and AKR controls were left untreated or stimulated with MDP. Although the amplitude of ultimate signal was similar between BMDMs from SAMP and AKR mice, SAMP mice showed a marked delay in NF-κB signaling (Fig. 3B). Immune homeostasis is in such tight regulation between different cell types in the intestinal tract and between the microbiome and the intestine, that even a 15- to 20-min delay in optimally responding to intracellular bacterial breakdown products could cause a wider inflammatory dysfunction.
Synergistic Cytokine Production upon MDP and LPS Costimulation Is Abrogated in SAMP Mice.
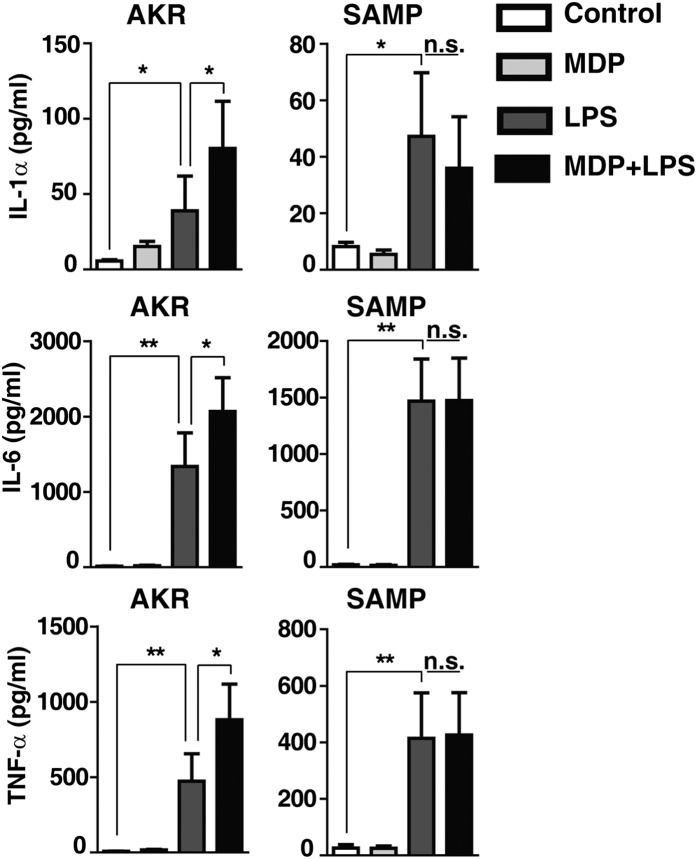
Mouse macrophages have been shown to produce low levels of cytokines in response to MDP. Furthermore, MDP and LPS costimulation has been shown to produce a synergistic effect in macrophages with enhanced production of proinflammatory cytokines (22, 23). Thus, we next studied the ability of SAMP BMDMs to secrete cytokines in response to the combination of MDP and LPS stimulation. BMDMs isolated from preinflamed SAMP mice and age-matched AKR control mice were left untreated or incubated with MDP, LPS, or the combination of MDP and LPS together for 24 h. Macrophages isolated from AKR mice showed a synergistic enhancement of cytokine production in response to costimulation with MDP and LPS; this effect was not observed in cells isolated from SAMP mice (Fig. 4). Given that SAMP mice have normal responses to LPS, these results indicate that the defective innate cytokine production is not a generalizable innate immune phenomenon.
Fig. 4.
Impaired synergism of MDP and LPS on innate cytokine production in SAMP vs. AKR BMDMs. BMDMs isolated from preinflamed SAMP and age-matched AKR control mice were stimulated with medium (control), MDP (10 μg/mL), LPS (10 ng/mL), or a combination of MDP and LPS (n ≥ 9). Cultured supernatants were collected after 24 h and were analyzed by ELISA for production of IL-1α, IL-6, and TNF-α. Data are represented as mean ± SEM (Kruskal–Wallis, pairwise Mann–Whitney). The single asterisk (*) and double asterisk (**) denote significant differences at P < 0.05 and P < 0.01, respectively.
NOD2-Dependent Intracellular Salmonella Killing Is Defective in SAMP Mice.
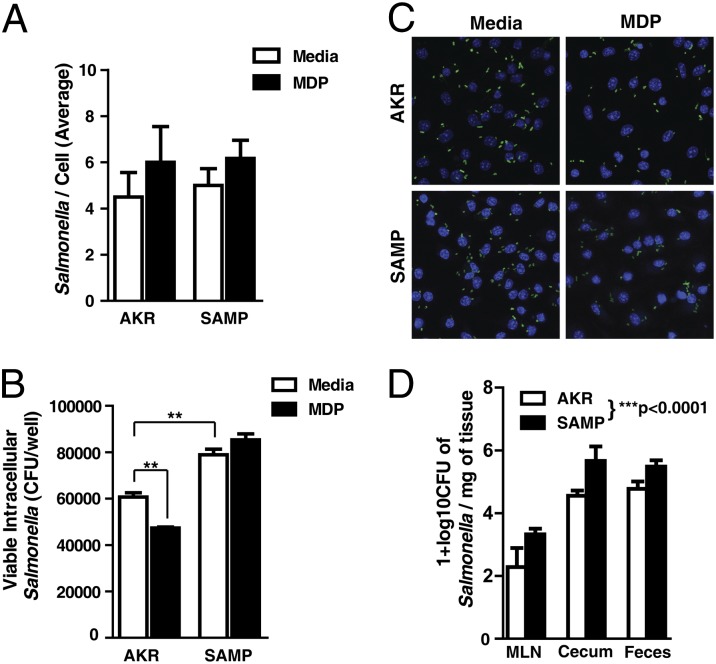
In addition to stimulating signaling pathways, MDP stimulation of NOD2 is known to enhance bacterial killing (9). Therefore, we examined whether the dysfunctional cytokine release in MDP-stimulated SAMP BMDMs also impeded the clearance of the intracellular pathogen, Salmonella typhimurium. BMDMs from preinflamed SAMP mice or AKR age-matched controls were infected with Salmonella in the presence or absence of MDP stimulation. Total bacterial loads were visualized by immunofluorescent confocal microscopy and viable intracellular Salmonella determined by gentamicin protection assay. No difference was observed in the total number of bacteria infecting BMDMs at this time point (Fig. 5 A and C). However, there was a significant decrease in the number of viable intracellular Salmonella recovered from AKR BMDMs that were stimulated with MDP (Fig. 5B). SAMP BMDMs had higher numbers of viable intracellular Salmonella than AKR BMDMs and were refractory to MDP stimulation. These results demonstrate reduced bacterial clearance in SAMP BMDMs, which is independent of bacterial internalization. MDP stimulation also fails to enhance bacterial killing in these cells, suggesting that NOD2 dysfunction plays a role in this defective bacterial clearance.
Fig. 5.
SAMP BMDMs have impaired intracellular bacterial killing and are unresponsive to MDP stimulation. BMDMs from preinflamed SAMP and AKR mice were infected with Salmonella typhimurium for 90 min in the presence and absence of MDP (10 μg/mL). (A) Quantification of immunofluorescent micrographs stained for total number of Salmonella per cell (six fields counted from two separate experiments; mean ± SEM). (B) Viable intracellular Salmonella recovered in gentamicin protection assays. (C) Confocal micrographs of infected BMDMs. Salmonella shown in red, and nuclei stained with DAPI (blue) (six independent experiments; mean ± SEM). The double asterisk (**) denotes significant differences at P < 0.01 (one-way ANOVA, pairwise Bonferroni). (D) SAMP and AKR mice were pretreated with streptomycin and infected with 109 CFU of Salmonella or with sterile PBS; bacterial loads from mesenteric lymph nodes (MLNs), cecum, and feces were calculated 2 d postinfection. SAMP mice were significantly more likely to yield higher Salmonella counts than AKR [linear regression, F(4,23), P < 0.00001, adjusted R2 = 0.7891].
SAMP Mice Are More Susceptible to Salmonella Invasion in Vivo.
To test whether SAMP mice have increased susceptibility to bacteria invasion in vivo, we infected SAMP mice and AKR controls intragastrically with 109 colony-forming units (CFU) of Salmonella. Bacterial loads from mesenteric lymph nodes (MLNs), cecum, and feces were calculated 2 d postinfection. As shown in Fig. 5D, Salmonella counts were significantly higher in MLNs, cecum, and feces of SAMP mice compared with those found in AKR controls. The increased bacterial burden in these tissues and fecal content demonstrates that SAMP mice are more susceptible to Salmonella invasion and possess a defective bacterial clearance in vivo.
Discussion
Although the precise molecular mechanisms responsible for the pathogenesis of CD remain unclear, increasing evidence supports the hypothesis that this chronic, relapsing inflammatory disease of the gut results from a primary defect in intestinal innate immunity. The most compelling support for this hypothesis comes from the clear genetic association of CD with carriage of polymorphisms within the CARD15 gene, which represent the most frequent genetic defect in CD (14, 24). CARD15 encodes the NOD2 protein, an innate immune PRR that detects intracellular peptidoglycan from the bacterial cell wall, of which MDP is the minimal activating component, and initiates a signaling cascade that results in NF-κB activation and cytokine production (4–6, 21), MHC cross-presentation (7), autophagy induction, and intracellular bacterial killing (8). The CD-associated NOD2 polymorphisms are considered a loss-of-function phenotype because they cause defective NF-κB activation and reduced cytokine production in response to MDP stimulation (4, 13). Although the NOD2 polymorphisms represent the first genetic risk factor associated with CD, they account for only ∼15–20% of CD cases (15). In the remaining 85% of CD patients that carry WT NOD2, either too much or too little NOD2 signaling might be deleterious and NOD2’s influence on innate immune signaling could be in such tight balance that any deviation, either positively or negatively, could cause immunologic dysfunction. In this context, we found evidence for a functional defect in NOD2 signaling in response to MDP stimulation in the SAMP mouse model of CD. Importantly, these unique inbred mice do not possess any mutations in the NOD2 gene, but develop a progressive, spontaneous CD-like ileitis histologically obvious after 10 wk of age, allowing us to study both preinflamed and inflamed disease states (16).
MDP-induced NOD2 signaling plays a protective role in certain animal models of colitis. As demonstrated previously, in vivo administration of MDP to mice leads to amelioration of both DSS- and TNBS-induced colitis (19). In fact, during earlier time points (i.e., 3 h after MDP pretreatment), MDP enhances the effects of subsequent TLR stimuli. In contrast, upon longer MDP pretreatment self- and cross-tolerance occurs as evidenced by up-regulation of inhibitory signaling molecules, such as IL-1 receptor-associated kinase 1, and subsequent down-regulation of inflammatory pathways (25). Further evidence for the down-regulatory effects of NOD2 signaling comes from ex vivo studies showing that MDP prestimulation of human monocyte-derived dendritic cells is followed by a diminished capacity of several TLR ligands to induce production of innate cytokines and also abolishes the subsequent ability of MDP to synergize with TLR3 and TLR9 in inducing IL-12, IL-6, and TNF-α (19). Interestingly, our results show that MDP administration is not protective against both the spontaneous SAMP CD-like ileitis and DSS-induced colitis in SAMP mice, consistent with the hypothesis that these mice possess an underlying functional defect in the NOD2 signaling pathway. We speculate that this defect is specific for NOD2 and does not involve other PRRs, including NOD1.
NOD2 is well known to be expressed in the cytosol of both professional antigen-presenting cells and, upon inflammatory stimulation, in intestinal epithelial cells (1). In the present study, we used BM chimera experiments to localize the defective response to MDP in SAMP mice to the hematopoietic compartment. This finding supports the concept that the inflammatory defect in CD is, in fact, systemic, even though the disease is principally localized to the gut (26). This is supported by a paper by Marks et al. (27) that showed that patients with CD had both impaired inflammatory responses in the colon and skin challenged by heat-killed bacteria. In these patients the ability to clear Escherichia coli at the site of injection was also impaired. Interestingly, we also observed impaired bacterial clearance in SAMP mice. In separate studies, Smith et al. (28) showed that macrophages derived from blood monocytes of CD patients fail to secrete proinflammatory cytokines and chemokines in response to bacteria or bacterial products. Of note, this phenotype was shared by all CD patients tested, regardless of their NOD2 genotype, and was markedly distinct from healthy controls. This parallels our findings that BMDMs from SAMP mice (which have a WT NOD2 genotype) are refractory to MDP-stimulated cytokine production and MDP-enhanced Salmonella clearance. Because NOD2 signaling is tightly linked to autophagy (9), it is possible that autophagic mechanisms are also impaired in SAMP mice. This hypothesis is actively being tested in our laboratory at the present time. Altogether, our findings strongly support the concept of a functional defect in innate immunity within the hematopoietic compartment of CD patients that renders patients unable to mount an effective immune response to acute bacterial injury. This functional defect of CD patients is mirrored in our SAMP mouse model of CD-like ileitis and suggests that NOD2 dysfunction in hematopoietic cells plays a critical role in disease pathogenesis.
Consistent with the in vivo studies, we found that MDP stimulation of BMDMs isolated from preinflamed SAMP mice resulted in abnormal cytokines responses. This dysfunction presented in acute signaling studies as an ∼20-min delay in BMDMs from SAMP mice responding to administration of MDP. Because intestinal immune homeostasis is in such tight balance with multiple cytokines and cell types influencing one another, even with ultimately normal amplitude, a delay in NOD2 signaling upon epithelial breach in vivo could cause a dysfunctional immune response. We propose that the delay in signaling may contribute to this defect by establishing a dysfunctional innate immune response that then amplifies as physiologic cytokines are not present in the proper time frame, context, or amount necessary for effective bacterial clearance.
Taken together, our study provides compelling evidence that CD may be initiated by a deficit in intestinal innate immunity, which is either genetic or functional in nature. In fact, we provide evidence that SAMP mice, which develop spontaneous CD-like ileitis in the absence of CARD15 genetic mutations, possess a NOD2 dysregulation that inhibits their ability to respond appropriately to bacterial stimulation. These findings shed important light on the initiating molecular events underlying CD and may have important therapeutic implications by facilitating the identification of patients with early disease who may benefit from interventions aimed at boosting innate immune responses and restoring physiological NOD2 function.
Materials and Methods
Experimental Animals.
SAMP and AKR mice were maintained under specific pathogen-free conditions, fed standard laboratory chow (Harlan Teklad), and kept on 12-h light/dark cycles. All procedures were approved by Case Western Reserve University’s Institutional Animal Care and Use Committee and Association for Assessment and Accreditation of Laboratory Animal Care guidelines. For a full description, see SI Materials and Methods.
Cells Isolation and Culture.
BM macrophages precursors were harvested from femurs of mice and cultured for 7 d in DMEM containing 10% FBS, 25 mM Hepes buffer, 1 mM sodium pyruvate, 5 × 10−5 2-ME, antibiotic, and 25% of LADMAC cell conditioned medium as a source of M-CSF. For a full description, see SI Materials and Methods.
ELISA.
BMDMs were stimulated for 24 h with MDP (1, 10, 100, 200 μg/mL) or LPS (10 ng/mL); secreted cytokines were measured by ELISA. For a full description, see SI Materials and Methods.
Western Blot Analysis.
Western blot was performed as described previously (29). Membranes were blotted with antibodies as follows: anti-P105, anti-phospho-IkBα, total-IκBα, and anti-actin (Cell Signaling). For a full description, see SI Materials and Methods.
Histology.
Colons and ilea from experimental mice were removed from mice and histologically evaluated as described (30). For a full description, see SI Materials and Methods.
Images Acquisition.
Images were obtained on an Olympus BX41 microscope. For a full description, see SI Materials and Methods.
Induction of Colitis and MDP Administration.
Induction of acute colitis was achieved in AKR, SAMP, and BM chimeric mice by exposing them to 3% DSS in their drinking water for 7 d. For a full description, see SI Materials and Methods.
Colonoscopic Investigation.
Colonoscopy was performed using a flexible digital ureteroscope on the day 7 of DSS treatment. For a full description, see SI Materials and Methods.
BM Chimeric Mice.
Mice receiving BM transfer were irradiated (900 radiation absorbed dose) immediately before transplantation. BM was harvested from femurs and tibias of 4-wk-old SAMP or AKR mice. For a full description, see SI Materials and Methods.
Myeloperoxidase Assay Activity.
Colon samples were assayed for myeloperoxidase (MPO) activity as previously described (31, 32). For a full description, see SI Materials and Methods.
Salmonella Infection Assays.
Salmonella infection assays were performed as previously described (9). For a full description, see SI Materials and Methods.
Salmonella Infection in Vivo.
SAMP and AKR control mice (4–6 wk) were infected with Salmonella for 2 d. For a full description, see SI Materials and Methods.
Statistical Analysis.
Analyses of continuous data were conducted using parametric Student t tests, one-way or two-way ANOVAs, or linear regression (when appropriate), or their nonparametric alternatives. For a full description, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Prof. Maria Grazia Cifone (University of L’Aquila) for scientific support; Dr. Marcello Chieppa for assistance with bone marrow chimeric mice; Dr. Amitabh Chak for help with the mouse colonoscopy; and Li-Guo Jia, Mitchell Guanzon, Dennis Gruszka, Sarah Kossak, Lindsey Kaydo, and Homer Craig for their technical support. This work was supported by National Institutes of Health Grants DK091222 (to F.C.), DK055812 (to F.C.), DK042191 (to F.C. and T.T.P.), and DK082437 (to C.M.), as well as the Howard Hughes Medical Institute “Med into Grad” Initiative.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311657110/-/DCSupplemental.
References
- 1.Gutierrez O, et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem. 2002;277(44):41701–41705. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- 2.Girardin SE, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278(11):8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 3.Inohara N, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278(8):5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 4.Inohara N, Nuñez G. NODs: Intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3(5):371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, Omori E, Matsumoto K, Núñez G, Ninomiya-Tsuji J. TAK1 is a central mediator of NOD2 signaling in epidermal cells. J Biol Chem. 2008;283(1):137–144. doi: 10.1074/jbc.M704746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JH, et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178(4):2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 7.Wagner CS, Cresswell P. TLR and nucleotide-binding oligomerization domain-like receptor signals differentially regulate exogenous antigen presentation. J Immunol. 2012;188(2):686–693. doi: 10.4049/jimmunol.1102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooney R, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16(1):90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 9. Homer CR, Richmond AL, Rebert NA, Achkar JP, McDonald C (2010) ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn's disease pathogenesis. Gastroenterology 139(5):1630–1641. [DOI] [PMC free article] [PubMed]
- 10.Hampe J, et al. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet. 2001;357(9272):1925–1928. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- 11.Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 12.Ogura Y, et al. Genetic variation and activity of mouse Nod2, a susceptibility gene for Crohn’s disease. Genomics. 2003;81(4):369–377. doi: 10.1016/s0888-7543(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 13.Bonen DK, et al. Crohn’s disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology. 2003;124(1):140–146. doi: 10.1053/gast.2003.50019. [DOI] [PubMed] [Google Scholar]
- 14.Barrett JC, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40(8):955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strober W, Kitani A, Fuss I, Asano N, Watanabe T. The molecular basis of NOD2 susceptibility mutations in Crohn’s disease. Mucosal Immunol. 2008;1(Suppl 1):S5–S9. doi: 10.1038/mi.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizarro TT, et al. SAMP1/YitFc mouse strain: A spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis. 2011;17(12):2566–2584. doi: 10.1002/ibd.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hisamatsu T, et al. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124(4):993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 18.Kim YG, et al. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28(2):246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118(2):545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson TS, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203(3):541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi K, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416(6877):194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 22.Netea MG, et al. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol. 2005;174(10):6518–6523. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- 23.Uehara A, et al. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol. 2005;7(1):53–61. doi: 10.1111/j.1462-5822.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 24.Strober W, Watanabe T. NOD2, an intracellular innate immune sensor involved in host defense and Crohn’s disease. Mucosal Immunol. 2011;4(5):484–495. doi: 10.1038/mi.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc Natl Acad Sci USA. 2007;104(49):19440–19445. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casanova JL, Abel L. Revisiting Crohn’s disease as a primary immunodeficiency of macrophages. J Exp Med. 2009;206(9):1839–1843. doi: 10.1084/jem.20091683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marks DJ, et al. Defective acute inflammation in Crohn’s disease: A clinical investigation. Lancet. 2006;367(9511):668–678. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- 28.Smith AM, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med. 2009;206(9):1883–1897. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutti JE, et al. IkappaB kinase beta phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-kappaB pathway. Mol Cell Biol. 2007;27(21):7451–7461. doi: 10.1128/MCB.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bamias G, et al. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128(3):654–666. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 31.Boughton-Smith NK, Wallace JL, Whittle BJ. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988;25(1–2):115–123. doi: 10.1007/BF01969102. [DOI] [PubMed] [Google Scholar]
- 32.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.